Evaluation of Cholera Toxin B Subunit as a Novel Carrier Protein for Polysaccharide Conjugate Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Expression Plasmid
2.2. Protein Expression and Purification
2.3. Polysaccharide Fermentation and Purification
2.4. Conjugation, Purification, and Vaccine Formulation
2.5. Rabbit Immunization Study
2.6. IgG Quantification by Multiplex Bead-Based Immunoassay
3. Results
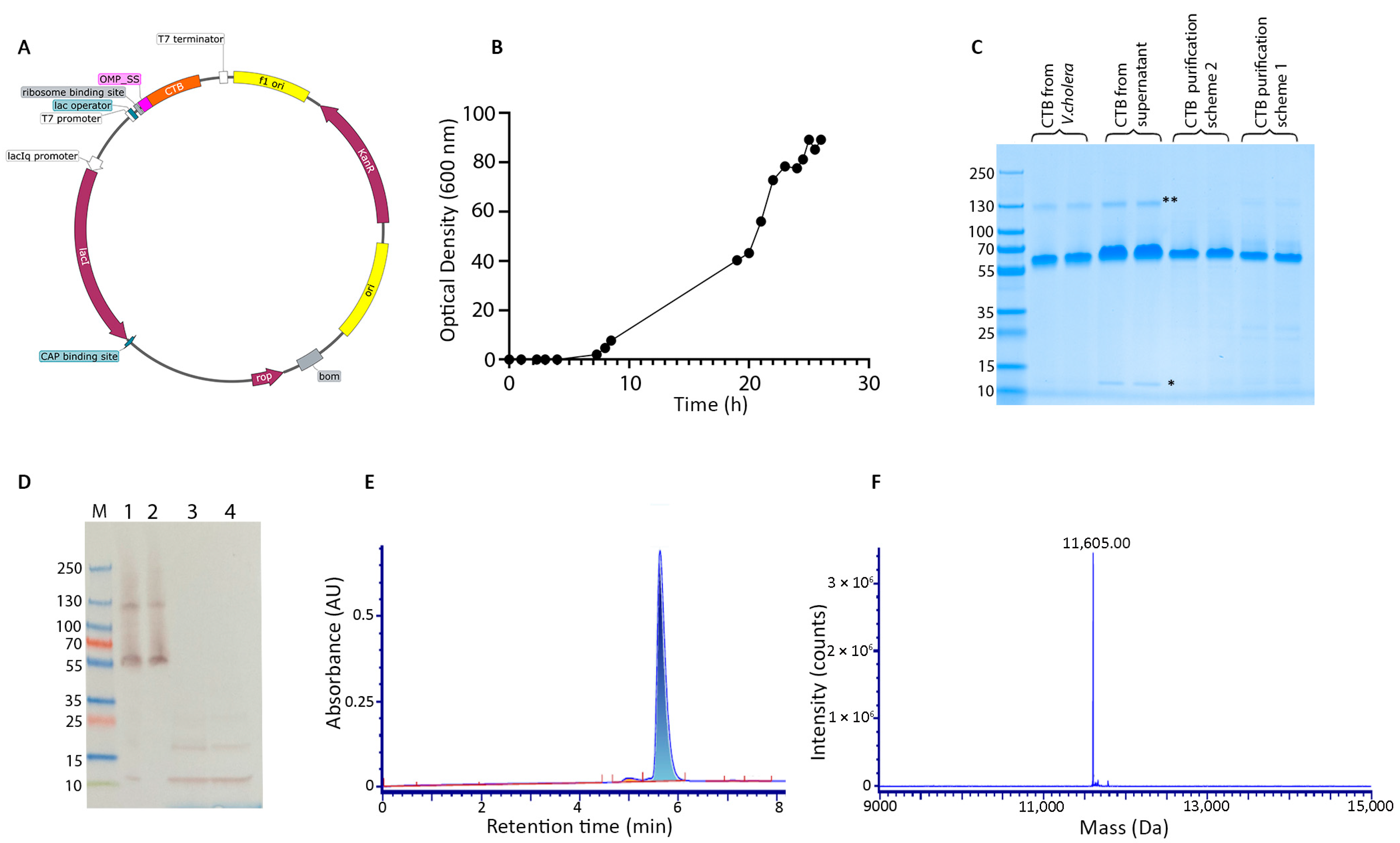
3.1. E. coli Fermentation and Protein Purification
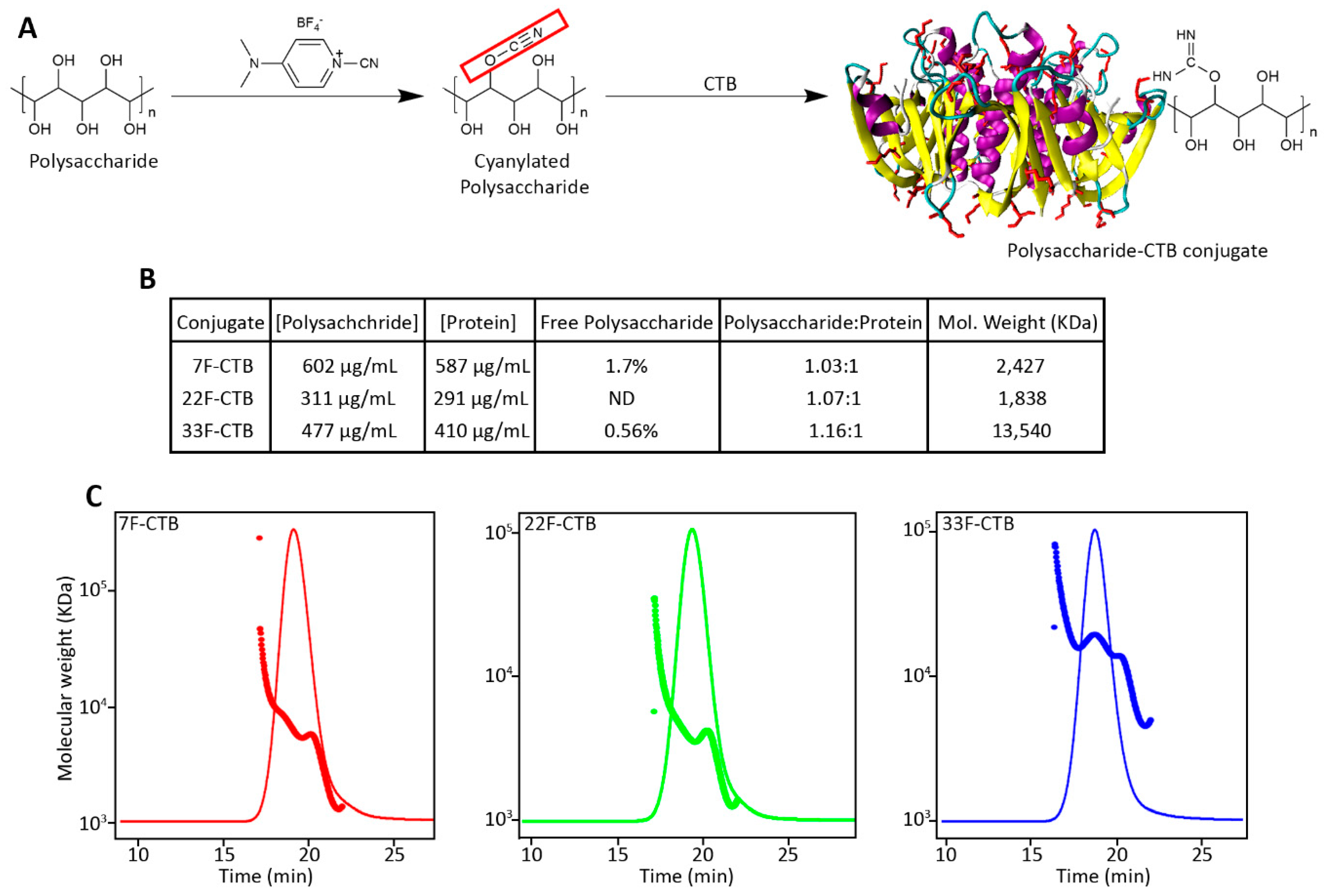
3.2. Conjugation of Pneumococcal Polysaccharide with CTB
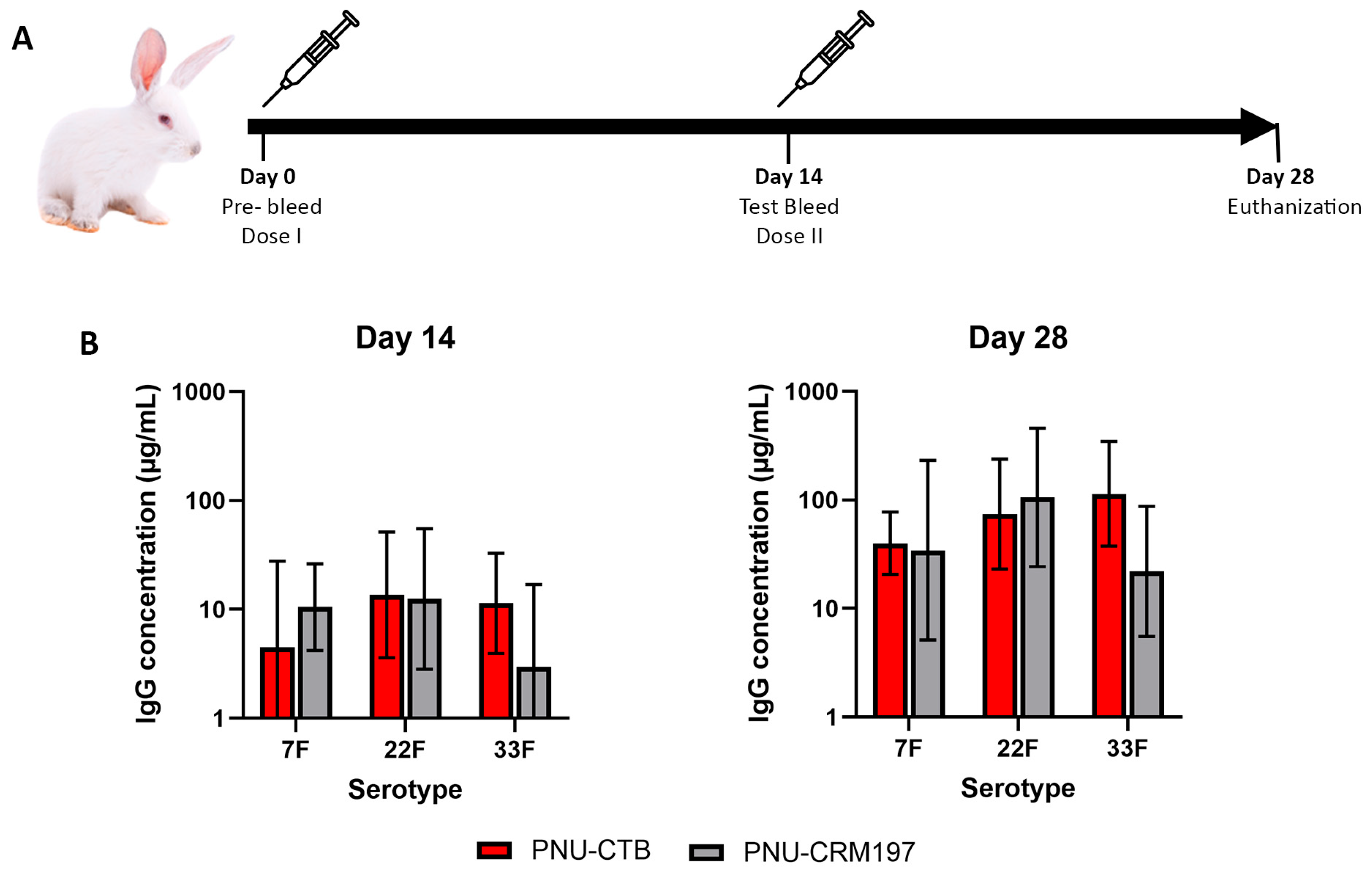
3.3. Pneumococcal Polysaccharide Conjugated to CTB Is Comparatively Immunogenic to CRM197 Conjugates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| CTB | Cholera toxin subunit B |
| CDAP | 1-Cyano-4-dimethylaminopyridinium tetrafluoroborate |
| CRM197 | Cross-reacting material 197 |
| OMPA | Outer membrane protein A |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| IgG | Immunoglobulin G |
References
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- André, F.E. Vaccination and global health. Vaccine 2003, 21, 593–595. [Google Scholar]
- Andre, F.E.; Booy, R.; Bock, H.L.; Clemens, J.; Datta, S.K.; John, T.J.; Lee, B.W.; Lolekha, S.; Peltola, H.; Ruff, T.A.; et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008, 86, 140–146. [Google Scholar] [CrossRef]
- Weisfelt, M.; De Gans, J.; Van Der Poll, T.; Van De Beek, D. Pneumococcal meningitis in adults: New approaches to management and prevention. Lancet Neurol. 2006, 5, 332–432. [Google Scholar] [CrossRef] [PubMed]
- Van Der Poll, T.; Opal, S.M. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 2009, 374, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- Musher, D.M.; Anderson, R.; Feldman, C. The remarkable history of pneumococcal vaccination: An ongoing challenge. Pneumonia 2022, 14, 5. [Google Scholar] [CrossRef]
- Weinberger, D.M.; Malley, R.; Lipsitch, M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011, 378, 1962–1973. [Google Scholar] [CrossRef]
- Feemster, K.; Buchwald, U.K.; Banniettis, N.; Joyce, J.G.; Velentgas, P.; Chapman, T.J.; Yildirim, I. Immunogenicity of Current and Next-Generation Pneumococcal Conjugate Vaccines in Children: Current Challenges and Upcoming Opportunities. Open Forum Infect. Dis. 2024, 11, ofae220. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, H.; Zhao, S.-H.; Yang, X.-Y.; Guo, Z. Recent progress in pneumococcal protein vaccines. Front. Immunol. 2023, 14, 1278346. [Google Scholar] [CrossRef]
- Mond, J.J.; Lees, A.; Snapper, C.M. T Cell-Independent Antigens Type 2. Annu. Rev. Immunol. 1995, 13, 655–692. [Google Scholar] [CrossRef]
- Siegrist, C.A. Vaccine Immunology. In Plotkin’s Vaccines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 16–34.e7. Available online: https://linkinghub.elsevier.com/retrieve/pii/B978032335761600002X (accessed on 7 August 2024).
- Pichichero, M.E. Protein carriers of conjugate vaccines: Characteristics, development and clinical trials. Hum. Vaccines Immunother. 2013, 9, 2505–2523. [Google Scholar] [CrossRef]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis-Kafeja, F.; Beutels, P.; Van Damme, P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998–2006). Vaccine 2007, 25, 2194–2212. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.K.; Moss, W.J.; Halsey, N. Haemophilus influenzae type b conjugate vaccine use and effectiveness. Lancet Infect. Dis. 2008, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, P.; Miglietta, A.; Neri, A.; Fazio, C.; Vacca, P.; Voller, F.; Rezza, G.; Stefanelli, P. Meningococcal C conjugate vaccine effectiveness before and during an outbreak of invasive meningococcal disease due to Neisseria meningitidis serogroup C/cc11, Tuscany, Italy. Vaccine 2018, 36, 4222–4227. [Google Scholar] [CrossRef]
- Rabian, C.; Tschöpe, I.; Lesprit, P.; Katlama, C.; Molina, J.-M.; Meynard, J.-L.; Delfraissy, J.-F.; Cheêne, G.; Lévy, Y. Cellular CD4 T Cell Responses to the Diphtheria-Derived Carrier Protein of Conjugated Pneumococcal Vaccine and Antibody Response to Pneumococcal Vaccination in HIV-Infected Adults. Clin. Infect. Dis. 2010, 50, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Shinefield, H.R. Overview of the development and current use of CRM197 conjugate vaccines for pediatric use. Vaccine 2010, 28, 4335–4339. [Google Scholar] [CrossRef]
- Taha, A.M.; Abouelmagd, K.; Mahmoud, A.M.; Elkasaby, M.H.; Nguyen, D.; Ahmed, R.; Patel, P.; Bonilla-Aldana, D.K.; Luna, C.; Rodriguez-Morales, A.J. Safety and immunogenicity of Vi-diphtheria toxoid typhoid conjugate vaccine among children below 2 years: A systematic review and meta-analysis. Front. Microbiol. 2024, 15, 1385834. [Google Scholar] [CrossRef]
- Chang, M.-J.; Ollivault-Shiflett, M.; Schuman, R.; Nguyen, S.N.; Kaltashov, I.A.; Bobst, C.; Rajagopal, S.P.; Przedpelski, A.; Barbieri, J.T.; Lees, A. Genetically detoxified tetanus toxin as a vaccine and conjugate carrier protein. Vaccine 2022, 40, 5103–5113. [Google Scholar] [CrossRef]
- Knuf, M.; Kowalzik, F.; Kieninger, D. Comparative effects of carrier proteins on vaccine-induced immune response. Vaccine 2011, 29, 4881–4890. [Google Scholar] [CrossRef]
- Pobre, K.; Tashani, M.; Ridda, I.; Rashid, H.; Wong, M.; Booy, R. Carrier priming or suppression: Understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines. Vaccine 2014, 32, 1423–1430. [Google Scholar] [CrossRef]
- Schutze, M.P.; Leclerc, C.; Jolivet, M.; Audibert, F.; Chedid, L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J. Immunol. 1985, 135, 2319–2322. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Y.; Hsi, J.; Wang, H.; Tao, R.; Shao, Y. Cholera toxin B subunit acts as a potent systemic adjuvant for HIV-1 DNA vaccination intramuscularly in mice. Hum. Vaccines Immunother. 2014, 10, 1274–1283. [Google Scholar] [CrossRef]
- Marinaro, M.; Staats, H.F.; Hiroi, T.; Jackson, R.J.; Coste, M.; Boyaka, P.N.; Okahashi, N.; Yamamoto, M.; Kiyono, H.; Bluethmann, H.; et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 1995, 155, 4621–4629. [Google Scholar] [CrossRef]
- Holmgren, J.; Lycke, N.; Czerkinsky, C. Cholera toxin and cholera B subunit as oral—Mucosal adjuvant and antigen vector systems. Vaccine 1993, 11, 1179–1184. [Google Scholar] [CrossRef]
- Bregenholt, S.; Wang, M.; Wolfe, T.; Hughes, A.; Bærentzen, L.; Dyrberg, T.; Von Herrath, M.G.; Petersen, J.S. The Cholera Toxin B Subunit is a Mucosal Adjuvant for Oral Tolerance Induction in Type 1 Diabetes. Scand. J. Immunol. 2003, 57, 432–438. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- Stratmann, T. Cholera Toxin Subunit B as Adjuvant––An Accelerator in Protective Immunity and a Break in Autoimmunity. Vaccines 2015, 3, 579–596. [Google Scholar] [CrossRef]
- George-Chandy, A.; Eriksson, K.; Lebens, M.; Nordström, I.; Schön, E.; Holmgren, J. Cholera Toxin B Subunit as a Carrier Molecule Promotes Antigen Presentation and Increases CD40 and CD86 Expression on Antigen-Presenting Cells. Infect. Immun. 2001, 69, 5716–5725. [Google Scholar] [CrossRef]
- Eriksson, K.; Fredriksson, M.; Nordström, I.; Holmgren, J. Cholera Toxin and Its B Subunit Promote Dendritic Cell Vaccination with Different Influences on Th1 and Th2 Development. Infect. Immun. 2003, 71, 1740–1747. [Google Scholar] [CrossRef]
- Sun, J.-B.; Flach, C.-F.; Czerkinsky, C.; Holmgren, J. B Lymphocytes Promote Expansion of Regulatory T Cells in Oral Tolerance: Powerful Induction by Antigen Coupled to Cholera Toxin B Subunit. J. Immunol. 2008, 181, 8278–8287. [Google Scholar] [CrossRef]
- Tepale-Segura, A.; Gajón, J.A.; Muñoz-Cruz, S.; Castro-Escamilla, O.; Bonifaz, L.C. The cholera toxin B subunit induces trained immunity in dendritic cells and promotes CD8 T cell antitumor immunity. Front. Immunol. 2024, 15, 1362289. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar]
- Wang, B.-Z.; Xu, R.; Quan, F.-S.; Kang, S.-M.; Wang, L.; Compans, R.W. Intranasal Immunization with Influenza VLPs Incorporating Membrane-Anchored Flagellin Induces Strong Heterosubtypic Protection. PLoS ONE 2010, 5, e13972. [Google Scholar] [CrossRef]
- Lee, A.; Chen, M. Successful immunization against gastric infection with Helicobacter species: Use of a cholera toxin B-subunit-whole-cell vaccine. Infect. Immun. 1994, 62, 3594–3597. [Google Scholar] [CrossRef]
- Hausdorff, W.P.; Bryant, J.; Paradiso, P.R.; Siber, G.R. Which Pneumococcal Serogroups Cause the Most Invasive Disease: Implications for Conjugate Vaccine Formulation and Use, Part, I. Clin. Infect. Dis. 2000, 30, 100–121. [Google Scholar] [CrossRef]
- Klugman, K.P. Contribution of vaccines to our understanding of pneumococcal disease. Philos. Trans. R. Soc. B 2011, 12, 2790–2798. [Google Scholar]
- Bogaert, D.; de Groot, R.; Hermans, P.W.M. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Astronomo, R.D.; Burton, D.R. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 2010, 9, 308–324. [Google Scholar] [CrossRef]
- Szu, S.C.; Li, X.R.; Schneerson, R.; Vickers, J.H.; Bryla, D.; Robbins, J.B. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect. Immun. 1989, 57, 3823–3827. [Google Scholar] [CrossRef]
- Micoli, F.; Costantino, P.; Adamo, R. Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Wu, C.-Y. Carbohydrate-based vaccines: Challenges and opportunities. Expert Rev. Vaccines 2010, 9, 1257–1274. [Google Scholar] [CrossRef]
- Price, G.A.; Bash, M.C. Development of an FHbp-CTB holotoxin-like chimera and the elicitation of bactericidal antibodies against serogroup B Neisseria meningitidis. Vaccine 2018, 36, 644–652. [Google Scholar] [CrossRef]
- Shen, X.; Lagergård, T.; Yang, Y.; Lindblad, M.; Fredriksson, M.; Holmgren, J. Group B Streptococcus Capsular Polysaccharide-Cholera Toxin B Subunit Conjugate Vaccines Prepared by Different Methods for Intranasal Immunization. Infect. Immun. 2001, 69, 297–306. [Google Scholar] [CrossRef]
- Bergquist, C.; Lagergård, T.; Lindblad, M.; Holmgren, J. Local and systemic antibody responses to dextran-cholera toxin B subunit conjugates. Infect. Immun. 1995, 63, 2021–2025. [Google Scholar] [CrossRef]
- Bergquist, C.; Lagergård, T.; Holmgren, J. Antibody responses in serum and lung to intranasal immunization with Haemophilus influenzae type b polysaccharide conjugated to cholera toxin B subunit and tetanus toxoid. APMIS 1998, 106, 800–806. [Google Scholar] [CrossRef]
- Lees, A.; Nelson, B.L.; Mond, J.J. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein—Polysaccharide conjugate vaccines and immunological reagents. Vaccine 1996, 14, 190–198. [Google Scholar] [CrossRef]
- Halhoul, M.; Kleinberg, I. Differential determination of glucose and fructose, and glucose- and fructose-yielding substances with anthrone. Anal. Biochem. 1972, 50, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Lamb, D.H.; Heller, R.; Pietrobon, P. Quantitation of low level unconjugated polysaccharide in tetanus toxoid-conjugate vaccine by HPAEC/PAD following rapid separation by deoxycholate/HCl. J. Pharm. Biomed. Anal. 2000, 21, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Dertzbaugh, M.T.; Cox, L.M. The affinity of cholera toxin for Ni2+ ion. Protein Eng. Des. Sel. 1998, 11, 577–581. [Google Scholar] [CrossRef]
- Khastar, A.; Jamshidain-Mojaver, M.; Farzin, H.; Jomhori Baloch, M.; Salamatian, I.; Akbarzadeh–Sherbaf, K. Production and Purifi-cation of Recombinant B Subunit of Vibrio cholerae Toxin in Escherichia coli. J. Cell Mol. Res. 2022, 13, 113–120. [Google Scholar] [CrossRef]
- Tamaki, Y.; Harakuni, T.; Yamaguchi, R.; Miyata, T.; Arakawa, T. Cholera toxin B subunit pentamer reassembled from Escherichia coli inclusion bodies for use in vaccination. Vaccine 2016, 34, 1268–1274. [Google Scholar] [CrossRef]
- Slos, P.; Speck, D.; Accart, N.; Kolbe, H.; Schubnel, D.; Bouchon, B.; Bischoff, R.; Kieny, M. Recombinant Cholera Toxin B-Subunit in Escherichia coli: High-Level Secretion, Purification, and Characterization. Protein Expr. Purif. 1994, 5, 518–526. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siriwardhana, C.; Bajracharya, A.; Seal, F.; Datta, A.; Kapre, S. Evaluation of Cholera Toxin B Subunit as a Novel Carrier Protein for Polysaccharide Conjugate Vaccines. Vaccines 2025, 13, 1159. https://doi.org/10.3390/vaccines13111159
Siriwardhana C, Bajracharya A, Seal F, Datta A, Kapre S. Evaluation of Cholera Toxin B Subunit as a Novel Carrier Protein for Polysaccharide Conjugate Vaccines. Vaccines. 2025; 13(11):1159. https://doi.org/10.3390/vaccines13111159
Chicago/Turabian StyleSiriwardhana, Chathuranga, Aakriti Bajracharya, Florence Seal, Anup Datta, and Subhash Kapre. 2025. "Evaluation of Cholera Toxin B Subunit as a Novel Carrier Protein for Polysaccharide Conjugate Vaccines" Vaccines 13, no. 11: 1159. https://doi.org/10.3390/vaccines13111159
APA StyleSiriwardhana, C., Bajracharya, A., Seal, F., Datta, A., & Kapre, S. (2025). Evaluation of Cholera Toxin B Subunit as a Novel Carrier Protein for Polysaccharide Conjugate Vaccines. Vaccines, 13(11), 1159. https://doi.org/10.3390/vaccines13111159




