Heterogeneity in Prevalence, Incidence, and Clearance of Anal Human Papillomavirus Among HIV-Negative and HIV-Positive Men Who Have Sex with Men in China: An Observational Cohort Study
Abstract
1. Background
2. Methods
2.1. Overview
2.2. Study Population
2.3. Data Sources and Measurement
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Description of Study Population
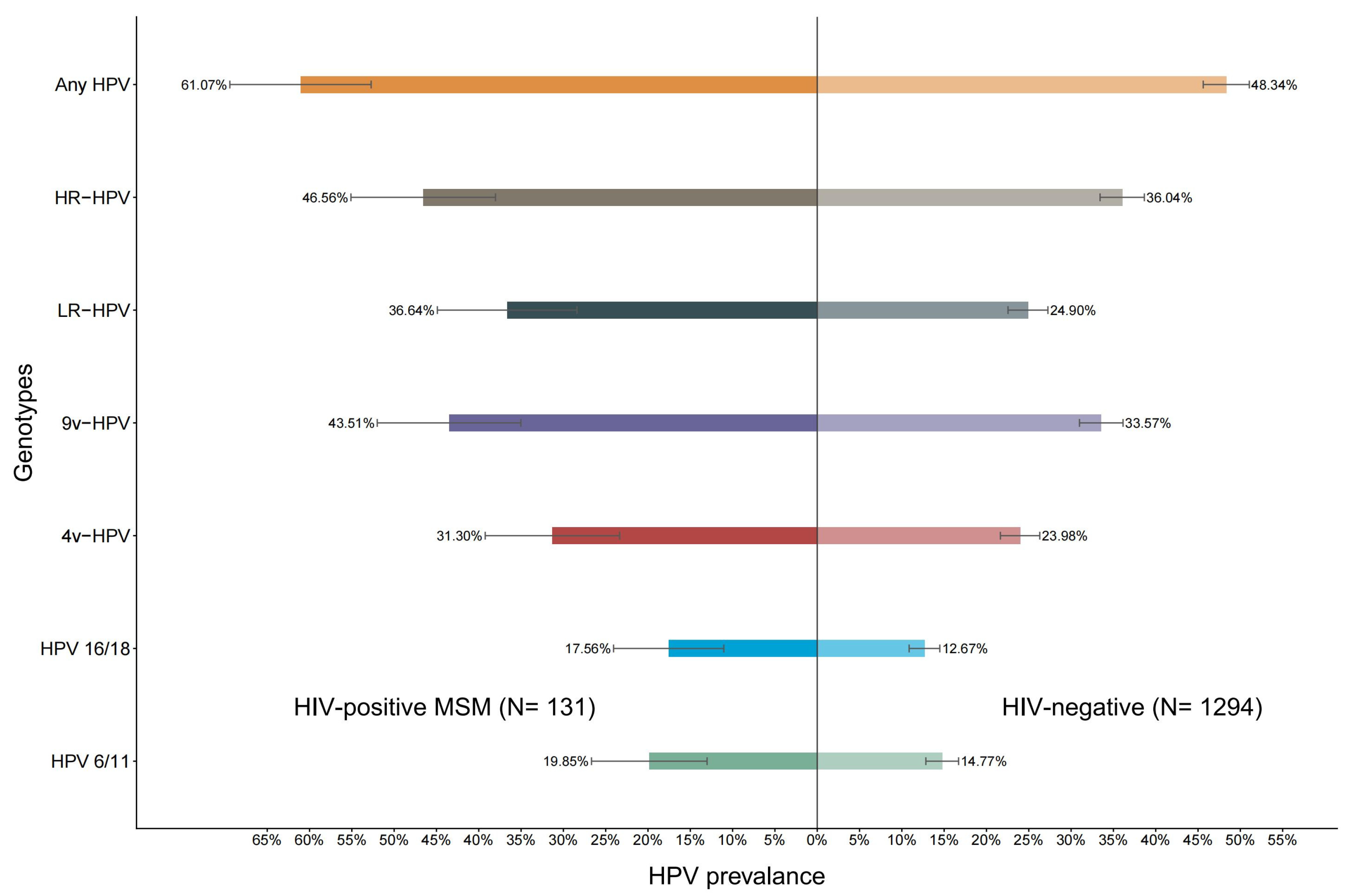
3.2. HPV Prevalence
3.3. HPV Incidence
3.4. HPV Clearance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPV | Human Papillomavirus |
| MSM | Men who have sex with men |
| NGO | Non-Governmental Organizations |
| PR | Prevalence ratio |
| IR | Incidence rate |
| CR | Clearance rate |
| IRR | Incidence rate ratio |
| IQR | Interquartile ranges |
References
- Biological Agents; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100, Pt B, pp. 1–441. [Google Scholar]
- McBride, A.A. Human papillomaviruses: Diversity, infection and host interactions. Nat. Rev. Microbiol. 2022, 20, 95–108. [Google Scholar] [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30 (Suppl. S5), F55–F70. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Hotca, A.; Goodman, K.A. Trends in Anal Cancer: Leveraging Public Health Efforts to Improve Cancer Care. J. Clin. Oncol. 2023, 41, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- Malagón, T.; Franco, E.L.; Tejada, R.; Vaccarella, S. Epidemiology of HPV-associated cancers past, present and future: Towards prevention and elimination. Nat. Rev. Clin. Oncol. 2024, 21, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Georges, D.; Shiels, M.S.; Engels, E.A.; Albuquerque, A.; Poynten, I.M.; de Pokomandy, A.; Easson, A.M.; Stier, E.A. A meta-analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. Int. J. Cancer 2021, 148, 38–47. [Google Scholar] [CrossRef]
- Dreyer, G. Clinical implications of the interaction between HPV and HIV infections. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 95–106. [Google Scholar] [CrossRef]
- Tian, T.; Mijiti, P.; Bingxue, H.; Fadong, Z.; Ainiwaer, A.; Guoyao, S.; Zhanlin, Z.; Mahan, Y.; Xiaoqin, T.; Zheng, G.; et al. Prevalence and risk factors of anal human papillomavirus infection among HIV-negative men who have sex with men in Urumqi city of Xinjiang Uyghur Autonomous Region, China. PLoS ONE 2017, 12, e0187928. [Google Scholar] [CrossRef]
- Kasavandi, A.; Foroohi, F.; Rahimi, T.; Ferdousi, A.; Mohammadian, T. An Insight into the Coinfections of Hepatitis B, C, and Mycobacterium Tuberculosis in Iranian HIV Patients. Iran. Red Crescent Med. J. (IRCMJ) 2024, 26, 1–7. [Google Scholar]
- Hargreaves, J.R.; Bonell, C.P.; Boler, T.; Boccia, D.; Birdthistle, I.; Fletcher, A.; Pronyk, P.M.; Glynn, J.R. Systematic review exploring time trends in the association between educational attainment and risk of HIV infection in sub-Saharan Africa. AIDS 2008, 22, 403–414. [Google Scholar] [CrossRef]
- Hu, H.; Hao, J.; Wang, D.; Liu, X.; Chen, H.; Li, F.; Chen, J.; Li, M.; Xin, P.; Li, Y.; et al. Pretreatment HIV Drug Resistance to Integrase Strand Transfer Inhibitors Among Newly Diagnosed HIV Individuals—China, 2018–2023. China CDC Wkly. 2025, 7, 31–39. [Google Scholar] [CrossRef]
- Shan, D.; Liu, Y.; Zang, C.; Zhao, Y.; Li, H.; Han, J.; Yang, J.; Liu, J.; Liu, Z.; Liu, Y. Characteristics and Predictors of Interprovincial Migration Following HIV Diagnosis Among Men Who Have Sex with Men—China, 2016–2022. China CDC Wkly. 2025, 7, 39–44. [Google Scholar] [CrossRef]
- Sandfort, T.G.; Nel, J.; Rich, E.; Reddy, V.; Yi, H. HIV testing and self-reported HIV status in South African men who have sex with men: Results from a community-based survey. Sex. Transm. Infect. 2008, 84, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Outlaw, A.Y.; Phillips, G., 2nd; Hightow-Weidman, L.B.; Fields, S.D.; Hidalgo, J.; Halpern-Felsher, B.; Green-Jones, M. Age of MSM sexual debut and risk factors: Results from a multisite study of racial/ethnic minority YMSM living with HIV. AIDS Patient Care STDS 2011, 25 (Suppl. 1), S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Serovich, J.M.; Laschober, T.C.; Kimberly, J.A. Age and racial disparities in substance use and self-reported viral suppression among men who have sex with men with HIV. Int. J. STD AIDS 2018, 29, 1174–1182. [Google Scholar] [CrossRef]
- Pankam, T.; Kerr, S.J.; Teeratakulpisan, N.; Rodbamrung, P.; Wongkanya, R.; Keelawat, S.; Ruangritchankul, K.; Hongchookiat, P.; Watanapokasin, R.; Phanuphak, N. Human papillomavirus in anal biopsy tissues and liquid-based cytology samples of HIV-positive and HIV-negative Thai men who have sex with men. Papillomavirus Res. 2017, 3, 149–154. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Yin, Y.P.; Feng, T.J.; Hong, F.C.; Jiang, N.; Wang, B.X.; Chen, X.S. HPV infections among MSM in Shenzhen, China. PLoS ONE 2014, 9, e96364. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Yang, Y.; Zhong, X.; Feng, B.; Xin, H.; Li, Z.; Jin, Q.; Gao, L. Anal HPV/HIV co-infection among Men Who Have Sex with Men: A cross-sectional survey from three cities in China. Sci. Rep. 2016, 6, 21368. [Google Scholar] [CrossRef]
- Donà, M.G.; Giuliani, M.; Rollo, F.; Vescio, M.F.; Benevolo, M.; Giglio, A.; Giuliani, E.; Morrone, A.; Latini, A. Incidence and clearance of anal high-risk Human Papillomavirus infection and their risk factors in men who have sex with men living with HIV. Sci. Rep. 2022, 12, 184. [Google Scholar]
- Wei, F.; Gaisa, M.M.; D’Souza, G.; Xia, N.; Giuliano, A.R.; Hawes, S.E.; Gao, L.; Cheng, S.H.; Donà, M.G.; Goldstone, S.E.; et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29,900 men according to HIV status, sexuality, and age: A collaborative pooled analysis of 64 studies. Lancet HIV 2021, 8, e531–e543. [Google Scholar] [CrossRef]
- Ucciferri, C.; Tamburro, M.; Falasca, K.; Sammarco, M.L.; Ripabelli, G.; Vecchiet, J. Prevalence of anal, oral, penile and urethral Human Papillomavirus in HIV infected and HIV uninfected men who have sex with men. J. Med. Virol. 2018, 90, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Geskus, R.B.; González, C.; Torres, M.; Del Romero, J.; Viciana, P.; Masiá, M.; Blanco, J.R.; Iribarren, M.; De Sanjosé, S.; Hernández-Novoa, B.; et al. Incidence and clearance of anal high-risk human papillomavirus in HIV-positive men who have sex with men: Estimates and risk factors. AIDS 2016, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Goodman, M.T.; Xia, N.; Zhang, J.; Giuliano, A.R.; D’Souza, G.; Hessol, N.A.; Schim van der Loeff, M.F.; Dai, J.; Neukam, K.; et al. Incidence and Clearance of Anal Human Papillomavirus Infection in 16 164 Individuals, According to Human Immunodeficiency Virus Status, Sex, and Male Sexuality: An International Pooled Analysis of 34 Longitudinal Studies. Clin. Infect. Dis. 2023, 76, e692–e701. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | HIV-Negative (N = 1294) | HIV-Positive (N = 131) | p Value | Total (N = 1425) |
|---|---|---|---|---|
| Age (years) * | 29.00 (24.00, 35.00) | 28.00 (23.00, 36.75) | 0.209 | 29.00 (24.00, 36.00) |
| Locality | 0.195 | |||
| Local resident | 630 (48.69) | 56 (42.75) | 686 (48.14) | |
| Non-local resident | 664 (51.31) | 75 (57.25) | 739 (51.86) | |
| Ethnicity | 0.395 | |||
| Han | 1112 (85.94) | 109 (83.21) | 1221 (85.68) | |
| Non-han | 182 (14.06) | 22 (16.79) | 204 (14.32) | |
| Educational level | <0.001 | |||
| High school or below | 262 (20.25) | 43 (32.82) | 305 (21.40) | |
| Some college | 359 (27.74) | 42 (32.06) | 401 (28.14) | |
| Bachelor’s degree and above | 673 (52.01) | 46 (35.11) | 719 (50.46) | |
| Employment | 0.815 | |||
| Employed | 1046 (80.83) | 107 (81.68) | 1153 (80.91) | |
| Unemployed | 248 (19.17) | 24 (18.32) | 272 (19.09) | |
| Salary (yuan/month) | 0.008 | |||
| ≤1000 | 161 (12.44) | 17 (12.98) | 178 (12.49) | |
| 1001~5000 | 610 (47.14) | 79 (60.31) | 689 (48.35) | |
| 5001~10,000 | 419 (32.38) | 32 (24.43) | 451 (31.65) | |
| ≥10,001 | 104 (8.04) | 3 (2.29) | 107 (7.51) | |
| Age at first anal intercourse (years) # | 20.00 (18.00, 23.00) | 20.00 (18.00, 22.00) | 0.016 | 20.00 (18.00, 23.00) |
| Sexual orientation | 0.188 | |||
| Gay | 1002 (77.43) | 108 (82.44) | 1110 (77.89) | |
| Bisexual or other | 292 (22.57) | 23 (17.56) | 315 (22.11) | |
| Sexual partner | 0.592 | |||
| Men only | 749 (57.88) | 79 (60.31) | 828 (58.11) | |
| Both men and women | 545 (42.12) | 52 (39.69) | 597 (41.89) | |
| Sexual partner during last year | 0.504 | |||
| Men only | 1056 (81.61) | 110 (83.97) | 1166 (81.82) | |
| Both men and women | 238 (18.39) | 21 (16.03) | 259 (18.18) | |
| Anal sex during last six months | 0.067 | |||
| Yes | 1041 (80.45) | 114 (87.02) | 1155 (81.05) | |
| No | 253 (19.55) | 17 (12.98) | 270 (18.95) | |
| Predominant role in anal sex | 0.402 | |||
| Mainly insertive | 853 (65.92) | 94 (71.76) | 947 (66.46) | |
| Mainly receptive | 247 (19.09) | 21 (16.03) | 268 (18.81) | |
| Insertive and receptive | 194 (14.99) | 16 (12.21) | 210 (14.74) | |
| Homosexual partner number during last six months & | 2.00 (1.00, 3.00) | 2.00 (1.00, 3.50) | 0.013 | 2.00 (1.00, 3.00) |
| Condom use | 0.139 | |||
| Yes | 782 (60.43) | 82 (62.60) | 864 (60.63) | |
| No | 259 (20.02) | 32 (24.43) | 291 (20.42) | |
| No anal sex during last six months | 253 (19.55) | 17 (12.98) | 270 (18.95) | |
| Frequence of condom use during last six months | 0.185 | |||
| Always | 588 (45.44) | 58 (44.27) | 646 (45.33) | |
| Sometimes | 186 (14.37) | 24 (18.32) | 210 (14.74) | |
| Never | 267 (20.63) | 32 (24.43) | 299 (20.98) | |
| No anal sex during last six months | 253 (19.55) | 17 (12.98) | 270 (18.95) | |
| Commercial sex | 0.049 | |||
| No | 1001 (77.36) | 113 (86.26) | 1114 (78.18) | |
| Yes | 57 (4.40) | 2 (1.53) | 59 (4.14) | |
| Unknown | 236 (18.24) | 16 (12.21) | 252 (17.68) | |
| Heterosexual sex during last six months | 0.797 | |||
| No | 1146 (88.56) | 117 (89.31) | 1263 (88.63) | |
| Yes | 148 (11.44) | 14 (10.69) | 162 (11.37) | |
| Substance | <0.001 | |||
| No | 956 (73.88) | 78 (59.54) | 1034 (72.56) | |
| Yes | 338 (26.12) | 53 (40.46) | 391 (27.44) | |
| VCT | 0.914 | |||
| Yes | 1131 (87.40) | 114 (87.02) | 1245 (87.37) | |
| No | 85 (6.57) | 8 (6.11) | 93 (6.53) | |
| Unknown | 78 (6.03) | 9 (6.87) | 87 (6.11) | |
| Circumcision | 0.602 | |||
| No | 782 (60.43) | 85 (64.89) | 867 (60.84) | |
| Yes | 487 (37.64) | 44 (33.59) | 531 (37.26) | |
| Unknown | 25 (1.93) | 2 (1.53) | 27 (1.89) | |
| Tobacco | 0.092 | |||
| Never | 622 (48.07) | 56 (42.75) | 678 (47.58) | |
| Sometimes | 295 (22.80) | 41 (31.30) | 336 (23.58) | |
| Everyday | 377 (29.13) | 34 (25.95) | 411 (28.84) | |
| Alcohol | 0.135 | |||
| Never | 279 (21.56) | 28 (21.37) | 307 (21.54) | |
| Sometimes | 977 (75.50) | 103 (78.63) | 1080 (75.79) | |
| Everyday | 38 (2.94) | 0 (0.00) | 38 (2.67) |
| HPV Genotypes | HIV-Positive (N = 131) | HIV-Negative (N = 1294) | Risk Ratio (HIV-Positive vs. HIV-Negative) | p Value |
|---|---|---|---|---|
| HPV16 | 12.21 (7.72–19.33) | 9.27 (7.82–11.00) | 1.32 (0.81–2.15) | 0.270 |
| HPV18 | 6.11 (3.12–11.95) | 4.25 (3.28–5.50) | 1.44 (0.70–2.95) | 0.324 |
| HPV31 | 6.11 (3.12–11.95) | 2.47 (1.76–3.48) | 2.47 (1.16–5.25) | 0.019 |
| HPV33 | 5.34 (2.60–10.99) | 3.40 (2.54–4.55) | 1.57 (0.72–3.42) | 0.254 |
| HPV45 | 5.34 (2.60–10.99) | 2.16 (1.50–3.12) | 2.47 (1.10–5.54) | 0.028 |
| HPV52 | 3.82 (1.62–9.02) | 4.33 (3.35–5.59) | 0.88 (0.36–2.16) | 0.784 |
| HPV58 | 12.98 (8.33–20.22) | 4.71 (3.69–6.02) | 2.75 (1.66–4.57) | <0.001 |
| HPV6 | 13.74 (8.95–21.10) | 9.13 (7.68–10.84) | 1.51 (0.95–2.39) | 0.083 |
| HPV11 | 9.16 (5.34–15.71) | 6.34 (5.14–7.81) | 1.45 (0.81–2.58) | 0.212 |
| HPV Genotypes | HIV-Positive (N = 131) | HIV-Negative (N = 1294) | Rate Ratio (HIV-Positive vs. HIV-Negative) | p Value |
|---|---|---|---|---|
| HPV16 | 25.00 (16.94–36.90) | 18.54 (16.11–21.35) | 1.35 (0.89–2.04) | 0.157 |
| HPV18 | 6.33 (2.71–14.78) | 11.03 (9.14–13.33) | 0.57 (0.24–1.37) | 0.210 |
| HPV31 | 15.19 (9.02–25.58) | 7.18 (5.66–9.10) | 2.12 (1.19–3.75) | 0.010 |
| HPV33 | 15.38 (9.14–25.89) | 7.01 (5.50–8.93) | 2.19 (1.24–3.90) | 0.007 |
| HPV45 | 11.11 (6.00–20.57) | 4.79 (3.57–6.44) | 2.32 (1.17–4.59) | 0.016 |
| HPV52 | 10.13 (5.25–19.53) | 13.86 (11.74–16.36) | 0.73 (0.37–1.44) | 0.364 |
| HPV58 | 10.81 (5.62–20.80) | 10.71 (8.84–12.96) | 1.01 (0.51–2.00) | 0.978 |
| HPV6 | 22.67 (14.92–34.43) | 17.76 (15.37–20.51) | 1.28 (0.82–1.99) | 0.279 |
| HPV11 | 13.92 (8.05–24.09) | 13.79 (11.68–16.29) | 1.01 (0.57–1.79) | 0.974 |
| HPV Genotypes | HIV-Positive (N = 131) | HIV-Negative (N = 1294) | Rate Ratio (HIV-Positive vs. HIV-Negative) | p Value |
|---|---|---|---|---|
| HPV16 | 74.07 (57.90–94.77) | 83.98 (76.80–91.83) | 0.88 (0.68–1.15) | 0.348 |
| HPV18 | 85.71 (63.88–115.0) | 93.58 (87.14–100.5) | 0.92 (0.68–1.24) | 0.570 |
| HPV31 | 76.92 (59.33–99.73) | 93.65 (84.16–104.2) | 0.82 (0.62–1.09) | 0.170 |
| HPV33 | 80.00 (59.50–107.6) | 91.76 (83.46–100.9) | 0.87 (0.64–1.19) | 0.387 |
| HPV45 | 88.89 (67.94–116.3) | 93.18 (82.75–104.9) | 0.95 (0.71–1.28) | 0.753 |
| HPV52 | 90.00 (66.66–121.5) | 88.89 (81.64–96.78) | 1.01 (0.74–1.38) | 0.938 |
| HPV58 | 57.14 (43.79–74.57) | 95.65 (89.03–102.8) | 0.60 (0.45–0.79) | <0.001 |
| HPV6 | 63.64 (47.68–84.93) | 85.42 (78.51–92.93) | 0.75 (0.55–1.01) | 0.055 |
| HPV11 | 84.62 (62.92–113.8) | 86.86 (79.38–95.05) | 0.97 (0.71–1.33) | 0.868 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, T.; Lu, Z.; He, J.; Fu, L.; Yu, W.; Zhang, Z.; Chen, Z.; Zou, H.; Dai, J. Heterogeneity in Prevalence, Incidence, and Clearance of Anal Human Papillomavirus Among HIV-Negative and HIV-Positive Men Who Have Sex with Men in China: An Observational Cohort Study. Vaccines 2025, 13, 1144. https://doi.org/10.3390/vaccines13111144
Tian T, Lu Z, He J, Fu L, Yu W, Zhang Z, Chen Z, Zou H, Dai J. Heterogeneity in Prevalence, Incidence, and Clearance of Anal Human Papillomavirus Among HIV-Negative and HIV-Positive Men Who Have Sex with Men in China: An Observational Cohort Study. Vaccines. 2025; 13(11):1144. https://doi.org/10.3390/vaccines13111144
Chicago/Turabian StyleTian, Tian, Zhen Lu, Jingjing He, Leiwen Fu, Wenhui Yu, Zewen Zhang, Zhen Chen, Huachun Zou, and Jianghong Dai. 2025. "Heterogeneity in Prevalence, Incidence, and Clearance of Anal Human Papillomavirus Among HIV-Negative and HIV-Positive Men Who Have Sex with Men in China: An Observational Cohort Study" Vaccines 13, no. 11: 1144. https://doi.org/10.3390/vaccines13111144
APA StyleTian, T., Lu, Z., He, J., Fu, L., Yu, W., Zhang, Z., Chen, Z., Zou, H., & Dai, J. (2025). Heterogeneity in Prevalence, Incidence, and Clearance of Anal Human Papillomavirus Among HIV-Negative and HIV-Positive Men Who Have Sex with Men in China: An Observational Cohort Study. Vaccines, 13(11), 1144. https://doi.org/10.3390/vaccines13111144






