Successive Efficacy Evaluation of Various Commercial Live-Attenuated Avian coronavirus Vaccination Schedules Against a Local GI-23.3 Challenge in SPF Broilers
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Vaccines and Strains of Viruses
2.3. Experimental Design
2.4. Evaluation of Tracheal Ciliostasis
2.5. IBV Shedding Titers
2.6. Pathological Examination
2.7. Statistical Analysis
3. Results
3.1. Genetic Similarity
3.2. Clinical Observations
3.3. Tracheal Post-Challenge Ciliary Activity
3.4. Quantification of IBV Shedding Using qRT–PCR
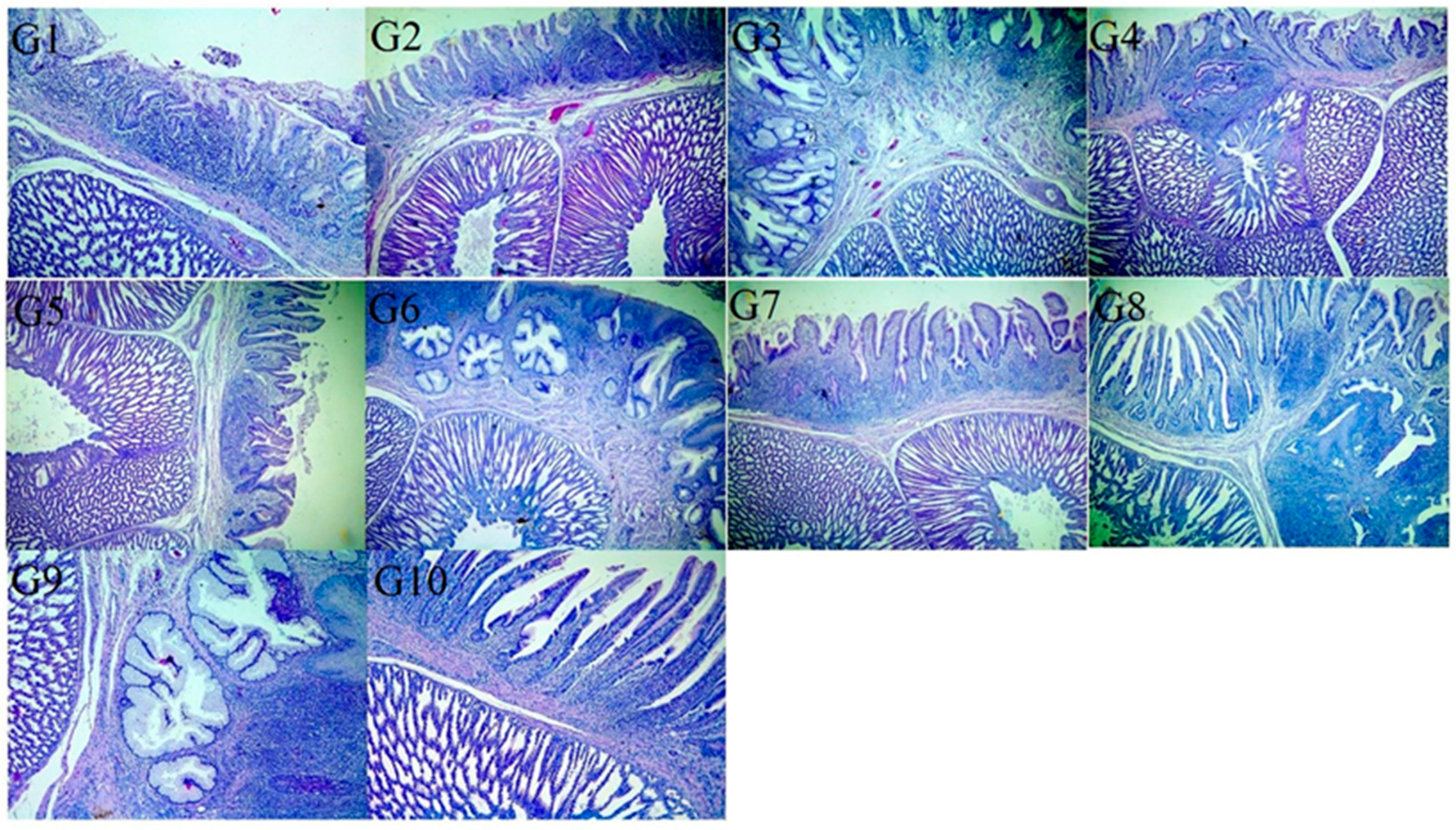
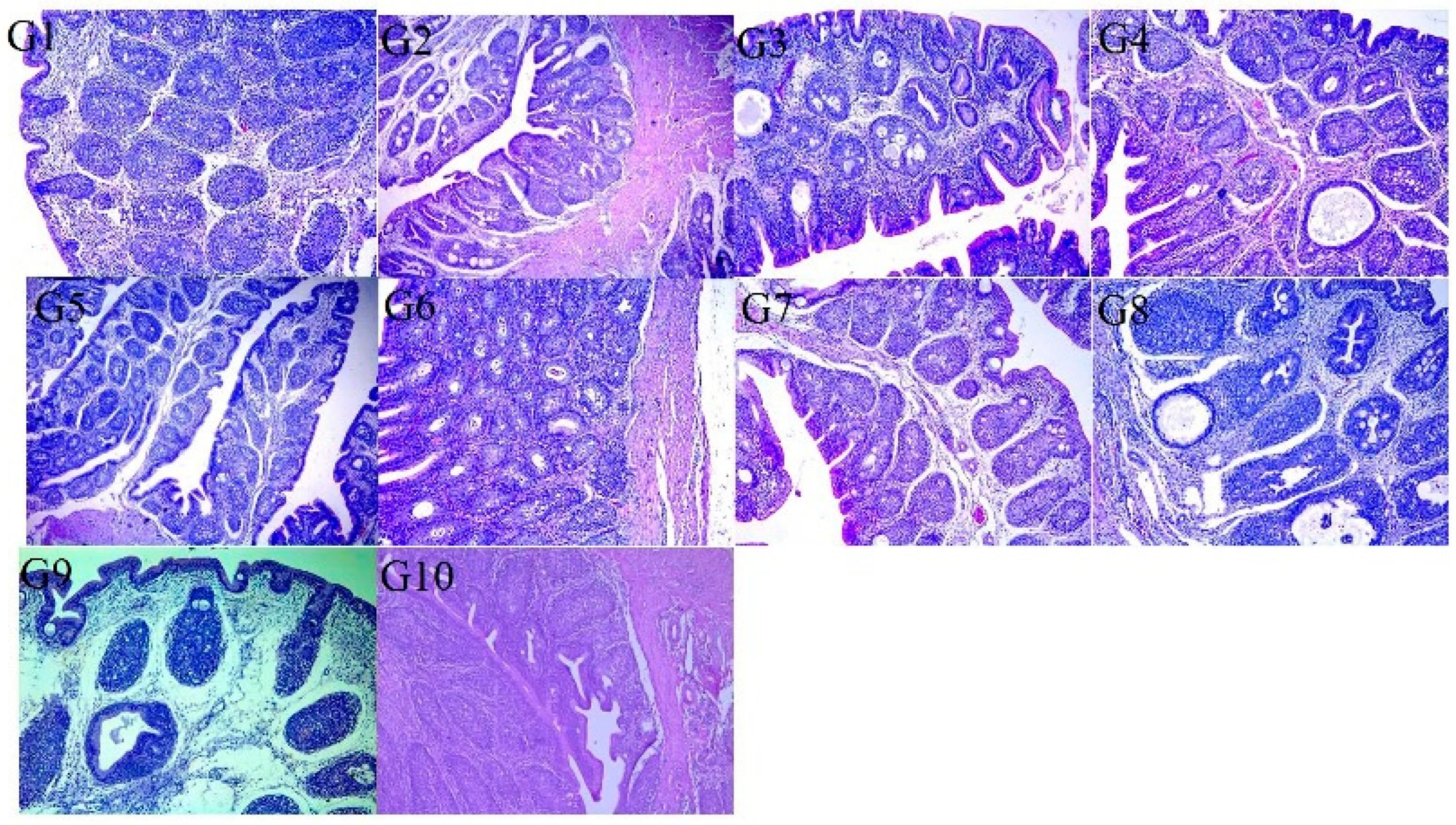
3.5. Histopathological Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abodalal, S.E.S.A.; Hafez, M.S.h.A.; Abd El-Munem Shosha, E.; Warda, F.F.; Hagag, N.M. Isolation and Molecular Characterization of Rabbit Haemorrhagic Disease Virus Strains Circulating in Rabbit Population Using Sequencing and Phylogenetic Analysis. J. World Poult. Res. 2021, 11, 302–311. [Google Scholar] [CrossRef]
- Badr, H.; AbdelMenamm Shosha, E.; Roshdy, H.; Abd El-Halem Mohammed, A.; Saad, N.; Mostafa Aboelenin, S.; Mohamed Soliman, M.; El-Tahan, A.M.; El-Saadony, M.T.; Yehia, N. Investigation of many bacterial and viral infections circulating in pigeons showing nervous symptoms. Saudi J. Biol. Sci. 2022, 29, 2911–2920. [Google Scholar] [CrossRef]
- Fotouh, A.; Shosha, E.A.E.; Zanaty, A.M.; Darwesh, M.M. Immunopathological investigation and genetic evolution of Avian leukosis virus Subgroup-J associated with myelocytomatosis in broiler flocks in Egypt. Virol. J. 2024, 21, 83. [Google Scholar] [CrossRef]
- Ramzy, N.M.; Ibrahim, A.I.; Shosha, E.A. Isolation and genetic diversity of fowlpox virus circulating in chicken flocks in Egypt. J. Adv. Vet. Res. 2024, 14, 799–806. Available online: https://www.advetresearch.com/index.php/AVR/article/view/1756 (accessed on 29 October 2025).
- Zhang, X.; Guo, M.; Zhao, J.; Wu, Y. Avian infectious bronchitis in China: Epidemiology, vaccination, and control. Avian Dis. 2021, 65, 654–658. [Google Scholar] [CrossRef]
- De Wit, J.J.; Cook, J.K.A. Spotlight on avian pathology: Infectious bronchitis virus. Avian Pathol. 2019, 48, 393–395. [Google Scholar] [CrossRef]
- Jackwood, M.; de Wit, J.J. Infectious bronchitis virus. In Diseases of Poultry, 14th ed.; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 167–188. [Google Scholar]
- Bhuiyan, M.S.; Amin, K.F.; Rodrigues, S.S.; Saallah, M.; Shaarani, S.S.; Siddiquee, S. Infectious bronchitis virus (gammacoronavirus) in poultry farming: Vaccination, immune response and measures for mitigation. Vet. Sci. 2021, 8, 273. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, Y.; Hu, Y.; Zhao, J.; Xue, J.; Zhang, G. The furin-S2′ site in avian coronavirus plays a key role in central nervous system damage progression. J. Virol. 2021, 95, e02447-20. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Zhang, G. Key Aspects of Coronavirus Avian Infectious Bronchitis Virus. Pathogens 2023, 12, 698. [Google Scholar] [CrossRef]
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef]
- Promkuntod, N.; van Eijndhoven, R.E.; de Vrieze, G.; Gröne, A.; Verheije, M.H. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology 2014, 448, 26–32. [Google Scholar] [CrossRef]
- Feng, K.; Chen, T.; Zhang, X.; Shao, G.; Cao, Y.; Chen, D.; Lin, W.; Chen, F.; Xie, Q. Molecular Characteristic and Pathogenicity Analysis of a Virulent Recombinant Avian Infectious Bronchitis Virus Isolated in China. Poult. Sci. 2018, 97, 3519–3531. [Google Scholar] [CrossRef]
- Laconi, A.; van Beurden, S.J.; Berends, A.J.; Krämer-Kühl, A.; Jansen, C.A.; Spekreijse, D.; Chénard, G.; Philipp, H.-C.; Mundt, E.; Rottier, P.J. Deletion of Accessory Genes 3a, 3b, 5a or 5b from Avian Coronavirus Infectious Bronchitis Virus Induces an Attenuated Phenotype Both in vitro and in vivo. J. Gen. Virol. 2018, 99, 1381. [Google Scholar] [CrossRef]
- Jackwood, M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012, 56, 634–641. [Google Scholar] [CrossRef]
- Abolnik, C. Genomic and single nucleotide polymorphism analysis of infectious bronchitis coronavirus. Infect. Genet. Evol. 2015, 32, 416–424. [Google Scholar] [CrossRef]
- Valastro, V.; Holmes, E.C.; Britton, P.; Fusaro, A.; Jackwood, M.W.; Cattoli, G.; Monne, I. S1 gene-based phylogeny of infectious bronchitis virus: An attempt to harmonize virus classification. Infect. Genet. Evol. 2016, 39, 349–364. [Google Scholar] [CrossRef]
- Ganapathy, K.; Ball, C.; Forrester, A. Genotypes of infectious bronchitis viruses circulating in the Middle East between 2009 and 2014. Virus Res. 2015, 210, 198–204. [Google Scholar] [CrossRef]
- Houta, M.H.; Hassan, K.E.; El-Sawah, A.A.; Elkady, M.F.; Kilany, W.H.; Ali, A.; Abdel-Moneim, A.S. The emergence, evolution and spread of infectious bronchitis virus genotype GI-23. Arch. Virol. 2021, 166, 9–26. [Google Scholar] [CrossRef]
- Madbouly, H.; Abdel-Moneim, A.S.; Gelb, J.; Ladman, B.S.; Nancy, B.R. Molecular characterization of three Egyptian infectious bronchitis virus isolates. Vet. Med. J. 2002, 50, 1053–1064. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.S.; El-Kady, M.F.; Ladman, B.S.; Gelb, J. S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol. J. 2006, 3, 78. [Google Scholar] [CrossRef]
- Zanaty, A.; Naguib, M.M.; El-Husseiny, M.H.; Mady, W.; Hagag, N.; Arafa, A.S. The sequence of the full spike S1 glycoprotein of infectious bronchitis virus circulating in Egypt reveals evidence of intra-genotypic recombination. Arch. Virol. 2016, 161, 3583–3587. [Google Scholar] [CrossRef]
- Hassan, K.E.; Shany, S.A.S.; Ali, A.; Dahshan, A.H.M.; El-Sawah, A.A.; El-Kady, M.F. Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poult. Sci. 2016, 95, 1271–1280. [Google Scholar] [CrossRef]
- Feng, K.; Xue, Y.; Wang, J.; Chen, W.; Chen, F.; Bi, Y.; Xie, Q. Development and efficacy of a novel live-attenuated QX-like nephropathogenic infectious bronchitis virus vaccine in China. Vaccine 2015, 33, 1113–1120. [Google Scholar] [CrossRef]
- Ali, A.; Kilany, W.H.; Zain El-Abideen, M.A.; Sayed MEl Elkady, M. Safety and efficacy of attenuated classic and variant 2 infectious bronchitis virus candidate vaccines. Poult. Sci. 2018, 97, 4238–4244. [Google Scholar] [CrossRef]
- Awad, F.; Hutton, S.; Forrester, A.; Baylis, M.; Ganapathy, K. Heterologous live infectious bronchitis virus vaccination in day-old commercial broiler chicks: Clinical signs, ciliary health, immune responses and protection against variant infectious bronchitis viruses. Avian Pathol. 2016, 45, 169–177. [Google Scholar] [CrossRef]
- Awad, F.; Chhabra, R.; Forrester, A.; Chantrey, J.; Baylis, M.; Lemiere, S.; Hussein, H.A.; Ganapathy, K. Experimental infection of IS/885/00-like infectious bronchitis virus in specific pathogen free and commercial broiler chicks. Res. Vet. Sci. 2016, 105, 15–22. [Google Scholar] [CrossRef]
- Habibi, M.; Karimi, V.; Langeroudi, A.G.; Ghafouri, S.A.; Hashemzadeh, M.; Farahani, R.K.; Maghsoudloo, H.; Abdollahi, H.; Seifouri, P. Combination of H120 and 1/96 avian infectious bronchitis virus vaccine strains protect chickens against challenge with IS/1494/06 (variant 2)-like infectious bronchitis virus. Acta Virol. 2017, 61, 150–160. [Google Scholar] [CrossRef]
- Bru, T.; Vila, R.; Cabana, M.; Geerligs, H.J. Protection of chickens vaccinated with combinations of commercial live infectious bronchitis vaccines containing Massachusetts, Dutch and QX-like serotypes against challenge with virulent infectious bronchitis viruses 793B and IS/1494/06 Israel variant 2. Avian Pathol. 2017, 46, 52–58. [Google Scholar] [CrossRef]
- Pohuang, T.; Tanasatian, S.; Sasipreeyajan, J. Efficacy of different vaccination programs of live 4/91 strain against Thai QX-like infectious bronchitis virus in broiler chickens. Thai J. Vet. Med. 2016, 46, 419–425. [Google Scholar] [CrossRef]
- Yang, C.Y.; Peng, P.; Liu, X.; Cao, Y.; Zhang, Y. Effect of monovalent and bivalent live attenuated vaccines against QX-like IBV infection in young chickens. Poult. Sci. 2023, 102, 102501. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, J.L.; Liu, X.Y.; Zhao, J.; Hu, Y.X.; Zhang, G.Z. Safety and efficacy of an attenuated Chinese QX-like infectious bronchitis virus strain as a candidate vaccine. Vet. Microbiol. 2015, 180, 49–58. [Google Scholar] [CrossRef]
- Huo, Y.F.; Huang, Q.H.; Lu, M.; Wu, J.Q.; Lin, S.Q.; Zhu, F.; Zhang, X.-M.; Huang, Y.-Y.; Yang, S.-H.; Xu, C.T. Attenuation mechanism of virulent infectious bronchitis virus strain with QX genotype by continuous passage in chicken embryos. Vaccine 2016, 34, 83–89. [Google Scholar] [CrossRef]
- Abozeid, H.H.; Paldurai, A.; Khattar, S.K.; Afifi, M.A.; El-Kady, M.F.; El-Deeb, A.H.; Samal, S.K. Complete genome sequences of two avian infectious bronchitis viruses isolated in Egypt: Evidence for genetic drift and genetic recombination in the circulating viruses. Infect. Genet. Evol. 2017, 53, 7–14. [Google Scholar] [CrossRef]
- Naguib, M.M.; El-Kady, M.F.; Lüschow, D.; Hassan, K.E.; Arafa, A.S.; El-Zanaty, A.; Hassan, M.K.; Hafez, H.M.; Grund, C.; Harder, T.C. New real time and conventional RT-PCRs for updated molecular diagnosis of infectious bronchitis virus infection (IBV) in chickens in Egypt associated with frequent co-infections with avian influenza and Newcastle Disease viruses. J. Virol. Methods 2017, 245, 19–27. [Google Scholar] [CrossRef]
- Abdelnasser, S.; Senosy, W.; Zanaty, A.M.; Shosha, E. Genetic evolution and phylogenetic analysis of Infectious Bronchitis Virus circulating in broiler flocks in New Valley Governorate, Egypt. New Val. Vet. J. 2024, 4, 202. [Google Scholar] [CrossRef]
- Legnardi, M.; Franzo, G.; Koutoulis, K.C.; Wisniewski, M.; Catelli, E.; Tucciarone, C.M.; Cecchinato, M. Vaccine or field strains: The jigsaw pattern of infectious bronchitis virus molecular epidemiology in Poland. Poult. Sci. 2019, 98, 6388–6392. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Han, Z.; Zhang, Q.; Shao, Y.; Kong, X.; Tong, G. Infectious bronchitis virus: S1 gene characteristics of vaccines used in China and efficacy of vaccination against heterologous strains from China. Avian Pathol. 2006, 35, 394–399. [Google Scholar] [CrossRef]
- Sultan, H.A.; Ali, A.; El Feil, W.K.; Bazid, A.H.I.; Zain El-Abideen, M.A.; Kilany, W.H. Protective Efficacy of Different Live Attenuated Infectious Bronchitis Virus Vaccination Regimes Against Challenge with IBV Variant-2 Circulating in the Middle East. Front. Vet. Sci. 2019, 6, 341. [Google Scholar] [CrossRef]
- Gelb, J.J.; Jackwood, M.W. Infectious bronchitis. In A Laboratory Manual for the Isolation, Identification, and Characterization of Avian Pathogens, 6th ed.; Williams, S.M., Dufour-Zavala, L., Jackwood, M.W., Lee, M.D., Lupiani, B., Reed, W.M., Eds.; Wiley: Hoboken, NJ, USA; American Association of Avian Pathologists: Jacksonville, FL, USA, 2016; pp. 146–149. [Google Scholar]
- Parvin, R.; Begum, J.A.; Nooruzzaman, M.; Kabiraj, C.K.; Chowdhury, E.H. Circulation of three genotypes and identification of unique mutations in neutralizing epitopes of infectious bronchitis virus in chickens in Bangladesh. Arch. Virol. 2021, 166, 3093–3103. [Google Scholar] [CrossRef]
- Eid, A.A.M.; Mahmoud, A.M.; Hamouda, E.E.; Metwally, M.; Ezz-Eldin, R.M.M.; ElBakrey, R.M. The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus. Open Vet. J. 2024, 14, 90–107. [Google Scholar] [CrossRef]
- Cook, J.K.A.; Orbell, S.J.; Woods, M.A.; Huggins, M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999, 28, 477–485. [Google Scholar] [CrossRef]
- De Wit, J.J.; Boelm, G.J.; van Gerwe, T.J.; Swart, W.A. The required sample size in vaccination-challenge experiments with infectious bronchitis virus, a metaanalysis. Avian Pathol. 2013, 42, 9–16. [Google Scholar] [CrossRef]
- Ameen, S.M.; Adel, A.; Selim, A.; Magouz, A.; AboElKhair, M.; Bazid, A.H. A multiplex real-time reverse transcription polymerase chain reaction assay for differentiation of classical and variant II strains of avian infectious bronchitis virus. Arch. Virol. 2022, 167, 2729–2741. [Google Scholar] [CrossRef]
- Lee, C.W.; Suarez, D.L. Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J. Virol. Methods 2004, 119, 151–158. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Technique, 7th ed.; Churchill, Livingston: London, UK; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Wilson, F.D.; Banda, A.; Hoerr, F.J.; Alvarado, I.; Orozco, E.; Mackey, R. Histopathologic lesion scoring and histomorphometric methods for measuring vaccine reactions in the trachea of broiler chickens. Avian Dis. 2021, 65, 18–25. [Google Scholar] [CrossRef]
- Moharam, I.; Sultan, H.; Hassan, K.; Ibrahim, M.; Shany, S.; Shehata, A.A.; Abo-ElKhair, M.; Pfaff, F.; Höper, D.; Kady, M.E. Emerging Infectious Bronchitis Virus (IBV) in Egypt: Evidence for an Evolutionary Advantage of a New S1 Variant with a Unique Gene 3ab Constellation. Infect. Genet. Evol. 2020, 85, 104433. [Google Scholar] [CrossRef]
- Amer, S.A.E.l.-M.; Ahmed, H.M.; Maatouq, A.M. Molecular Genotyping and Pathogenicity Study for Avian Infectious Bronchitis Virus Currently Epidemic in Chicken Flocks in North Egypt during 2021. Egypt. J. Vet. Sci. 2024, 55, 223–232. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Randi Clark Sunny, C.h.e.n.g.; Brian, J. Jordan Protection following simultaneous vaccination with three or four different attenuated live vaccine types against infectious bronchitis virus. Avian Pathol. 2020, 49, 335–341. [Google Scholar] [CrossRef]
- Houta, M.H.; Hassan, K.E.; Kilany, W.H.; Shany, S.A.S.; El-Sawah, A.A.; ElKady, M.F.; Abdel-Moneim, A.S.; Ali, A. Evaluation of different heterologous-homologous vaccine regimens against challenge with GI-23 lineage infectious bronchitis virus. Virology 2024, 598, 110193. [Google Scholar] [CrossRef]
- Thomrongsuwannakij, T.; Phu, D.H.; Chansiripornchai, N. Evaluation of the efficacy of commercial live vaccines against the local Thai QX field strain for the protection of specific pathogen-free chicks. Vet. World 2024, 17, 771–777. [Google Scholar] [CrossRef]
- Afifi, M.A.; Zouelfakar, S.A.; Morsy, E.A.; Salem, H.M.; Abozeid, H.H.; Ahmed, K.A. Protectotype efficacy of commercial infectious bronchitis virus (IBV) liveattenuated vaccines against field classic and variant IBV challenges in Egypt. In Proceedings of the XXIst WVPAC, Bitec, Bangkok, Thailand, 16–20 September 2019. [Google Scholar]
- Sawerus, M.G.; Shehata, O.; Ahmed, W.M.S.; Shany, S.; Hassan, K.E.; Mahdi, E.A.; Mohamed, A.H. The modulatory effect of carvacrol on viral shedding titer and acute phase response in broiler chickens experimentally infected with infectious bronchitis virus. Microb. Pathog. 2022, 163, 105410. [Google Scholar] [CrossRef]
- Bande, F.; Arshad, S.S.; Omar, A.R.; Hair-Bejo, M.; Mahmuda, A.; Nair, V. Global distributions and strain diversity of avian infectious bronchitis virus: A review. Anim. Health Res. Rev. 2017, 18, 70–83. [Google Scholar] [CrossRef]
- Bali, K.; Balint, A.; Farsang, A.; Marton, S.; Nagy, B.; Kaszab, E.; Belak, S.; Palya, V.; Banyai, K. Recombination events shape the genomic evolution of infectious bronchitis virus in Europe. Viruses 2021, 13, 535. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Han, Z.; Wang, Y.; Liang, S.; Jiang, L.; Hu, Y.; Kong, X.; Liu, S. Recombinant duck enteritis viruses expressing major structural proteins of the infectious bronchitis virus provide protection against infectious bronchitis in chickens. Antivir. Res. 2016, 130, 19–26. [Google Scholar] [CrossRef]
- Li, J.; Helal, Z.H.; Karch, C.P.; Mishra, N.; Girshick, T.; Garmendia, A.; Burkhard, P.; Khan, M.I. A self-adjuvanted nanoparticle based vaccine against infectious bronchitis virus. PLoS ONE 2018, 13, e0203771. [Google Scholar] [CrossRef]
- Qin, Y.; Tu, K.; Teng, Q.; Feng, D.; Zhao, Y.; Zhang, G. Identification of novel T-cell epitopes on infectious bronchitis virus N protein and development of a multi-epitope vaccine. J. Virol. 2021, 95, e0066721. [Google Scholar] [CrossRef]
- Benyeda, Z.; Mató, T.; Süveges, T.; Szabo, E.; Kardi, V.; Abonyi-Toth, Z.; Rusvai, M.; Palya, V. Comparison of the pathogenicity of QX-like, M41 and 793/B infectious bronchitis strains from different pathological conditions. Avian Pathol. 2009, 38, 449–456. [Google Scholar] [CrossRef]
- Lee, H.J.; Youn, H.N.; Kwon, J.S.; Lee, Y.J.; Kim, J.H.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Characterization of a novel live attenuated infectious bronchitis virus vaccine candidate derived from a Korean nephropathogenic strain. Vaccine 2010, 28, 2887–2894. [Google Scholar] [CrossRef]
- Zanaty, A.; Arafa A-s Selim, A.A.; Khalifa, M.; El-Kady, M.F. Evaluation of the Protection conferred by heterologous attenuated live infectious bronchitis viruses againest an Egyptian variant IBV [EG/1212B]. J. Am. Sci. 2013, 9, 599–606. Available online: https://www.jofamericanscience.org/journals/am-sci/am0906/076_18736am0906_599_606.pdf (accessed on 29 October 2025).
- Sedeik, M.E. Evaluation the breadth of protection of some available commercial live IB vaccines against an Egyptian variant (EG/1212B) of IBV. Int. J. Curr. Res. 2018, 7, 11608–11617. [Google Scholar]
- Chhabra, R.; Chantrey, J.; Ganapathy, K. Immune responses to virulent and vaccine strains of infectious bronchitis viruses in chickens. Viral Immunol. 2015, 28, 478–488. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, K.; Pan, L.; Qi, K.; Liu, H.; Chen, H. Co-infection of H9N2 subtype avian influenza virus and infectious bronchitis virus decreases SP-A expression level in chickens. Vet. Microbiol. 2017, 203, 110–116. [Google Scholar] [CrossRef]
- Ismail, Z.M.; El-Deeb, A.H.; El-Safty, M.M.; Hussein, H.A. Enhanced pathogenicity of low-pathogenic H9N2 avian influenza virus after vaccination with infectious bronchitis live attenuated vaccine. Vet. World 2018, 11, 977–985. [Google Scholar] [CrossRef]
- Lockyear, O.; Breedlove, C.; Joiner, K.; Toro, H. Distribution of Infectious Bronchitis Virus Resistance in a Naïve Chicken Population. Avian Dis. 2022, 66, 101–105. [Google Scholar] [CrossRef]
- Chousalkar, K.K.; Roberts, J.R.; Reece, R. Comparative histopathology of two serotypes of infectious bronchitis virus (T and n1/88) in laying hens and cockerels. Poult. Sci. 2007, 86, 50–58. [Google Scholar] [CrossRef]
- Cheng, J.; Huo, C.; Zhao, J.; Liu, T.; Li, X.; Yan, S.; Wang, Z.; Hu, Y.; Zhang, G. Pathogenicity differences between QX-like and Mass-type infectious bronchitis viruses. Vet. Microbiol. 2018, 213, 129–135. [Google Scholar] [CrossRef]
- Tang, X.; Qi, J.; Sun, L.; Zhao, J.; Zhang, G. Pathological effect of different avian infectious bronchitis virus strains on the bursa of Fabricius of chickens. Avian Pathol. 2022, 51, 339–348. [Google Scholar] [CrossRef]


| Group | Vaccination Regime | Number of Chicks | Age at Vaccination | Number of Challenged Chicks | |
|---|---|---|---|---|---|
| 1 Day | 14 Days | ||||
| 1 | IB Primer + 4/91 | 15 in each group | IB Primer + 4/91 | - | 15 |
| 2 | H120 + 4/91 | H120 + 4/91 | - | 15 | |
| 3 | IB Primer + IB Var II (Mevac) | IB Primer + IB Var II (Mevac) | - | 15 | |
| 4 | H120 + IB Var II (Mevac) | H120 + IB Var II (Mevac) | - | 15 | |
| 5 | IB Primer + 4/91 | IB Primer | 4/91 | 15 | |
| 6 | IB Primer + IB Var II (Mevac) | IB Primer | IB Var II (Mevac) | 15 | |
| 7 | H120 + 4/91 | H120 | 4/91 | 15 | |
| 8 | H120 + IB Var II (Mevac) | H120 | IB Var II (Mevac) | 15 | |
| 9 | Positive control | Non-vaccinated challenged group | 15 | ||
| 10 | Negative control | Non-vaccinated and non-challenged group | 15 | ||
| Score | Ciliary Activity |
|---|---|
| 0 | 100% ciliary activity, all cilia beating (complete protection) |
| 1 | 75 percent of cilia beating |
| 2 | 50 percent of cilia beating |
| 3 | 25 percent of cilia beating |
| 4 | 0 percent ciliary activity, non-beating cilia (complete lack of protection) |
| Score | Trachea (% of Wall Affected) |
|---|---|
| 0 | No change |
| 1 | 25% |
| 2 | 26–50% |
| 3 | 51–75% |
| 4 | 76–100% |
| Sample ID | NewValley-1-EGYIBV-GI23.3 | IBV-H120-GI-1 | IBV-D274, GI-12 | IBV-4/91-GI-13 | IBV-GI-23-EG/1212B-2012 | ||
|---|---|---|---|---|---|---|---|
| Nucleotide identity (%) | |||||||
| 1 | PQ093637: NewValley-1-EGYIBV-GI-23.3-2023 | ID | 84% | 87% | 86% | 90% | 1 |
| 2 | IBV- H120-GI-1 | 75% | ID | 85% | 83% | 82% | 2 |
| 3 | IBV- D274, GI-12 | 81% | 78% | ID | 86% | 86% | 3 |
| 4 | IBV- 4/91-GI-13 (4/91 primer vaccine) | 78% | 75% | 79% | ID | 83% | 4 |
| 5 | IBV-GI-23-EG/1212B-2012 (IB Var II vaccine) | 86% | 74% | 82% | 75% | ID | 5 |
| Amino acid identity (%) | |||||||
| Group | Vaccination | Ciliary Score (±SD) | Ciliary Protection |
|---|---|---|---|
| G1 a | IB Primer + 4/91 (1D) | 1.4 ± 0.08 | 65% |
| G2 b | H120 + 4/91 (1D) | 2.1 ± 0.07 | 48% |
| G3 b | IB Primer + IB Var II (1D) | 2 ± 0.01 | 50% |
| G4 c | H120 + IB Var II (1D) | 2.5 ± 0.08 | 38% |
| G5 a | IB Primer + 4/91 (1D-14D) | 1.3 ± 0.14 | 68% |
| G6 ab | IB Primer + IB Var II (1D-14D) | 1.5 ± 0.08 | 63% |
| G7 bc | H120 + 4/91 (1D-14D) | 1.9 ± 0.08 | 53% |
| G8 d | H120 + IB Var II (1D-14D) | 2.75 ± 0.12 | 31% |
| G9 e | Positive control | 4 ± 0.49 | 0% |
| G10 f | Negative control | 0.7 ± 0.08 | 83% |
| Groups and Vaccination Regime (1 Day Old) | Virus Shedding Titers (EID50/mL) at Specific Time Points | ||
|---|---|---|---|
| 3 DPC | 5 DPC | 7 DPC | |
| G1: IB Primer + 4/91 a | 3.53 ± 0.21 | 1.81 ± 0.23 | 1.11 ± 0.18 |
| G2: H120 + 4/91 b | 5.67 ± 0.45 | 4.64 ± 0.40 | 3.74 ± 0.36 |
| G3: IB Primer + IB Var II b | 4.93 ± 0.36 | 4.53 ± 0.31 | 3.45 ± 0.27 |
| G4: H120 + IB Primer Var II c | 5.87 ± 0.37 | 4.76 ± 0.31 | 4.16 ± 0.29 |
| Groups and vaccination regime (1 + 14 days old) | |||
| G5: IB Primer + 4/91 a | 3.15 ± 0.13 | 1.65 ± 0.12 | 0.9 ± 0.10 |
| G6: IB Primer + IB Var II ab | 3.72 ± 0.18 | 2.12 ± 0.18 | 1.52 ± 0.14 |
| G7: H120 + 4/91 bc | 3.98 ± 0.23 | 3.76 ± 0.21 | 3.03 ± 0.20 |
| G8: H120 + IB Var II d | 6.04 ± 0.31 | 4.81 ± 0.28 | 4.82 ± 0.24 |
| G9: Positive control e | 6.16 ± 0.13 | 5.49 ± 0.60 | 4.85 ± 0.33 |
| G10: Negative control f | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shosha, E.A.E.; Abdelnaser, S.; Zanaty, A.M.; ElZanaty, A.E.; Selim, K.; Eldaghayes, I. Successive Efficacy Evaluation of Various Commercial Live-Attenuated Avian coronavirus Vaccination Schedules Against a Local GI-23.3 Challenge in SPF Broilers. Vaccines 2025, 13, 1132. https://doi.org/10.3390/vaccines13111132
Shosha EAE, Abdelnaser S, Zanaty AM, ElZanaty AE, Selim K, Eldaghayes I. Successive Efficacy Evaluation of Various Commercial Live-Attenuated Avian coronavirus Vaccination Schedules Against a Local GI-23.3 Challenge in SPF Broilers. Vaccines. 2025; 13(11):1132. https://doi.org/10.3390/vaccines13111132
Chicago/Turabian StyleShosha, Eman Abd ElMenum, Sara Abdelnaser, Ali Mahmoud Zanaty, Abd Elfattah ElZanaty, Karim Selim, and Ibrahim Eldaghayes. 2025. "Successive Efficacy Evaluation of Various Commercial Live-Attenuated Avian coronavirus Vaccination Schedules Against a Local GI-23.3 Challenge in SPF Broilers" Vaccines 13, no. 11: 1132. https://doi.org/10.3390/vaccines13111132
APA StyleShosha, E. A. E., Abdelnaser, S., Zanaty, A. M., ElZanaty, A. E., Selim, K., & Eldaghayes, I. (2025). Successive Efficacy Evaluation of Various Commercial Live-Attenuated Avian coronavirus Vaccination Schedules Against a Local GI-23.3 Challenge in SPF Broilers. Vaccines, 13(11), 1132. https://doi.org/10.3390/vaccines13111132







