Pilot Studies Testing Novel Minimized Pan-Coronavirus (CoV) Vaccines in Feline Immunodeficiency Virus-Infected Cats With or Without Feline CoV Serotype-1 (FCoV1) Coinfection and in Specific-Pathogen-Free Cats Against Pathogenic FCoV2
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunoblot Strips for CoV RBDs and FIPV2 Whole-Virus
2.3. Cross-Neutralizing Antibodies (XNAbs) to FIPV2-UCD2 Using Fc9 Cell Line
2.4. Feline IFNγ and IL-2 ELISpot Analyses
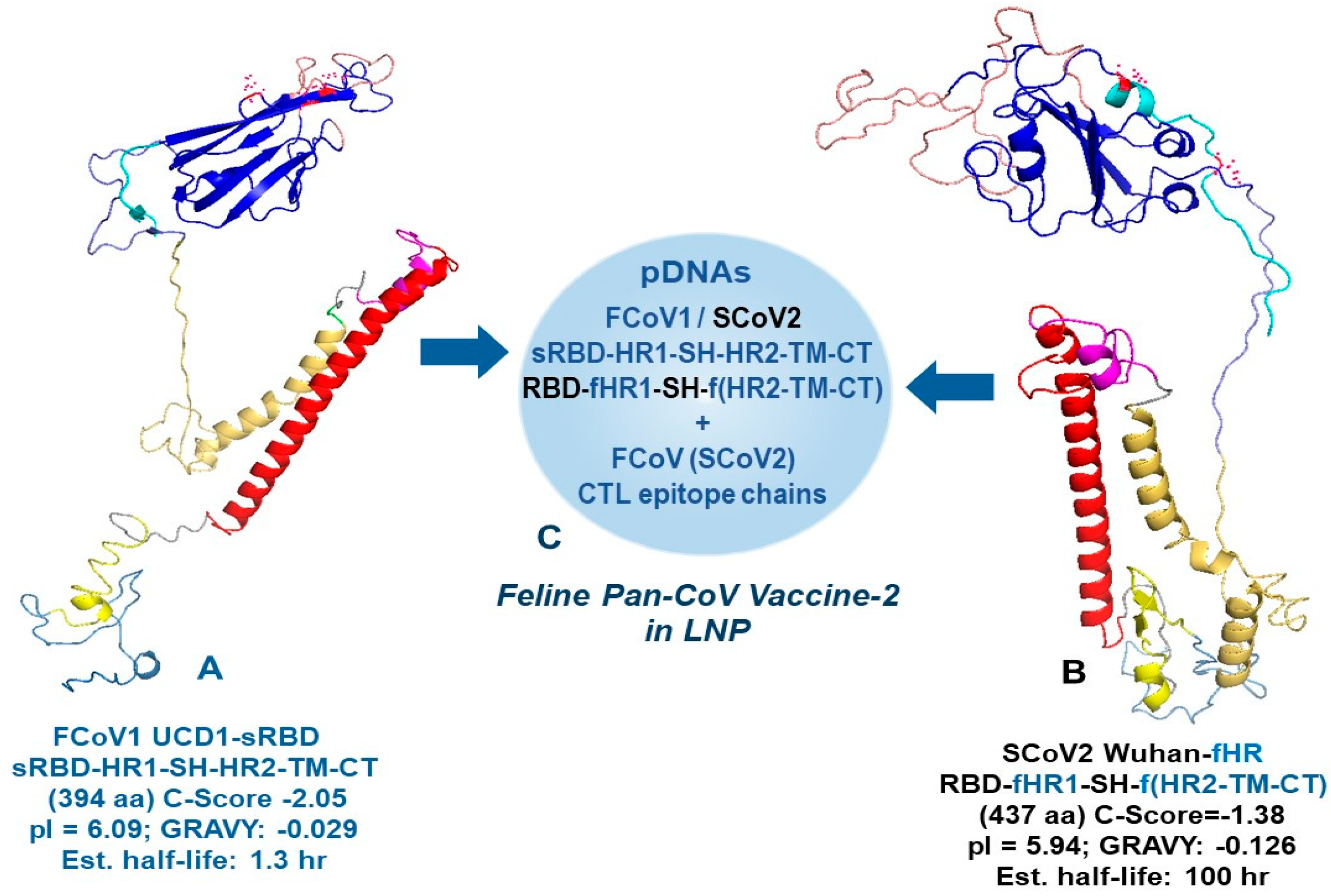
2.5. Developing and In Vitro Testing of Minimized pan-CoV Vaccines
2.5.1. Developing B-Cell Epitopes of Minimized pan-CoV Vaccines
2.5.2. Developing CTL Epitopes for All pan-CoV Vaccines
2.5.3. The 35 CTL Peptide Epitopes for the First Two Vaccinations in Study 1
2.5.4. Plasmid Construction
2.5.5. Production of pDNA-LNP-Based pan-CoV Vaccines
2.5.6. In Vitro Testing of Minimized pan-CoV Vaccines in Feline Cell Line
2.6. FIPV RT-PCR and Immunohistochemistry for Detection of FIPV2
2.7. Pilot Study 1
2.8. Pilot Study 2
2.9. Statistical Analyses
3. Results
3.1. Developing and In Vitro Testing of Minimized Pan-CoV Vaccines
3.1.1. Developing B-Cell Epitopes for pan-CoV Vaccine-1
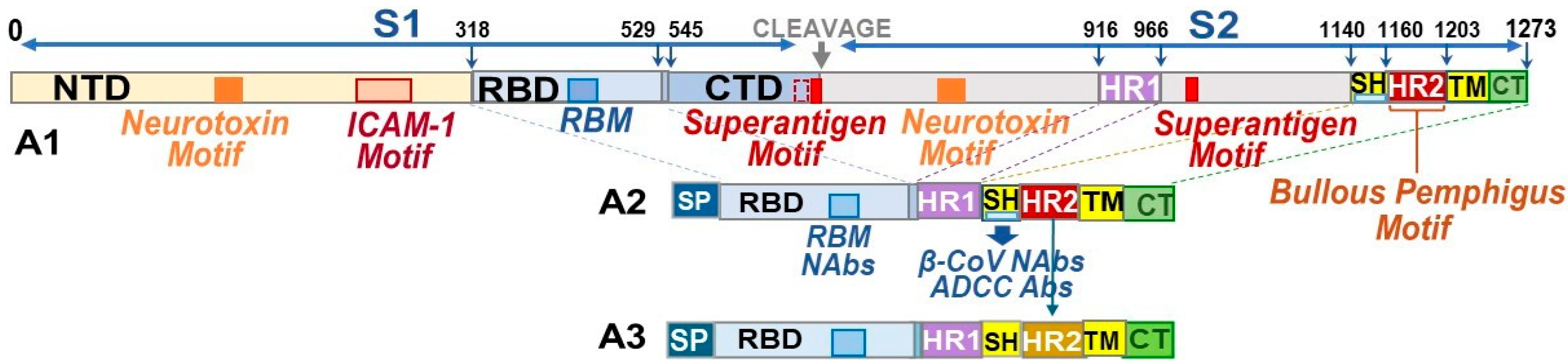
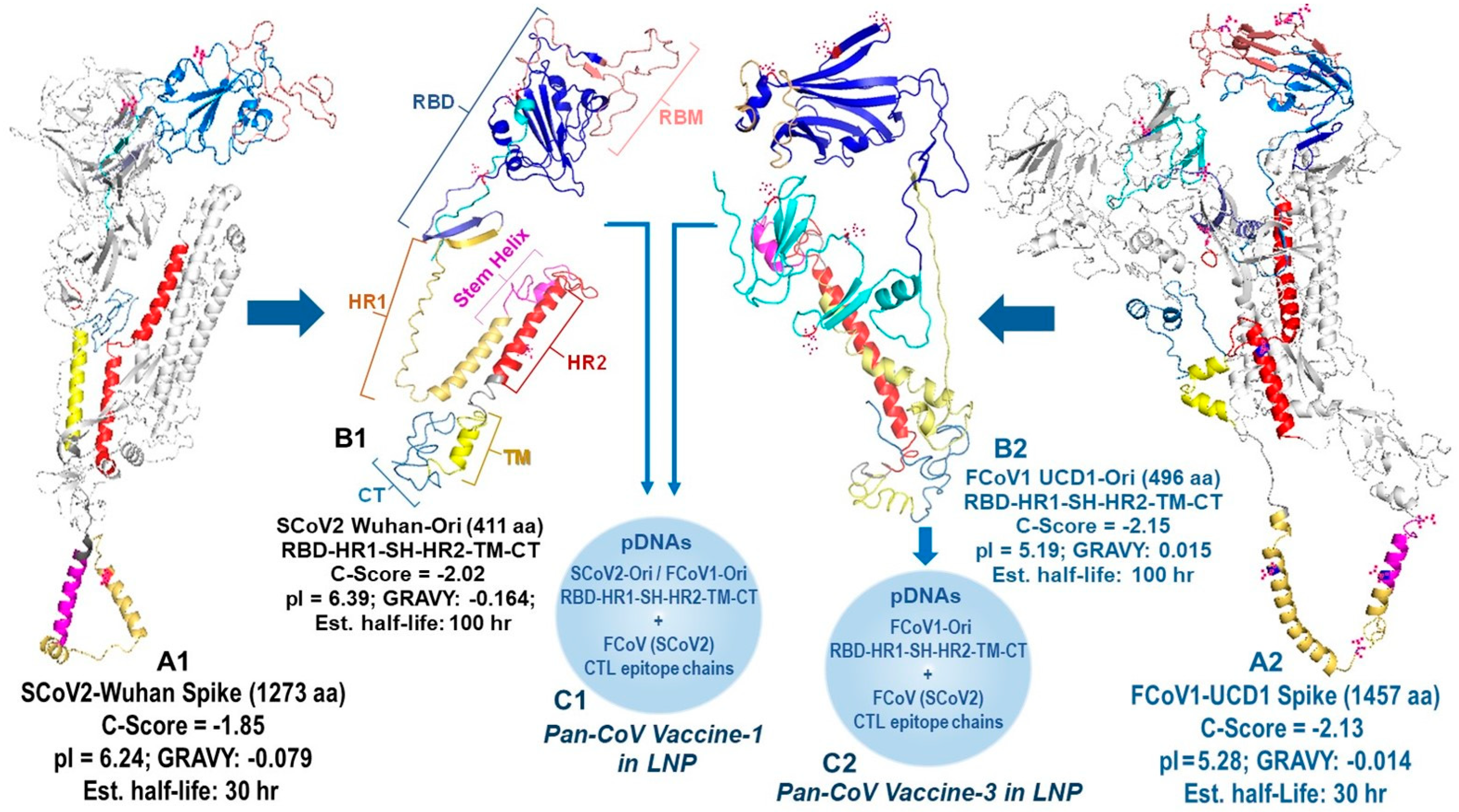
3.1.2. Developing B-Cell Epitopes for pan-CoV Vaccine-2
3.1.3. Developing B-Cell Epitopes for pan-CoV Vaccine-3
3.1.4. Developing CTL Epitopes for All pan-CoV Vaccines
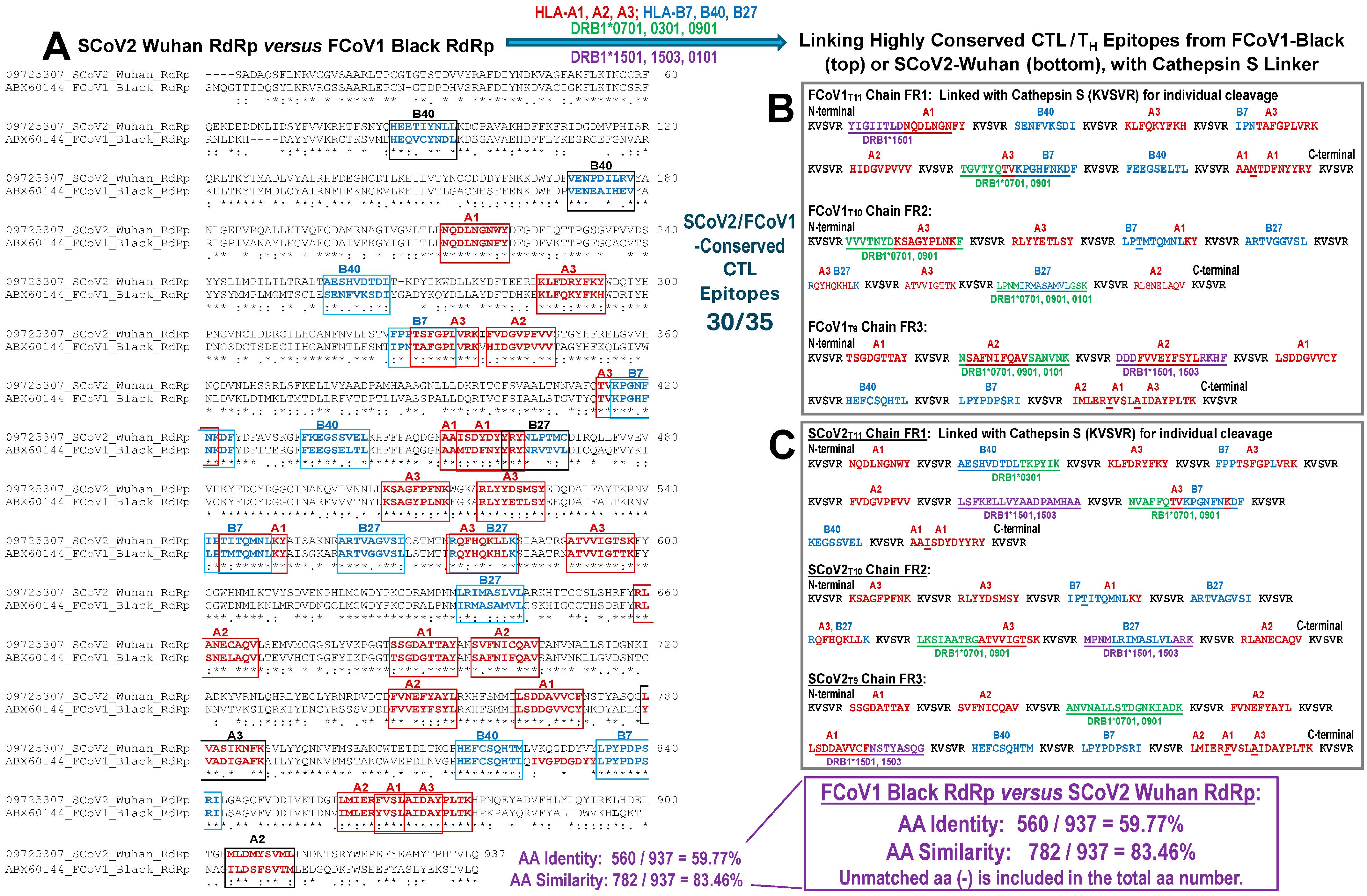
3.1.5. The In Vitro Expression of Minimized pan-CoV Vaccines in Feline Cell Line
3.2. Pilot Study 1: Testing of pan-CoV Vaccine-1 in FIV-Infected Cats With or Without FCoV1 Coinfection
3.2.1. Pilot Study 1: Schedule, Cat Distribution, and Vaccine-Induced Adverse Effects Four
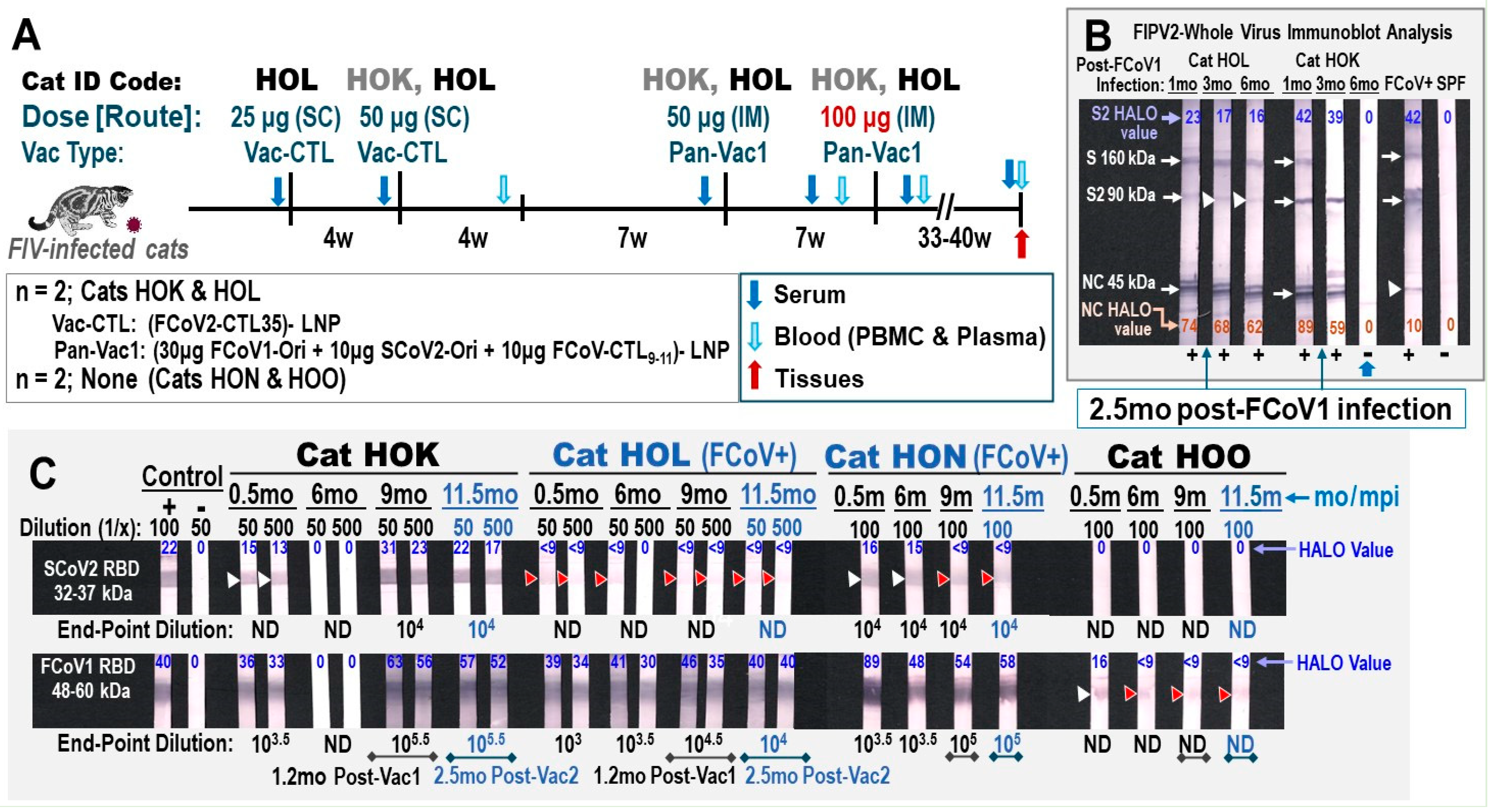
3.2.2. Pilot Study 1: Pan-CoV Vaccine-1-Induced Antibodies
3.2.3. Pilot Study 1: T-Cell Immunity Induced by FCoV1/SCoV2 RBDs
3.3. Pilot Study 2: Testing of pan-CoV Vaccines in SPF Cats
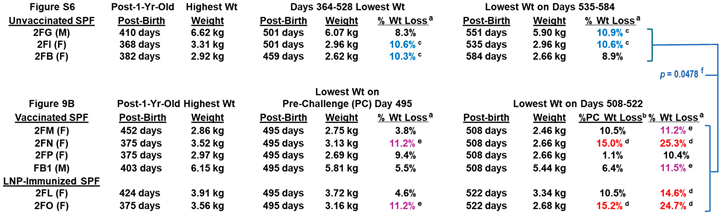
3.3.1. Pilot Study 2 Schedule, Vaccine-Induced Adverse Effects, and Clinical Signs Post-Challenge
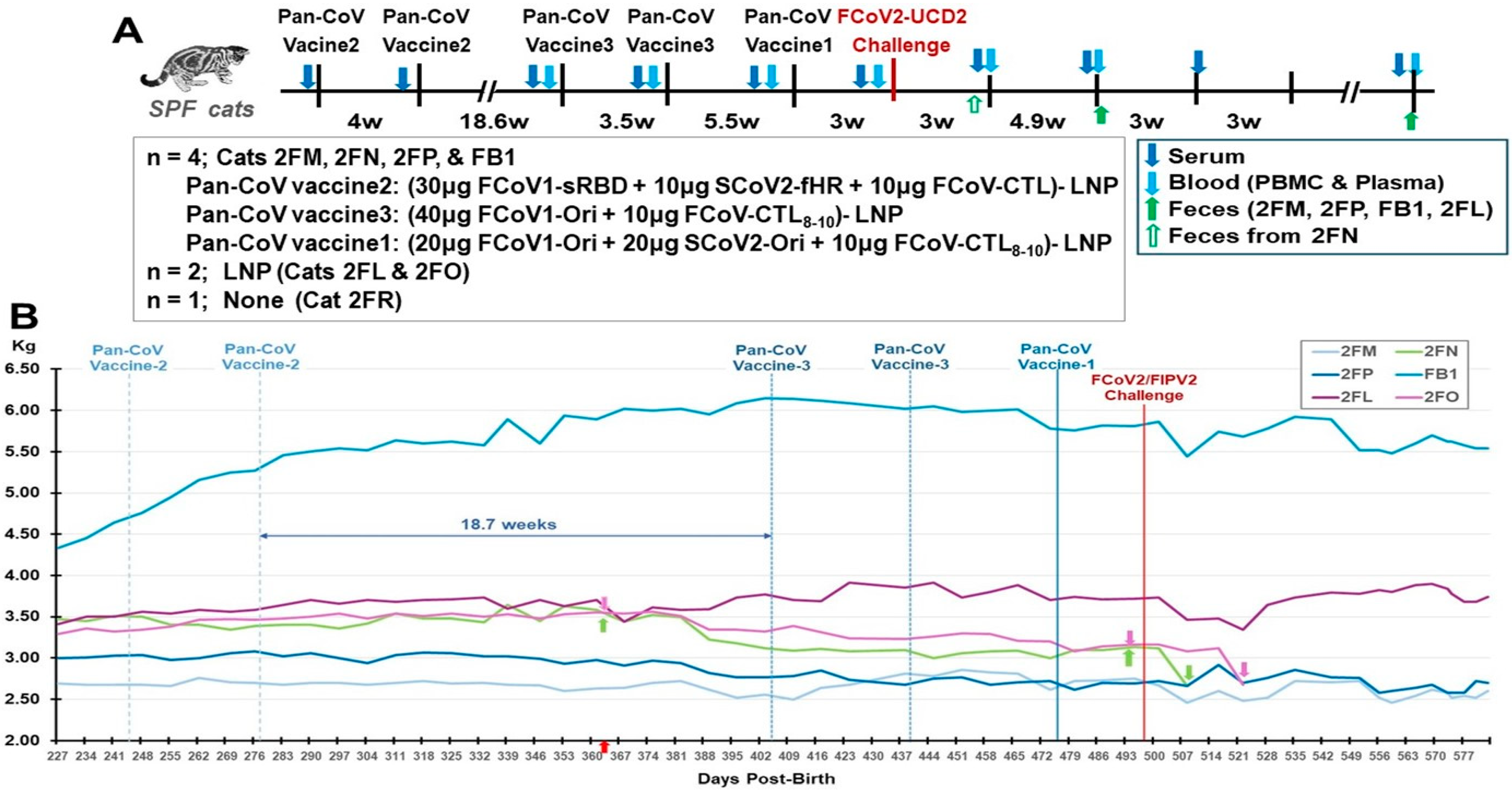
3.3.2. Pilot Study 2: B-Cell-Based pan-CoV Vaccine Abs

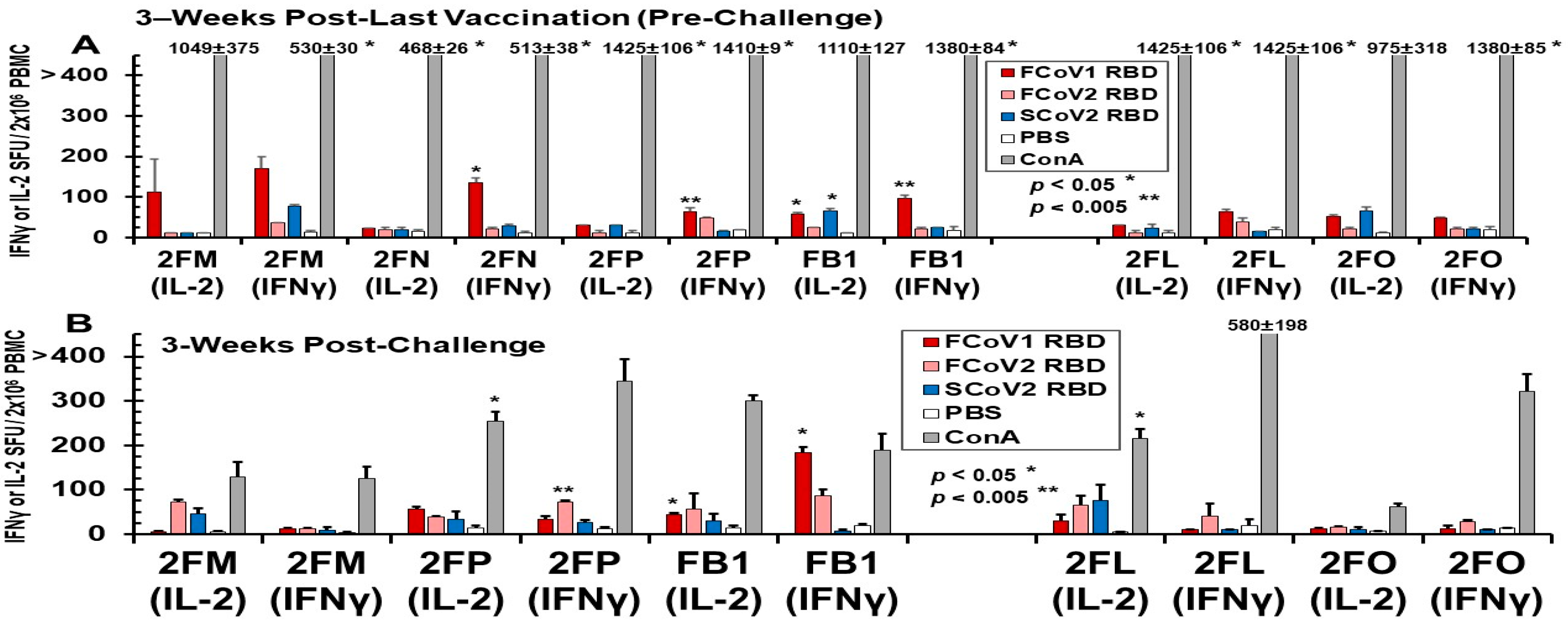
3.3.3. Pilot Study 2: T-Cell Immunity Induced by FCoV1/SCoV2 RBDs of B-Cell Constructs
3.3.4. Pilot Study 2: FIPV2 Challenge Infection Demonstrated by Fecal FIPV2 Load and High-Dose FIPV2 Immunoblot Strips
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nooruzzaman, M.; Diel, D.G. Infection dynamics, pathogenesis, and immunity to SARS-CoV-2 in naturally susceptible animal species. J. Immunol. 2023, 211, 1195–1201. [Google Scholar] [CrossRef]
- Sweet, A.N.; André, N.M.; Stout, A.E.; Licitra, B.N.; Whittaker, G.R. Clinical and molecular relationships between COVID-19 and feline infectious peritonitis (FIP). Viruses 2022, 14, 481. [Google Scholar] [CrossRef]
- Hosie, M.J.; Hofmann-Lehmann, R.; Hartmann, K.; Egberink, H.; Truyen, U.; Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Anthropogenic infection of cats during the 2020 COVID-19 pandemic. Viruses 2021, 3, 185. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Kuroda, Y.; Uda, A.; Kaku, Y.; Okutani, A.; Hotta, A.; Tatemoto, K.; Ishijima, K.; Inoue, Y.; Harada, M.; et al. The comparison of pathogenicity among SARS-CoV-2 variants in domestic cats. Sci. Rep. 2024, 14, 21815. [Google Scholar] [CrossRef]
- Pedersen, N.C. An update on feline infectious peritonitis: Virology and immunopathogenesis. Vet. J. 2014, 201, 123–132. [Google Scholar] [CrossRef]
- Hartmann, K. Feline infectious peritonitis. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 39–79. [Google Scholar] [CrossRef] [PubMed]
- Zuzzi-Krebitz, A.M.; Buchta, K.; Bergmann, M.; Krentz, D.; Zwicklbauer, K.; Dorsch, R.; Wess, G.; Fischer, A.; Matiasek, K.; Hönl, A.; et al. Short treatment of 42 days with oral GS-441524 results in equal efficacy as the recommended 84-day treatment in cats suffering from feline infectious peritonitis with effusion-a prospective randomized controlled study. Viruses 2024, 6, 1144. [Google Scholar] [CrossRef]
- Murphy, B.G.; Perron, M.; Murakami, E.; Bauer, K.; Park, Y.; Eckstrand, C.; Liepnieks, M.; Pedersen, N.C. The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet. Microbiol. 2018, 1219, 226–233. [Google Scholar] [CrossRef]
- Taylor, S.S.; Coggins, S.; Barker, E.N.; Gunn-Moore, D.; Jeevaratnam, K.; Norris, J.M.; Hughes, D.; Stacey, E.; MacFarlane, L.; O’Brien, C.; et al. Retrospective study and outcome of 307 cats with feline infectious peritonitis treated with legally sourced veterinary compounded preparations of remdesivir and GS-441524 (2020–2022). J. Feline Med. Surg. 2023, 25, 10986. [Google Scholar] [CrossRef]
- Scherk, M.A.; Ford, R.B.; Gaskell, R.M.; Hartmann, K.; Hurley, K.F.; Lappin, M.R.; Levy, J.K.; Little, S.E.; Nordone, S.K.; Sparkes, A.H. 2013 AAFP Feline Vaccination Advisory Panel Report. J. Feline Med. Surg. 2013, 15, 785–808. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.E.A.; Brummet, G.O.; Carozza, E.M.; Kass, P.H.; Petersen, E.P.; Sykes, J.; Westman, M.E. 2020 AAHA/AAFP Feline Vaccination Guidelines. Not Recommended Vaccines. JAAHA. Available online: https://www.aaha.org/aaha-guidelines/2020-aahaaafp-feline-vaccination-guidelines/feline-vaccination-home/ (accessed on 27 June 2025).
- O’Brian, A.; Mettelman, R.C.; Volk, A.; Andre, N.M.; Whittaker, G.R.; Baker, S.C. Characterizing replication kinetics and plaque production of type I feline infectious virus in three feline cell lines. Virology 2018, 525, 1–9. [Google Scholar] [CrossRef]
- Thiel, V.; Thiel, H.-J.; Tekes, G. Tackling feline infectious peritonitis via reverse genetics. Bioengineered 2014, 5, 396–400. [Google Scholar] [CrossRef]
- Addie, D.D.; Jarrett, J.O. Use of a reverse-transcriptase polymerase chain reaction for monitoring feline coronavirus shedding by healthy cats. Vet. Rec. 2001, 148, 649–653. [Google Scholar] [CrossRef]
- Drechsler, Y.; Alcaraz, A.; Bossong, F.J.; Collisson, E.W.; Diniz, P.P.V.P. Feline coronavirus in multicat environments. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1133–1169. [Google Scholar] [CrossRef] [PubMed]
- Barrs, V.R.; Peiris, M.; Tam, K.W.S.; Law, P.Y.T.; Brackman, C.J.; To, E.M.W.; Yu, V.Y.T.; Chu, D.K.W.; Perera, R.A.P.M.; Sit, T.H.C. SARS-CoV-2 in quarantined domestic cats from COVID-19 households or close contacts, Hong Kong, China. Emerg. Infect. Dis. 2020, 26, 3071–3074. [Google Scholar] [CrossRef]
- Klaus, J.; Meli, M.L.; Willi, B.; Nadeau, S.; Beisel, C.; Stadler, T.; SARS-CoV-Sequencing Team; Egberink, H.; Zhao, S.; Lutz, H.; et al. Detection and genome sequencing of SARS-CoV-2 in a domestic cat with respiratory signs in Switzerland. Viruses 2021, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.K.; Edison, L.K.; Rowe-Haas, D.K.; Takano, T.; Gilor, C.; Crews, C.D.; Tuanyok, A.; Arukha, A.P.; Shiomitsu, S.; Walden, H.D.S.; et al. Both feline coronavirus serotypes 1 and 2 infected domestic cats develop cross-reactive antibodies to SARS-CoV-2 receptor binding domain: Its implication to pan-CoV vaccine development. Viruses 2023, 15, 914. [Google Scholar] [CrossRef]
- Singh, A.K.; Goel, K.; Dhanawat, M. Plasmid DNA and mRNA delivery: Approaches and challenges. Adv. Immunol. 2025, 165, 63–87. [Google Scholar]
- Starr, T.N.; Czudnochowski, N.; Liu, Z.; Zatta, F.; Park, Y.J.; Addetia, A.; Pinto, D.; Beltramello, M.; Hernandez, P.; Greaney, A.J.; et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature 2021, 597, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Zhang, S.; Porritt, R.A.; Rivas, M.N.; Paschold, L.; Wilscher, E.; Binder, M.; Arditi, M.; Bahar, I. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 25254–25262. [Google Scholar] [CrossRef]
- Tu, T.H.; Bennani, F.E.; Masroori, N.; Liu, C.; Nemati, A.; Rozza, N.; Grunbaum, A.M.; Kremer, R.; Milhalcioiu, C.; Roy, D.C.; et al. The identification of a SARs-CoV2 S2 protein derived peptide with super-antigen-like stimulatory properties on T-cells. Commun. Biol. 2025, 8, 14. [Google Scholar] [CrossRef]
- Ma, X.; Zou, F.; Yu, F.; Li, R.; Yuan, Y.; Zhang, Y.; Zhang, X.; Deng, J.; Chen, T.; Song, Z.; et al. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses. Immunity 2020, 53, 1315–1330. [Google Scholar] [CrossRef]
- He, C.; Yang, J.; Hong, W.; Chen, Z.; Peng, D.; Lei, H.; Alu, A.; He, X.; Bi, Z.; Jiang, X.; et al. A self-assembled trimeric protein vaccine induces protective immunity against Omicron variant. Nat. Commun. 2022, 13, 5459. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Güler, A.; et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Q. The key site variation and immune challenges in SARS-CoV-2 evolution. Vaccines 2023, 11, 1472. [Google Scholar] [CrossRef]
- HALO Image Analysis Platform, version 4.1.5944 and HALO AI version 4.1.5944; Indica Labs, Inc.: Albuquerque, NM, USA, 2025.
- Nair, S.; Sahay, B.; Arukha, A.P.; Edison, L.K.; Crews, C.D.; Morris, J.G., Jr.; Kariyawasam, S.; Yamamoto, J.K. Interferon-γ/IL-2 and mRNA responses to the SARS-CoV2, feline coronavirus serotypes 1 (FCoV1), and FCoV2 receptor binding domains by the T cells from COVID-19-vaccinated humans and FCoV1-infected cats. Methods Mol. Biol. 2024, 2768, 135–151. [Google Scholar] [PubMed]
- Sahay, B.; Aranyos, A.M.; McAvoy, A.; Yamamoto, J.K. Utilization of feline ELISpot to evaluate the immunogenicity of a T cell-based FIV MAP vaccine. Methods Mol. Biol. 2018, 1808, 197–219. [Google Scholar]
- I-TASSER Server for Protein Structure & Function Predictions (Zhang Lab, University of Michigan, Ann Arbor, MI and National University of Singapore, Kent Ridge, Singapore). Available online: https://zhanggroup.org/I-TASSER/ (accessed on 30 May 2025).
- Schrodinger LLC. PyMOL 2.5 and 3.0.3 Systems. Available online: https://pymol.org/2/ (accessed on 26 June 2025).
- Pinto, D.; Sauer, M.M.; Czudnochowski, N.; Low, J.S.; Tortorici, M.A.; Housley, M.P.; Noack, J.; Walls, A.C.; Bowen, J.E.; Guarino, B.; et al. Broad betacoronavirus neutralization by a stem helix-specific human antibody. Science 2021, 373, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Expasy ProtParam Tool. Available online: https://web.expasy.org/protparam/ (accessed on 25 June 2025).
- Kida, K.; Hohdatsu, T.; Fujii, K.; Koyama, H. Selection of antigenic variants of the S glycoprotein of feline infectious peritonitis virus and analysis of antigenic sites involved in neutralization. J. Vet. Med. Sci. 1999, 61, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Corapi, W.V.; Darteil, R.J.; Audonnet, J.C.; Chappuis, G.E. Localization of antigenic sites of the S glycoprotein of feline infectious peritonitis virus involved in neutralization and antibody-dependent enhancement. J. Virol. 1995, 69, 2858–2862. [Google Scholar] [CrossRef] [PubMed]
- DTU Health, Tech Department of Health Technology, Lyngby, Denmark, NetMHCpan 4.1 Server. Available online: https://services.healthtech.dtu.dk/services/NetMHCpan-4.1/ (accessed on 26 June 2025).
- DTU Health, Tech Department of Health Technology, Lyngby, Denmark, NetCTL 1.2 Server. Available online: https://services.healthtech.dtu.dk/services/NetCTL-1.2/ (accessed on 26 June 2025).
- DTU Health, Tech Department of Health Technology, Lyngby, Denmark, NetMHCIIpan 4.0 Server. Available online: https://services.healthtech.dtu.dk/services/NetMHCIIpan-4.0/ (accessed on 27 June 2025).
- DTU Health, Tech Department of Health Technology, Lyngby, Denmark, NetMHCII 2.3 Server. Available online: https://services.healthtech.dtu.dk/services/NetMHCII-2.3/ (accessed on 27 June 2025).
- JustBio Server, AgileBio LLC, San Diego, CA. Available online: https://justbio.com/ (accessed on 24 June 2025).
- Marsh, S.G.E.; Parham, P.; Barber, L.D. The HLA Facts Book; Academic Press: London, UK, 2000; pp. 103–241. [Google Scholar]
- Allele Frequencies in Worldwide Populations Database Provides Top 2-3 HLA Alleles Under Population of USA Caucasian and USA African American in Their Surveys. Available online: http://www.allelefrequencies.net/hla6006a.asp (accessed on 27 June 2025).
- National Marrow Donor Program: High-Resolution HLA Alleles and Haplotypes in the US Populations as of 2006. Available online: https://network.nmdp.org/services-support/bioinformatics-immunobiology/haplotype-frequencies/high-resolution-hla-alleles-and-haplotypes-in-the-us-populations-as-of-2006 (accessed on 26 June 2025).
- Aranyos, A.M.; Roff, S.R.; Pu, R.; Owen, J.L.; Coleman, J.K.; Yamamoto, J.K. An initial examination of the potential role of T-cell immunity in protection against feline immunodeficiency virus (FIV) infection. Vaccine 2016, 34, 1480–1488. [Google Scholar] [CrossRef]
- Takara Xfect Transfectiton Reagent Protocol-At-A-Glance (PT5003-2, Takara Bio USA, Inc. San Jose, CA). Available online: https://www.takarabio.com/documents/User%20Manual/Xfect%20Transfection%20Reagent%20Protocol/Xfect%20Transfection%20Reagent%20Protocol-At-A-Glance_103012.pdf (accessed on 1 July 2025).
- Tanaka, Y.; Sasaki, T.; Matsuda, R.; Uematsu, Y.; Yamaguchi, T. Molecular epidemiological study of feline coronavirus strains in Japan using RT-PCR targeting nsp14 gene. BMC Vet. Res. 2015, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Masangkay, J.S.; Nagata, N.; Morikawa, S.; Mizutani, T.; Fukushi, S.; Alviola, P.; Omatsu, T.; Ueda, N.; Iha, K.; et al. Bat coronaviruses and experimental infection of bats, the Philippines. Emerg. Infect. Dis. 2010, 16, 1217–1223. [Google Scholar] [CrossRef]
- Holbrook, M.G.; Anthony, S.J.; Navarrele-Macias, I.; Bestebroer, T.; Muster, V.J.; van Doremalen, N. Updated and validated pan-coronavirus PCR assay to detect all coronavirus genera. Viruses 2021, 13, 599. [Google Scholar] [CrossRef]
- Steiner, S.; Kratzel, A.; Barut, G.T.; Lang, R.M.; Moreira, E.A.; Thomann, L.; Kelly, J.N.; Thiel, V. Sars-CoV-2 biology and host interactions. Nat. Rev. Microbiol. 2024, 22, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, H.; Pyrc, K.; van der Hoek, L.; Geier, M.; Berkhout, B.; Pöhlmann, S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [Google Scholar] [CrossRef]
- Wu, K.; Li, W.; Peng, G.; Li, F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc. Natl. Acad. Sci. USA 2009, 106, 19970–19974. [Google Scholar] [CrossRef]
- Lützner, N.; Kalbacher, H. Quantifying cathepsin S activity in antigen presenting cells using a novel specific substrate. J. Biol. Chem. 2008, 283, 36185–36194. [Google Scholar] [CrossRef]
- Huang, C.; Conlee, D.; Gill, M.; Chu, H.J. Dual-subtype feline immunodeficiency virus vaccine provides 12 months of protective immunity against heterologous challenge. J. Feline Med. Surg. 2010, 12, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-based nanoparticles for delivery of vaccine adjuvants and antigens: Toward multicomponent vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Zhang, X.; Hasoksuz, M.; Nagesha, H.S.; Haynes, L.M.; Fang, Y.; Lu, S.; Saif, L.J. Two-way antigenic cross-reactivity between severe acute respiratory syndrome coronavirus (SARS-CoV) and Group 1 animal CoVs is mediated through an antigenic site in the N-terminal region of the SARS-CoV nucleoprotein. J. Virol. 2007, 81, 13365–13377. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, W.; Schuurman, N.; van Kuppeveld, F.; Bosch, B.J.; Egberink, H. Serological Screening for coronavirus infections in cats. Viruses 2019, 11, 743. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Kurotani, A.; Sato, K.I. Protein pI and intracellular localization. Front. Mol. Biosci. 2021, 8, 775736. [Google Scholar] [CrossRef]
- Kurotani, A.; Tokmakov, A.A.; Sato, K.I.; Stefanov, V.E.; Yamada, Y.; Sakurai, T. Localization-specific distributions of protein pI in human proteome are governed by local pH and membrane charge. BMC Mol. Cell Biol. 2019, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 920–1931. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Gao, F.-X.; Wu, R.-X.; Shen, M.-Y.; Huang, J.-J.; Li, T.T.; Hu, C.; Luo, F.-Y.; Song, S.-Y.; Mu, S.; Hao, Y.-N.; et al. Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. iScience 2022, 25, 105479. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y.; Jiang, S. Recent advances in lipid nanoparticles and their safety concerns for mRNA delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef]
- Tahtinen, S.; Tong, A.-J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Sherding, R.G. Feline infectious peritonitis (Feline coronavirus). In Saunders Manual of Small Animal Practice, 3rd ed.; Birchard, S.J., Sherding, R.G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2006; Chapter 10; pp. 132–143. [Google Scholar]
- Meli, M.; Kipar, A.; Muller, C.; Jenal, K.; Gonczi, E.; Borel, N.; Gunn-Moore, D.; Chalmers, S.; Lin, F.; Reinacher, M.; et al. High viral loads despite absence of clinical and pathological findings in cats experimentally infected with feline coronavirus (FCoV) type I and in naturally FCoV-infected cats. J. Feline Med. Surg. 2004, 6, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Felten, S.; Klein-Richers, U.; Hofmann-Lehmann, R.; Bergmann, M.; Unterer, S.; Leutenegger, C.M.; Hartmann, K. Correlation of feline coronavirus shedding in feces with coronavirus antibody titer. Pathogens 2020, 9, 598. [Google Scholar] [CrossRef]
- Stranieri, A.; Scavone, D.; Paltrinieri, S.; Giordano, A.; Bonsembiante, F.; Ferro, S.; Gelain, M.E.; Meazzi, S.; Lauzi, S. Concordance between histology, immunohistochemistry, and RT-PCR in the diagnosis of feline infectious peritonitis. Pathogens 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Mônica Slaviero, M.; Cony, F.G.; da Silva, R.C.; De Lorenzo, C.; de Almeida, B.A.; Bertolini, M.; Driemeier, D.; Pavarini, S.P.; Sonne, L. Pathological findings and patterns of feline infectious peritonitis in the respiratory tract of cats. J. Comp. Pathol. 2024, 210, 15–24. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Parodi, M.C.; Cammarata, G. In vivo diagnosis of feline infectious peritonitis by comparison of protein content, cytology, and direct immunofluorescence test on peritoneal and pleural effusions. J. Vet. Diagn. Investig. 1999, 11, 358–361. [Google Scholar] [CrossRef]
- Takano, T.; Yamada, S.; Doki, T.; Hohdatsu, T. Pathogenesis of oral type I feline infectious peritonitis virus (FIPV) infection: Antibody dependent enhancement infection of cats with type I FIPV via the oral route. J. Vet. Med. Sci. 2019, 81, 911–915. [Google Scholar] [CrossRef]
- Sahay, B.; Aranyos, A.M.; Mishra, M.; McAvoy, A.C.; Martin, M.M.; Pu, R.; Shiomitsu, S.; Shiomitsu, K.; Dark, M.J.; Sanou, M.P.; et al. Immunogenicity and efficacy of a novel multi-antigenic peptide vaccine based on cross-reactivity between feline and human immunodeficiency viruses. Viruses 2019, 11, 136. [Google Scholar] [CrossRef]




 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, P.; Prevedello, M.B.; Arukha, A.P.; Stevenson, V.; Keisling, K.F.; Nycum, T.G.; Beam, N.M.; Barras, E.D.; Sahay, B.; Yamamoto, J.K. Pilot Studies Testing Novel Minimized Pan-Coronavirus (CoV) Vaccines in Feline Immunodeficiency Virus-Infected Cats With or Without Feline CoV Serotype-1 (FCoV1) Coinfection and in Specific-Pathogen-Free Cats Against Pathogenic FCoV2. Vaccines 2025, 13, 1172. https://doi.org/10.3390/vaccines13111172
Sinha P, Prevedello MB, Arukha AP, Stevenson V, Keisling KF, Nycum TG, Beam NM, Barras ED, Sahay B, Yamamoto JK. Pilot Studies Testing Novel Minimized Pan-Coronavirus (CoV) Vaccines in Feline Immunodeficiency Virus-Infected Cats With or Without Feline CoV Serotype-1 (FCoV1) Coinfection and in Specific-Pathogen-Free Cats Against Pathogenic FCoV2. Vaccines. 2025; 13(11):1172. https://doi.org/10.3390/vaccines13111172
Chicago/Turabian StyleSinha, Pranaw, Marco B. Prevedello, Ananta P. Arukha, Valentina Stevenson, Karen F. Keisling, Taylor G. Nycum, Nina M. Beam, Elise D. Barras, Bikash Sahay, and Janet K. Yamamoto. 2025. "Pilot Studies Testing Novel Minimized Pan-Coronavirus (CoV) Vaccines in Feline Immunodeficiency Virus-Infected Cats With or Without Feline CoV Serotype-1 (FCoV1) Coinfection and in Specific-Pathogen-Free Cats Against Pathogenic FCoV2" Vaccines 13, no. 11: 1172. https://doi.org/10.3390/vaccines13111172
APA StyleSinha, P., Prevedello, M. B., Arukha, A. P., Stevenson, V., Keisling, K. F., Nycum, T. G., Beam, N. M., Barras, E. D., Sahay, B., & Yamamoto, J. K. (2025). Pilot Studies Testing Novel Minimized Pan-Coronavirus (CoV) Vaccines in Feline Immunodeficiency Virus-Infected Cats With or Without Feline CoV Serotype-1 (FCoV1) Coinfection and in Specific-Pathogen-Free Cats Against Pathogenic FCoV2. Vaccines, 13(11), 1172. https://doi.org/10.3390/vaccines13111172









