Primary and Booster COVID-19 Vaccination in Patients with Sjögren’s Disease: Data from the Longitudinal SAFER Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion Criteria
2.3. Exclusion Criteria
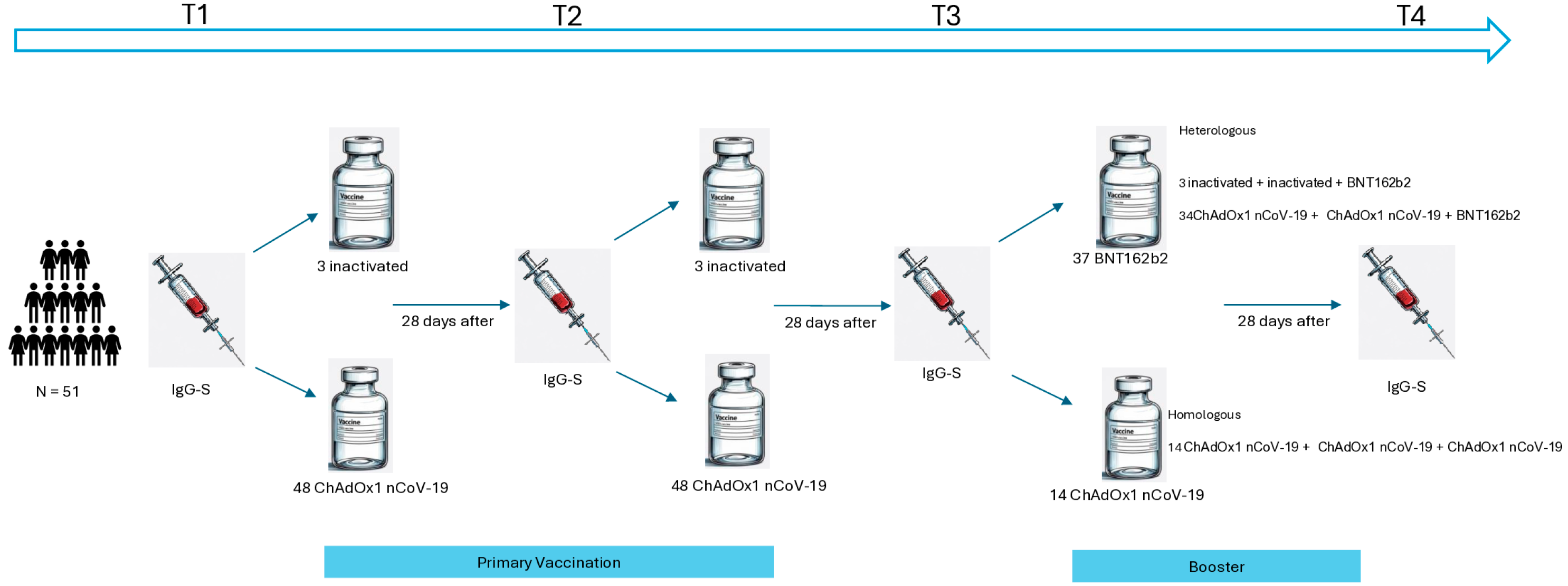
2.4. Vaccination Schedules
2.5. Ethical Procedures
2.6. Clinical Data
2.7. Immunogenicity Assessment
2.8. Vaccine Effectiveness Assessment
2.9. Safety
2.10. Statistical Analysis
- (1)
- Humoral immunogenicity was evaluated using ln-transformed IgG titers and geometric mean fold increase (FI-GMT); group and time comparisons were conducted with Wilcoxon/Mann–Whitney non-parametric tests, an approach suited to small sample sizes and non-normal distributions. Bonferroni correction was applied in the presence of multiple paired comparisons.
- (2)
- Seroconversion (SC) was summarized descriptively as the proportion of previously seronegative participants who converted in each group.
- (3)
- Predictors of IgG at T4 were explored by simple linear regression, with biologically relevant factors subsequently included in multiple regression models.
- (4)
- Vaccine effectiveness, defined as RT-PCR–confirmed COVID-19 cases, and
- (5)
- Safety, based on local and systemic post-vaccination symptoms, was analyzed with Fisher’s exact test to account for small cell counts.
- (6)
- Disease activity was described using ESSDAI and physician global assessment, adjusting for baseline activity.
3. Results
3.1. Vaccination Regimens
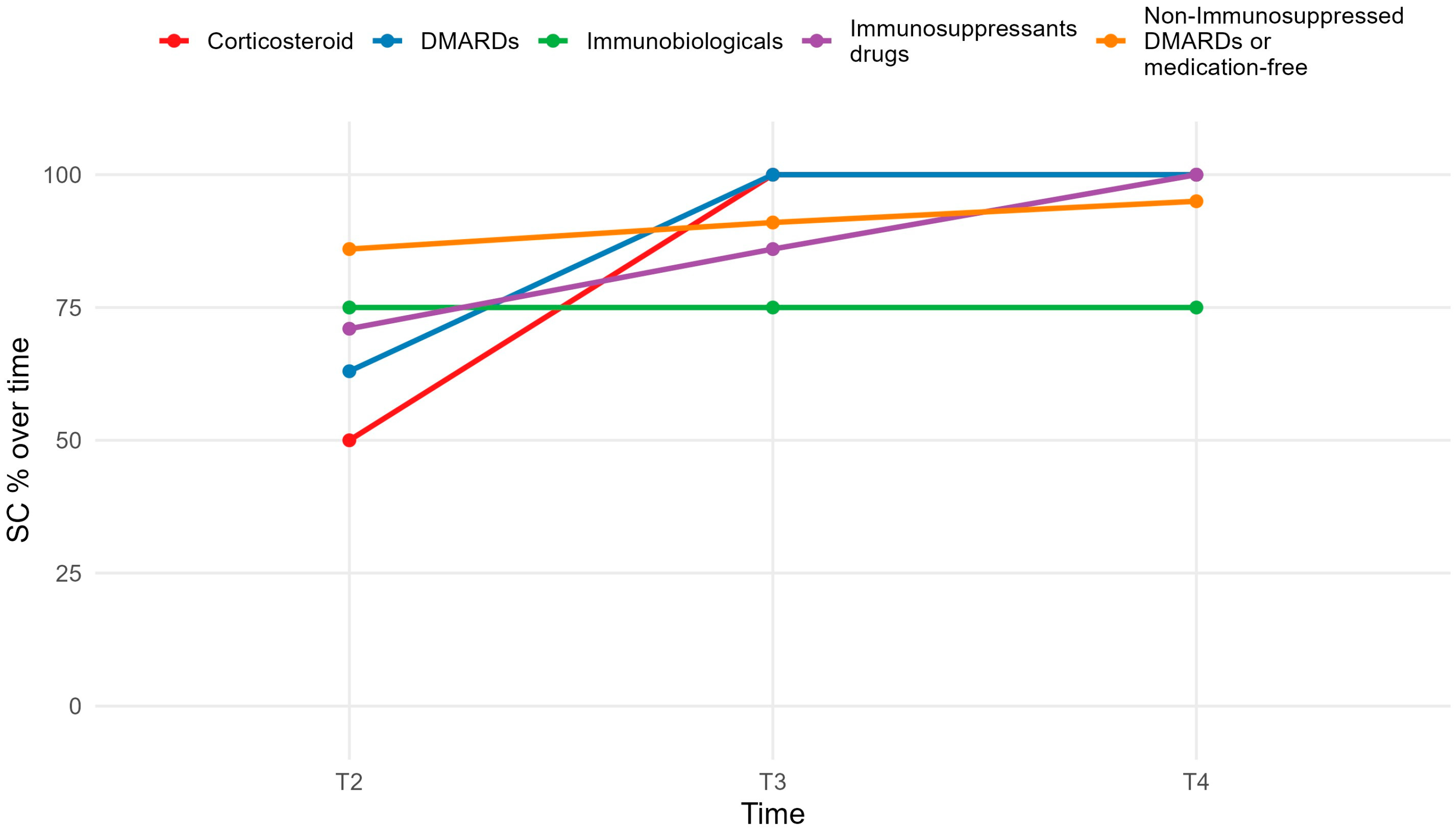
3.2. Seroconversion Rates by Medication
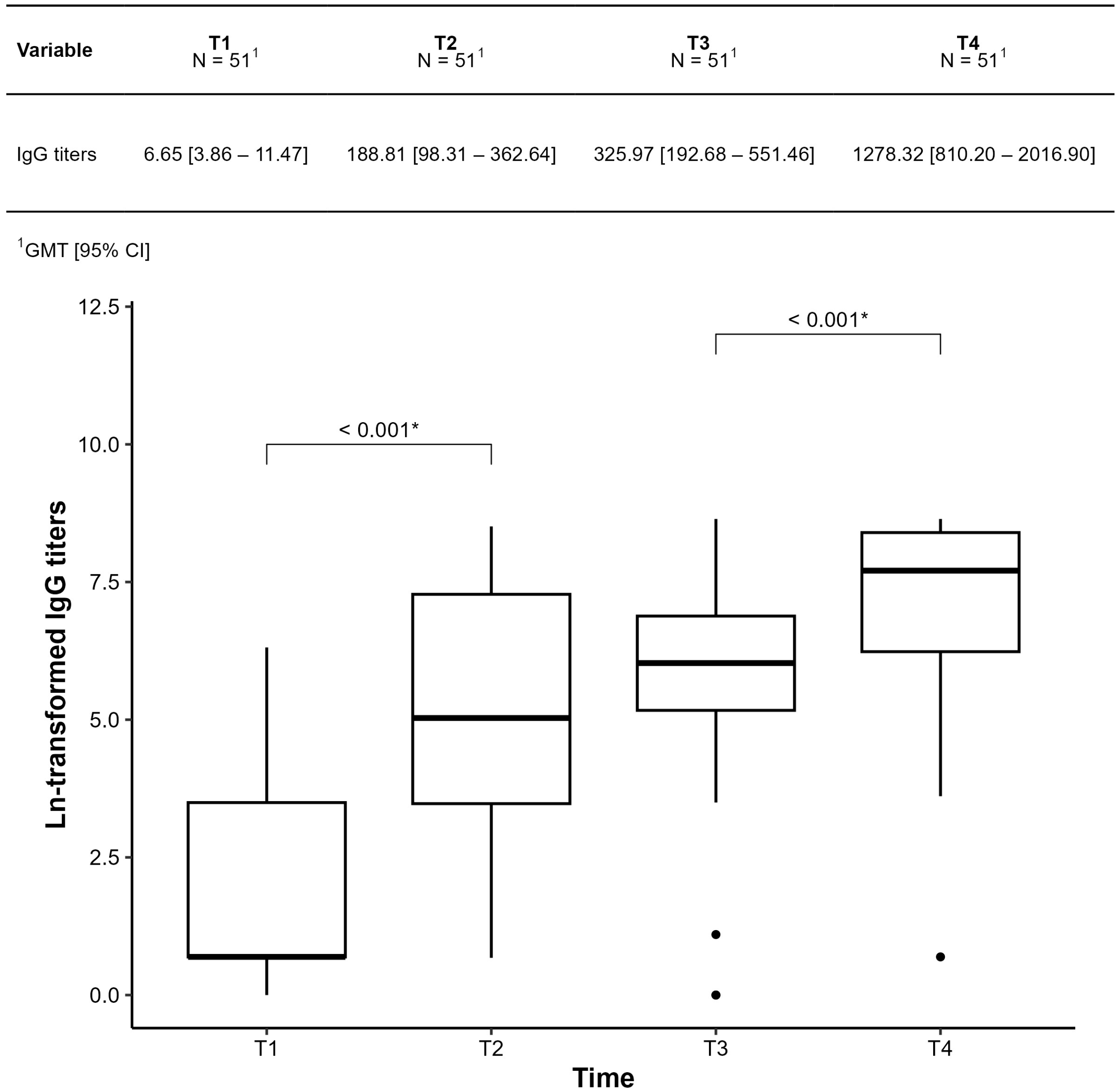
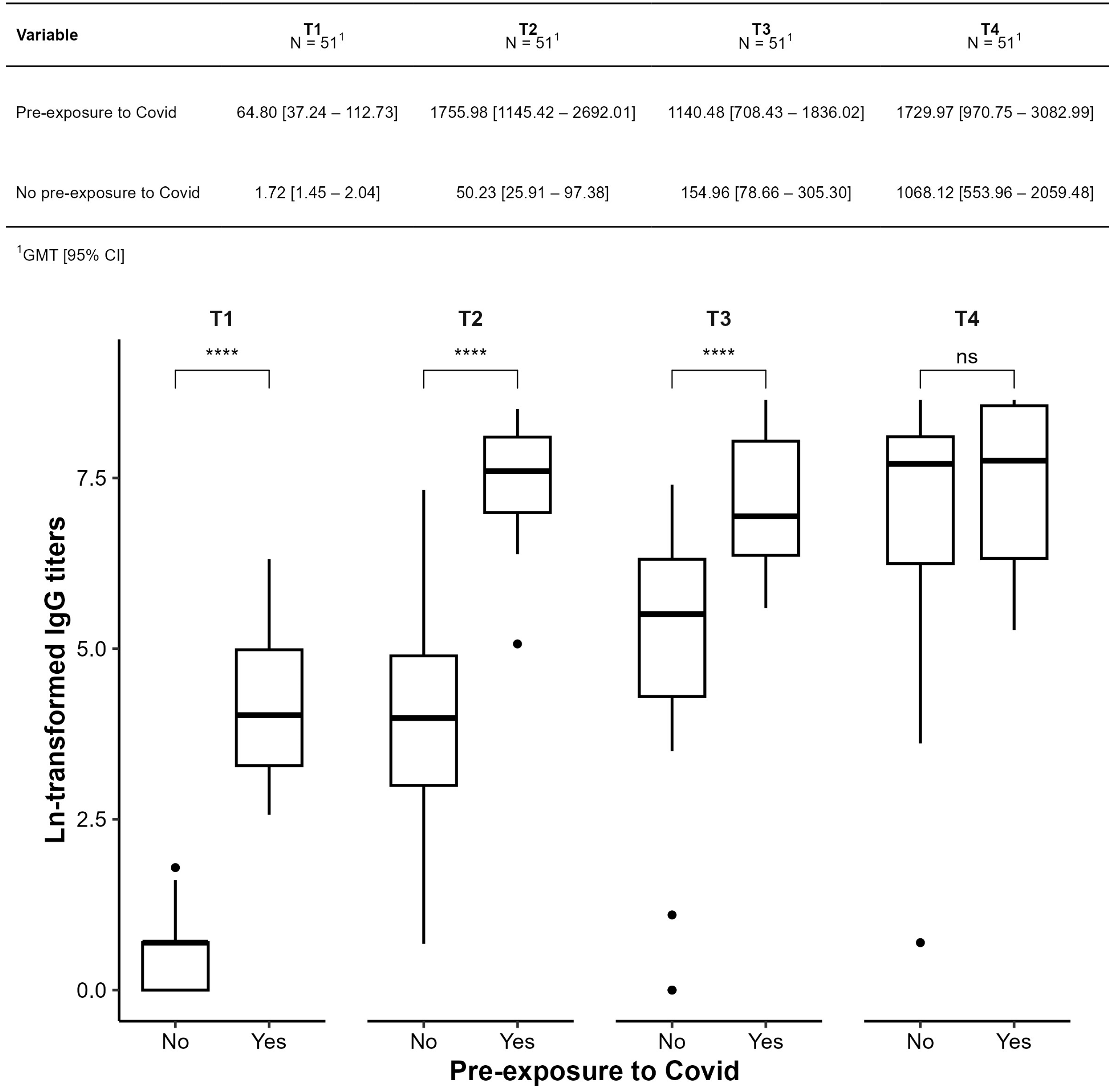
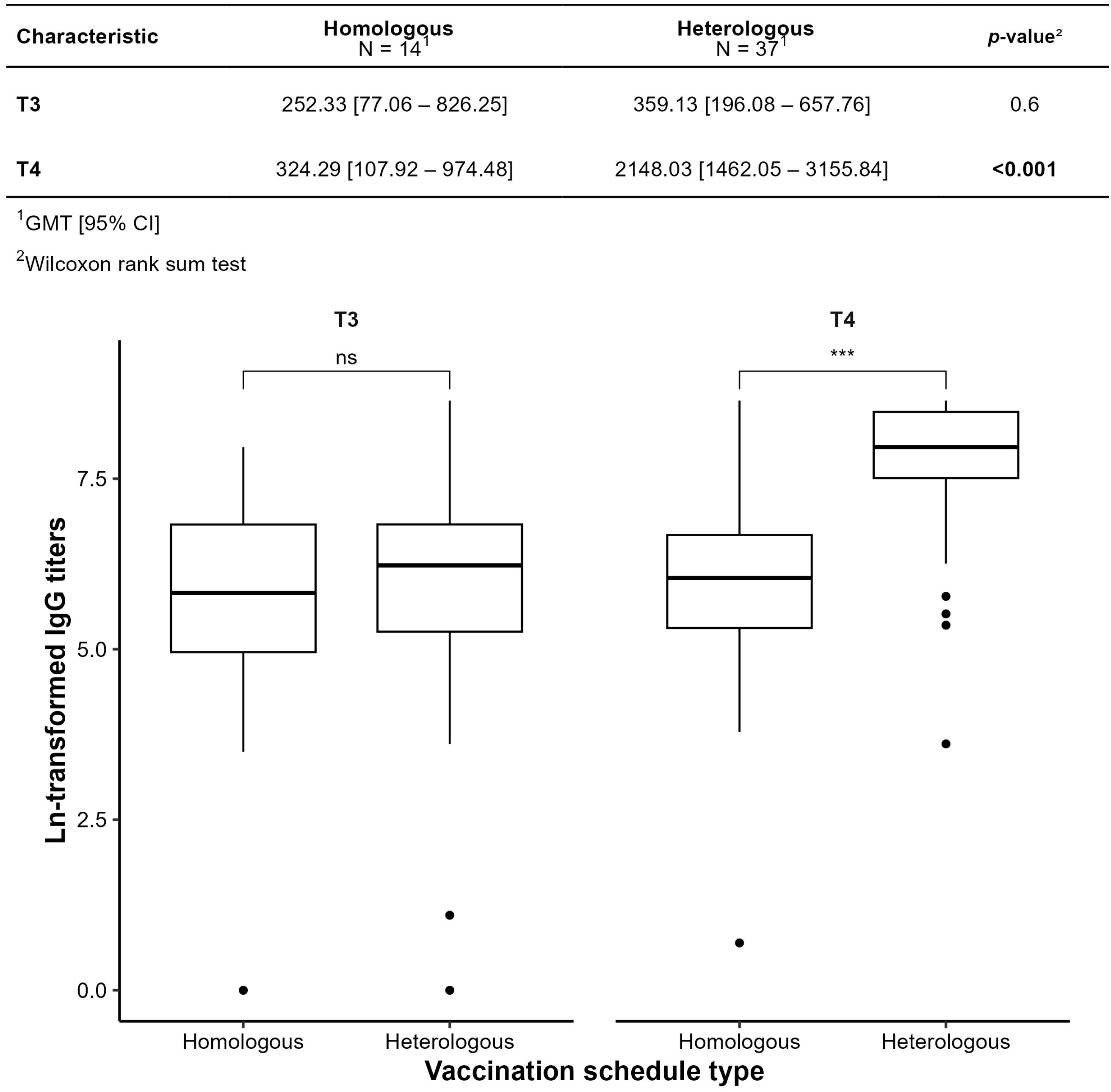
3.3. Humoral Immunogenicity Evaluation
3.4. Vaccine Effectiveness
3.5. Vaccine Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef]
- Demetriou, C.A.; Achilleos, S.; Quattrocchi, A.; Gabel, J.; Critselis, E.; Constantinou, C.; Nicolaou, N.; Ambrosio, G.; Bennett, C.M.; Le Meur, N.; et al. Impact of the COVID-19 pandemic on total, sex- and age-specific all-cause mortality in 20 countries worldwide during 2020: Results from the C-MOR project. Int. J. Epidemiol. 2023, 52, 664–676, Erratum in Int. J. Epidemiol. 2024, 53, dyae001. https://doi.org/10.1093/ije/dyae001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marques, C.D.L.; Kakehasi, A.M.; Pinheiro, M.M.; Mota, L.M.H.; Albuquerque, C.P.; Silva, C.R.; Santos, G.P.J.; Reis-Neto, E.T.; Matos, P.; Devide, G.; et al. High levels of immunosuppression are related to unfavourable outcomes in hospitalised patients with rheumatic diseases and COVID-19: First results of ReumaCoV Brasil registry. RMD Open 2021, 7, e001461. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. Plano Nacional de Operacionalização da Vacinação Contra a COVID-19, 15th ed.; Ministério da Saúde: Brasília, Brazil, 2022. [Google Scholar]
- Valim, V.; Zandonade, E.; Pereira, A.M.; de Brito Filho, O.H.; Serrano, E.V.; Musso, C.; Giovelli, R.A.; Ciconelli, R.M. Primary Sjögren’s Syndrome Prevalence in a Major Metropolitan Area in Brazil. Rev. Bras. Reumatol. 2013, 53, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Fulvio, G.; La Rocca, G.; Ferro, F. Update on the pathophysiology and treatment of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2024, 20, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Melchor, S.; Seror, R.; Priori, R.; Solans, R.; Kostov, B.; Baldini, C.; Carubbi, F.; Callejas, J.L.; Guisado-Vasco, P.; et al. SARS-CoV-2 infection in patients with primary Sjögren syndrome: Characterization and outcomes of 51 patients. Rheumatology 2021, 60, 2946–2957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arnold, J.; Winthrop, K.; Emery, P. COVID-19 vaccination and antirheumatic therapy. Rheumatology 2021, 60, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Pasoto, S.G.; Halpern, A.S.R.; Guedes, L.K.N.; Ribeiro, A.C.M.; Yuki, E.N.F.; Saad, C.G.S.; da Silva, C.A.A.; Kupa, L.d.V.K.; Villamarín, L.E.B.; Martins, V.A.d.O.; et al. Inactivated SARS-CoV-2 vaccine in primary Sjögren’s syndrome: Humoral response, safety, and effects on disease activity. Clin. Rheumatol. 2022, 41, 2079–2089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verstappen, G.M.; de Wolff, L.; Arends, S.; Heiermann, H.M.; van Sleen, Y.; Visser, A.; Terpstra, J.H.; A Diavatopoulos, D.; van der Heiden, M.; Vissink, A.; et al. Immunogenicity and safety of COVID-19 vaccination in patients with primary Sjögren’s syndrome. RMD Open 2022, 8, e002265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inanc, N.; Kostov, B.; Priori, R.; Flores-Chavez, A.; Carubbi, F.; Szántó, A.; Valim, V.; Bootsma, H.; Praprotnik, S.; Trevisani, V.F.M.; et al. Safety and efficacy of SARS-CoV-2 vaccination in 1237 patients with primary Sjögren syndrome. Clin. Exp. Rheumatol. 2022, 40, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.L.L.L.; Burian, A.P.N.; Martins-Filho, O.A.; Mill, J.G.; Ferreira, L.B.; Tapia, K.R.L.; Moulin, A.C.S.; Moulaz, I.R.; Ribeiro, P.D.C.; Magalhães, V.d.O.; et al. Immunogenicity and safety according to immunosuppressive drugs and different COVID-19 vaccine platforms in immune-mediated disease: Data from SAFER cohort. Vaccines 2024, 12, 1367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, C.; Schönbrunn, A.; Fischer, D.; Liu, Y.; Hocher, J.G.; Weinerth, J.; Klemm, K.; von Baehr, V.; Krämer, B.K.; Elitok, S.; et al. Immune response of heterologous versus homologous prime-boost regimens with adenoviral vectored and mRNA COVID-19 vaccines in immunocompromised patients. Front. Immunol. 2023, 14, 1187880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bayani, F.; Hashkavaei, N.S.; Arjmand, S.; Rezaei, S.; Uskoković, V.; Alijanianzadeh, M.; Uversky, V.N.; Siadat, S.O.R.; Mozaffari-Jovin, S.; Sefidbakht, Y. An Overview of the Vaccine Platforms to Combat COVID-19 with a Focus on the Subunit Vaccines. Prog. Biophys. Mol. Biol. 2023, 178, 32–49. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Bowman, S.J.; Brito-Zeron, P.; Theander, E.; Bootsma, H.; Tzioufas, A.; Gottenberg, J.-E.; Ramos-Casals, M.; Dörner, T.; Ravaud, P.; et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): A user guide. RMD Open 2015, 1, e000022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pileggi, G.S.; Da Mota, L.M.H.; Kakehasi, A.M.; De Souza, A.W.; Rocha, A.; de Melo, A.K.G.; da Fonte, C.A.M.; Bortoletto, C.; Brenol, C.V.; Marques, C.D.L.; et al. Brazilian recommendations on the safety and effectiveness of the yellow fever vaccination in patients with chronic immune-mediated inflammatory diseases. Adv. Rheumatol. 2019, 59, 17. [Google Scholar] [CrossRef] [PubMed]
- Al-Haideri, M.; Mohammad, T.A.M.; Darvishzadehdeldari, S.; Karbasi, Z.; Alimohammadi, M.; Faramarzi, F.; Khorasani, S.; Rasouli, A.; Tahmasebi, S.; Darvishi, M.; et al. Immunogenicity of COVID-19 vaccines in adult patients with autoimmune inflammatory rheumatic diseases: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2023, 26, 1227–1234. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338, Erratum in Ann. Rheum. Dis. 2022, 81, e133. https://doi.org/10.1136/annrheumdis-2021-220647corr1. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection against COVID-19 by BNT162b2 booster across age groups. N. Engl. J. Med. 2021, 385, 2421–2430. [Google Scholar] [CrossRef]
- Machado, P.M.; Lawson-Tovey, S.; Strangfeld, A.; Mateus, E.F.; Hyrich, K.L.; Gossec, L.; Rodrigues, A.; Raffeiner, B.; Duarte, C.; Hachulla, E.; et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: Results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann. Rheum. Dis. 2022, 81, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Sattui, S.E.; Liew, J.W.; Kennedy, K.; Sirotich, E.; Putman, M.; Moni, T.T.; Akpabio, A.; Alpízar-Rodríguez, D.; Berenbaum, F.; Bulina, I.; et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: Results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open 2021, 7, e001814. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; A Triccas, J.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe. 2022, 3, e52–e61. [Google Scholar] [CrossRef] [PubMed]
- Murari, T.B.; Fonseca, L.M.D.S.; Pereira, H.B.B.; Nascimento Filho, A.S.; Saba, H.; Scorza, F.A.; de Almeida, A.-C.G.; Maciel, E.L.N.; Mendes, J.F.F.; Filho, T.M.R.; et al. Retrospective cohort study of COVID-19 in patients of the Brazilian public health system with SARS-CoV-2 Omicron variant infection. Vaccines 2022, 10, 1504. [Google Scholar] [CrossRef]
- Tiozzo, G.; Louwsma, T.; Konings, S.R.A.; Vondeling, G.T.; Perez Gomez, J.; Postma, M.J.; Freriks, R.D. Evaluating the reactogenicity of COVID-19 vaccines from network-meta analyses. Expert. Rev. Vaccines 2023, 22, 410–418. [Google Scholar] [CrossRef]
- Younis, A.A.; Ridha, A.A.; Humadi, Y.A.; Jassim, N.A.; Awadh, N.I.; Maroof, A.; Alqazzaz, A.M.H.; Gorial, F.I.; Qaradaghi, T.A.; Abdulzahra, Z.S.; et al. Safety of COVID-19 vaccine in patients with rheumatic and musculoskeletal diseases. Mediterr. J. Rheumatol. 2024, 35, 123–133. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. Plano Nacional de Operacionalização da Vacinação Contra COVID-19—01.02.2022. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/c/covid-19/publicacoes-tecnicas/guias-e-planos/plano-nacional-de-operacionalizacao-da-vacinacao-contra-covid-19.pdf/view (accessed on 16 October 2025).
- Honfi, D.; Gémes, N.; Szabó, E.; Neuperger, P.; Balog, J.Á.; Nagy, L.I.; Toldi, G.; Puskás, L.G.; Szebeni, G.J.; Balog, A. Comparison of homologous and heterologous booster SARS-CoV-2 vaccination in autoimmune rheumatic and musculoskeletal patients. Int. J. Mol. Sci. 2022, 23, 11411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Serban, A.; Mihai, A.; Dima, A.; Balaban, D.V.; Jinga, M.; Jurcut, C. The impact of the COVID-19 pandemic on patients with primary Sjögren syndrome. Rheumatol. Int. 2021, 41, 1933–1940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, J.H.; Rha, M.S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.-H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]


| Characteristic | N = 51 1 | Characteristic | N = 51 1 |
|---|---|---|---|
| Age | 45.86 (±11.72) | Diagnosis time (In years) | 7.0 [4.0–11.0] |
| Gender | Immunosuppression level | ||
| Female | 46/51 (90%) | No/Low | 37/51 (73%) |
| Race | High | 14/51 (27%) | |
| Brown | 29/51 (57%) | Medication | |
| White | 21/51 (41%) | Immunobiologicals | 5/51 (9.8%) |
| Black | 1/51 (2.0%) | Immunosuppressive Drugs | 9/51 (18%) |
| Comorbidities | 21/51 (41%) | Corticosteroid | 3/51 (18%) |
| BMI—Categorical | DMARDs | 10/51 (20%) | |
| 1—Underweight (BMI < 18.5) | 1/51 (2.0%) | HCQ | 33/51 (65%) |
| 2—Healthy Weight (BMI 18.5–24.9) | 11/51 (22%) | Free | 8/51 (16%) |
| 3—Overweight (BMI 25–29.9) | 23/51 (45%) | ESSDAI | |
| 4—Obesity (BMI > 30) | 16/51 (31%) | No or low activity | 31/35 (89%) |
| Smoking | 0/51 (0%) | Moderate to high activity | 4/35 (11%) |
| Alcoholism | 3/51 (5.9%) | Immunological biomarkers | |
| Heart disease | 4/51 (7.8%) | Antinuclear Factor (ANA) positive | 33/35 (94%) |
| Diabetes mellitus | 3/51 (5.9%) | Rheumatoid Factor positive | 17/38 (45%) |
| Chronic renal disease | 1/51 (2.0%) | Anti-Ro positive | 25/44 (57%) |
| Systemic arterial hypertension | 7/51 (14%) | Anti-La positive | 9/32 (28%) |
| Previous history of COVID-19 | 16/51 (31%) | C3 low | 3/40 (7.50%) |
| C4 low | 2/39 (5.13%) |
| Characteristic | N = 51 1 |
|---|---|
| Primary Vaccination | |
| ChAdOx1 nCoV-19 | 48/51 (94%) |
| Inactivated SARS-CoV2 vaccine | 3/51 (5.9%) |
| Schedule type | |
| Heterologous | 37/51 (73%) |
| Homologous | 14/51 (27%) |
| Vaccination schedule | |
| ChAdOx1 nCoV-19 + ChAdOx1 nCoV-19 + BNT162b2 | 34/51 (67%) |
| ChAdOx1 nCoV-19 + ChAdOx1 nCoV-19 + ChAdOx1 nCoV-19 | 14/51 (27%) |
| inactivated + inactivated + BNT162b2 | 3/51 (5.9%) |
| Characteristic | N | Beta | 95% CI 1 | p-Value |
|---|---|---|---|---|
| Gender | 51 | |||
| Male | — | — | ||
| Female | −0.43 | −1.9, 1.1 | 0.6 | |
| Age | 51 | 0.02 | −0.02, 0.06 | 0.3 |
| Comorbidity | 51 | −0.45 | −1.4, 0.46 | 0.3 |
| COVID infection until T4 | 51 | 0.19 | −2.1, 2.5 | 0.9 |
| Pre-exposure to COVID | 51 | 0.48 | −0.44, 1.4 | 0.3 |
| First dose | 51 | |||
| Inactivated | — | — | ||
| ChAdOx1 | −0.89 | −2.8, 1.0 | 0.4 | |
| Booster dose | 51 | |||
| ChAdOx | — | — | ||
| BNT162b2 | 1.9 | 1.0, 2.7 | <0.001 | |
| Immunobiologicals | 51 | −1.3 | −2.8, 0.16 | 0.087 |
| Corticosteroid | 51 | 0.77 | −1.5, 3.1 | 0.5 |
| Non-Immunosuppressed DMARDs or medication-free | 51 | 0.08 | −1.1, 1.2 | 0.9 |
| DMARDs | 51 | −0.19 | −1.3, 0.94 | 0.7 |
| Immunosuppressants drugs | 51 | −0.23 | −1.4, 0.95 | 0.7 |
| Primary Vaccination | Booster | |||||
|---|---|---|---|---|---|---|
| Characteristic | Inactivated N = 3 1 | ChAdOx1 N = 48 1 | p-Value 2 | ChAdOx1 N = 14 1 | BNT162b2 N = 37 1 | p-Value 2 |
| Local reactions | 3/3 (100%) | 44/48 (92%) | >0.9 | 10/14 (71%) | 27/33 (82%) | 0.5 |
| Systemic reactions | 1/3 (33%) | 44/48 (92%) | 0.033 | 10/14 (71%) | 27/33 (82%) | 0.5 |
| Characteristic | Inactivated (N = 3 1) | ChAdOx1 (N = 48 1) | p-Value 2 |
|---|---|---|---|
| Erythema | 0/3 (0%) | 12/48 (25%) | >0.9 |
| Ecchymosis | 0/3 (0%) | 5/48 (10%) | >0.9 |
| Lesion | 1/3 (33%) | 8/48 (17%) | 0.4 |
| Itching | 0/1 (0%) | 4/8 (50%) | >0.9 |
| Swelling | 0/3 (0%) | 21/48 (44%) | 0.3 |
| Induration | 0/3 (0%) | 17/48 (35%) | 0.5 |
| Local pain | 2/3 (67%) | 43/48 (90%) | 0.3 |
| Nausea/Vomiting | 1/3 (33%) | 23/48 (48%) | >0.9 |
| Tiredness | 1/3 (33%) | 35/48 (73%) | 0.2 |
| Headache | 1/3 (33%) | 35/48 (73%) | 0.2 |
| Muscle pain | 1/3 (33%) | 33/48 (69%) | 0.3 |
| Joint pain | 1/3 (33%) | 35/48 (73%) | 0.2 |
| Fever | 0/3 (0%) | 19/48 (40%) | 0.3 |
| Dizziness | 0/3 (0%) | 17/48 (35%) | 0.5 |
| Other complaints | 0/3 (0%) | 21/48 (44%) | 0.3 |
| Characteristic | ChAdOx (N = 14 1) | BNT162b2 (N = 37 1) | p-Value 2 |
|---|---|---|---|
| Local reactions | 10/14 (71%) | 27/33 (82%) | 0.5 |
| Erythema | 5/14 (36%) | 11/33 (33%) | >0.9 |
| Ecchymosis | 3/14 (21%) | 9/33 (27%) | >0.9 |
| Lesion | 2/14 (14%) | 3/33 (9.1%) | 0.6 |
| Itching | 0/2 (0%) | 2/3 (67%) | 0.4 |
| Swelling | 4/14 (29%) | 17/33 (52%) | 0.15 |
| Induration | 5/14 (36%) | 15/33 (45%) | 0.5 |
| Local pain | 10/14 (71%) | 26/33 (79%) | 0.7 |
| Nausea/Vomiting | 2/14 (14%) | 5/33 (15%) | >0.9 |
| Tiredness | 7/14 (50%) | 16/33 (48%) | >0.9 |
| Headache | 8/14 (57%) | 18/33 (55%) | 0.9 |
| Muscle pain | 6/14 (43%) | 20/33 (61%) | 0.3 |
| Joint pain | 7/14 (50%) | 17/33 (52%) | >0.9 |
| Fever | 2/14 (14%) | 5/33 (15%) | >0.9 |
| Dizziness | 3/14 (21%) | 8/33 (24%) | >0.9 |
| Other complaints | 0/3 (0%) | 21/48 (44%) | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa Beloni Lirio, M.; Machado, K.L.L.L.; Assis Martins-Filho, O.; Miyamoto, S.T.; Gurtler Pinheiro de Oliveira, Y.; Vieira Serrano, É.; Geraldo Mill, J.; Rosemarie Lallemand Tapia, K.; Baptista Ferreira, L.; Ribeiro de Oliveira, J.; et al. Primary and Booster COVID-19 Vaccination in Patients with Sjögren’s Disease: Data from the Longitudinal SAFER Cohort Study. Vaccines 2025, 13, 1152. https://doi.org/10.3390/vaccines13111152
Barbosa Beloni Lirio M, Machado KLLL, Assis Martins-Filho O, Miyamoto ST, Gurtler Pinheiro de Oliveira Y, Vieira Serrano É, Geraldo Mill J, Rosemarie Lallemand Tapia K, Baptista Ferreira L, Ribeiro de Oliveira J, et al. Primary and Booster COVID-19 Vaccination in Patients with Sjögren’s Disease: Data from the Longitudinal SAFER Cohort Study. Vaccines. 2025; 13(11):1152. https://doi.org/10.3390/vaccines13111152
Chicago/Turabian StyleBarbosa Beloni Lirio, Maressa, Ketty Lysie Libardi Lira Machado, Olindo Assis Martins-Filho, Samira Tatiyama Miyamoto, Yasmin Gurtler Pinheiro de Oliveira, Érica Vieira Serrano, José Geraldo Mill, Karina Rosemarie Lallemand Tapia, Lunara Baptista Ferreira, Juliana Ribeiro de Oliveira, and et al. 2025. "Primary and Booster COVID-19 Vaccination in Patients with Sjögren’s Disease: Data from the Longitudinal SAFER Cohort Study" Vaccines 13, no. 11: 1152. https://doi.org/10.3390/vaccines13111152
APA StyleBarbosa Beloni Lirio, M., Machado, K. L. L. L., Assis Martins-Filho, O., Miyamoto, S. T., Gurtler Pinheiro de Oliveira, Y., Vieira Serrano, É., Geraldo Mill, J., Rosemarie Lallemand Tapia, K., Baptista Ferreira, L., Ribeiro de Oliveira, J., Gouvea, M. d. P. G., Rodrigues Aguiar, L. G., Souza, B. O., Cruz, V. A., Xavier, R. M., Teixeira Carvalho, A., de Souza, V. A., Ferreira, G. A., Monticielo, O. A., ... Valim, V. (2025). Primary and Booster COVID-19 Vaccination in Patients with Sjögren’s Disease: Data from the Longitudinal SAFER Cohort Study. Vaccines, 13(11), 1152. https://doi.org/10.3390/vaccines13111152






