Production Optimization, Adjuvant Screening and Immunogenicity Evaluation of a Virus-like Vesicle Rabies Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viral Strains, and Experimental Subjects
2.2. Antibodies and Reagents
2.3. Investigations of Virus Propagation in Cell Culture and Shake Flask Systems
2.4. Infection and Proliferation of SFV-RVG in a Bioreactor System
2.5. Virus Ultrafiltration Concentration
2.6. Virus Chromatography Purification
2.7. SDS-PAGE and Western Blotting
2.8. Animal Immunization
2.8.1. Immunization and Protection in Mice
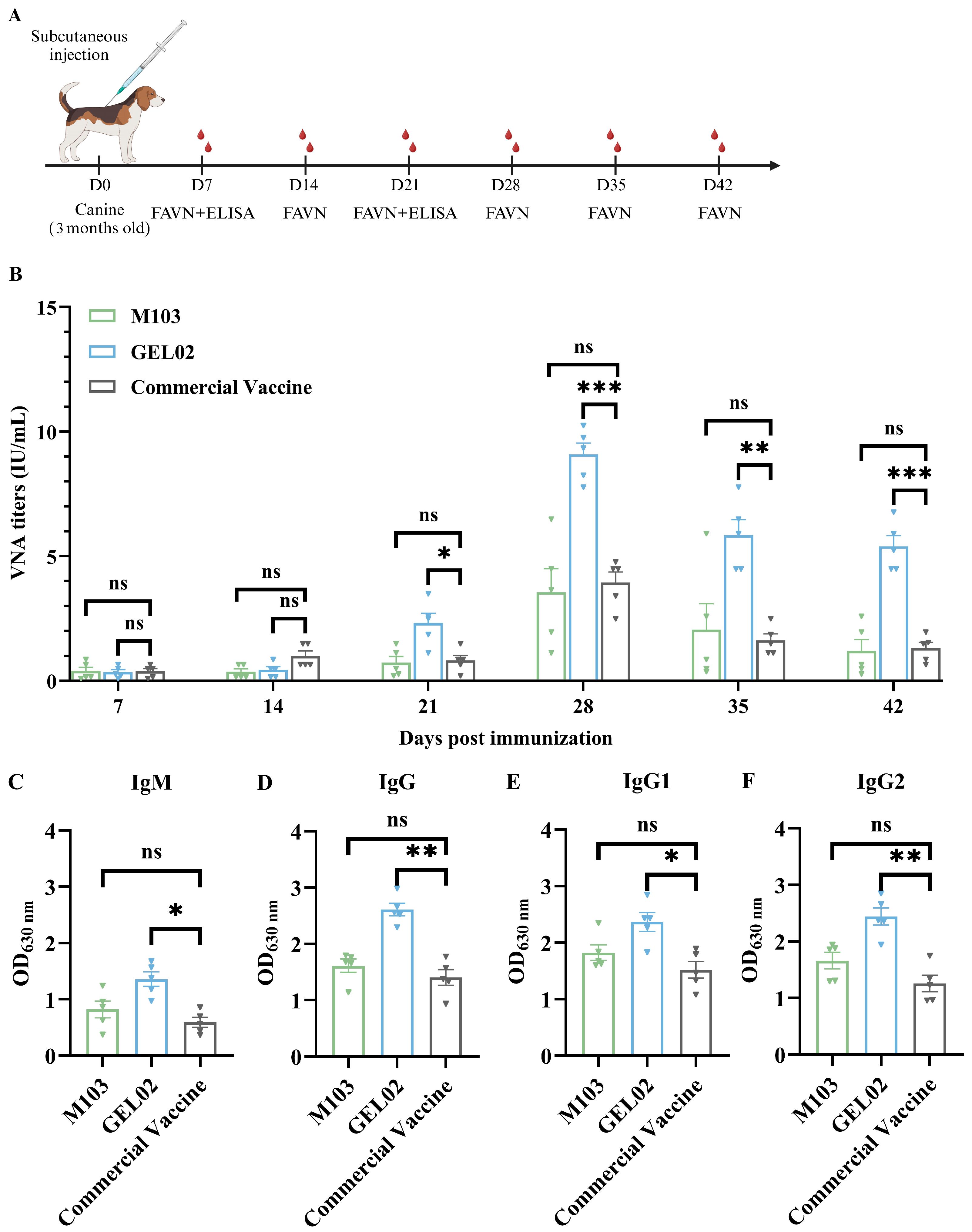
2.8.2. Immunization in Canines
2.9. Titration of RABV-Specific VNA
2.10. ELISA for Determining RABV Specific Antibodies
2.11. Detection of IFN-γ and IL-4
2.12. Statistical Examination
2.13. Ethical Statement
3. Results
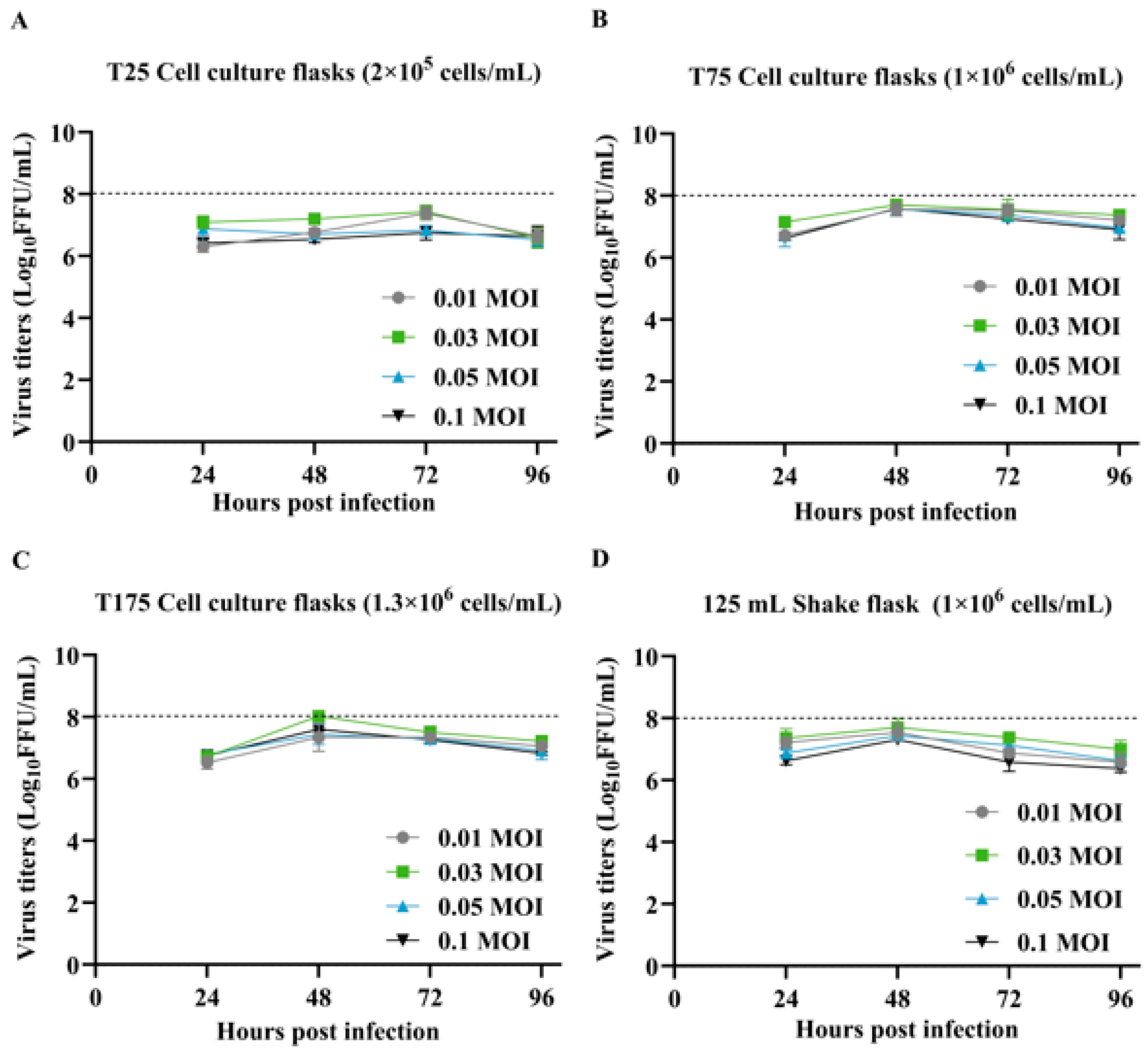
3.1. Optimization of Cell Density, MOI, and Infection Duration for SFV-RVG Amplification
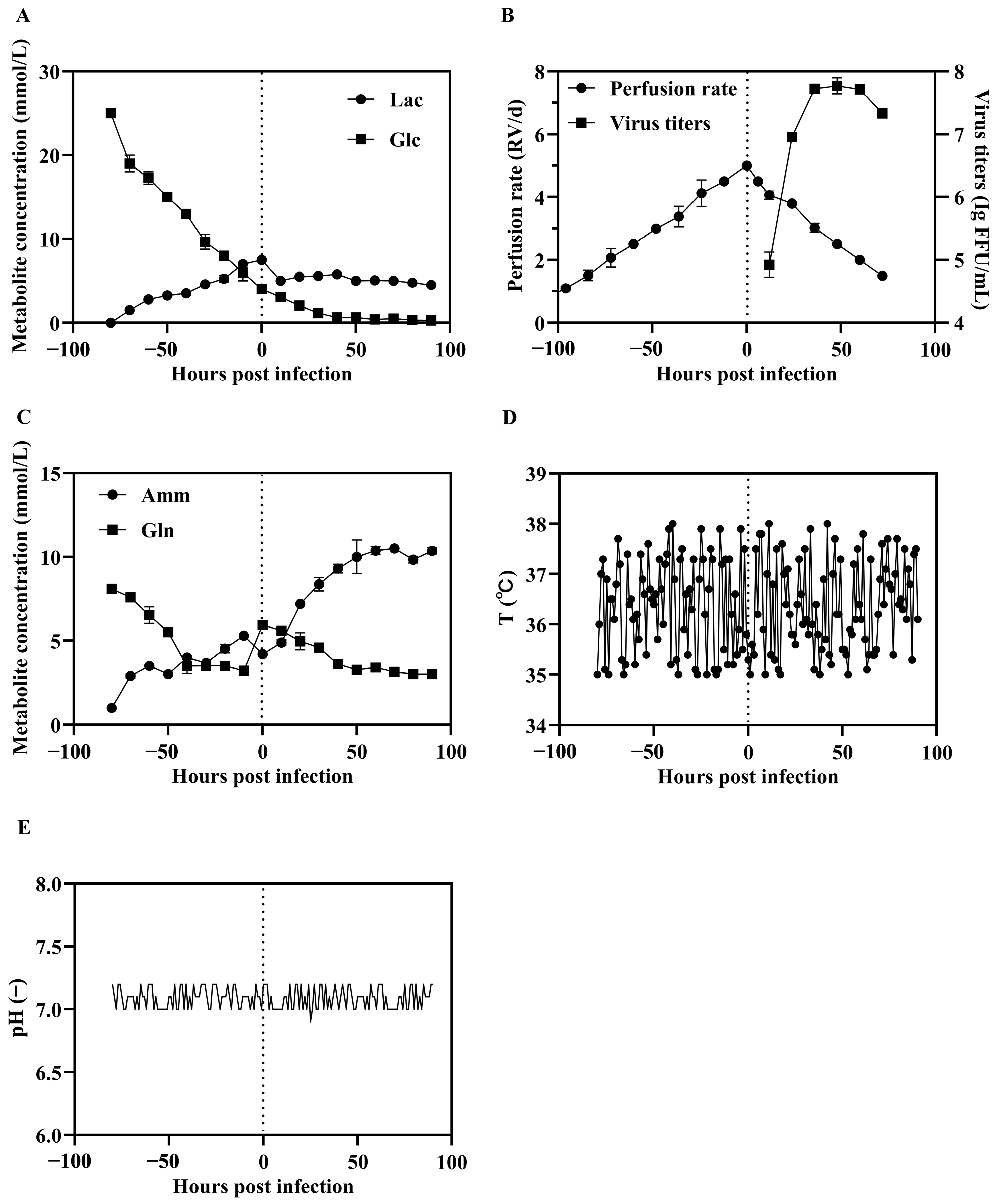
3.2. Large Scale Production of SFV-RVG in a Perfusion Bioreactor System
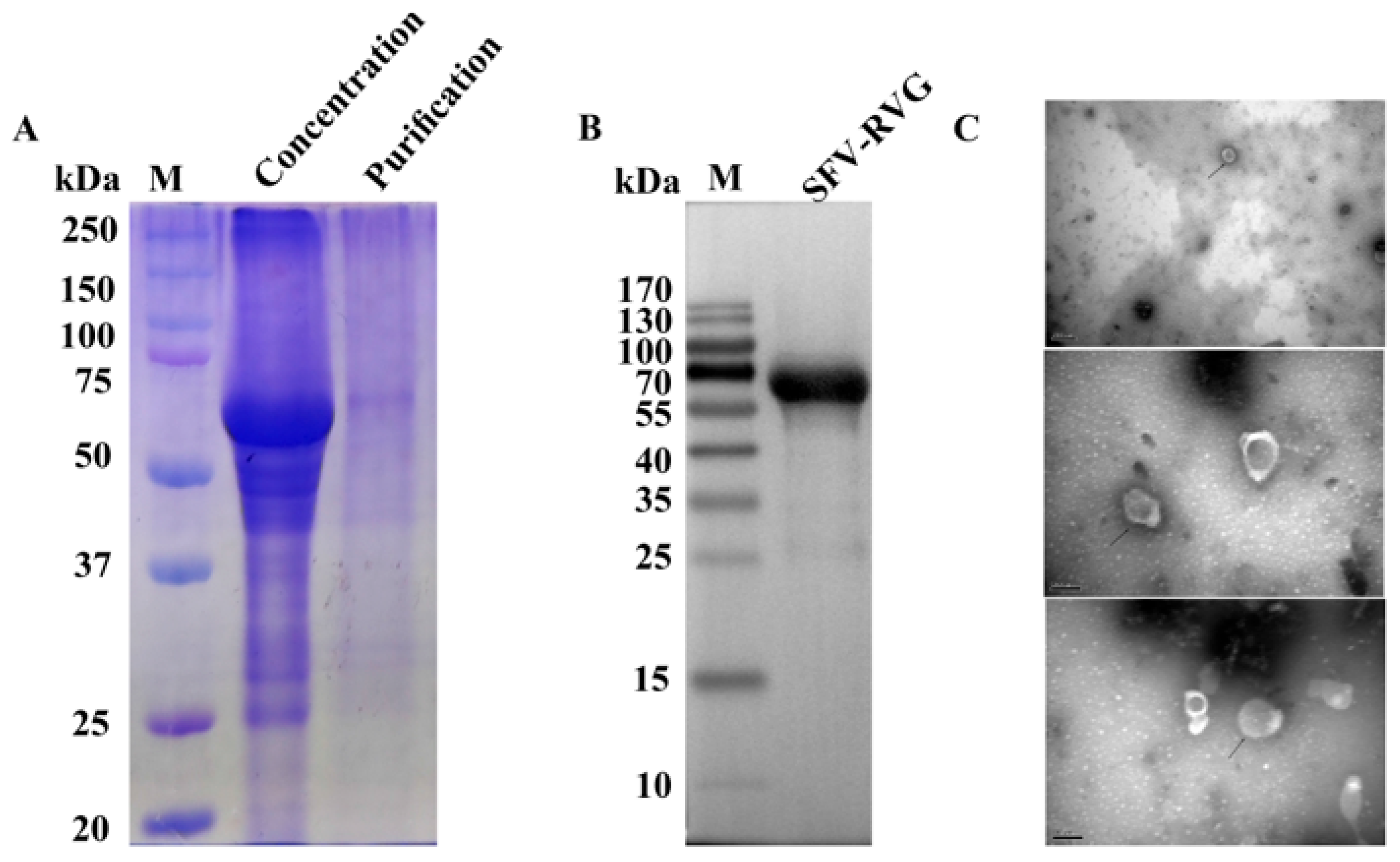
3.3. Purification and Verification of the Expression of G Protein by SFV-RVG
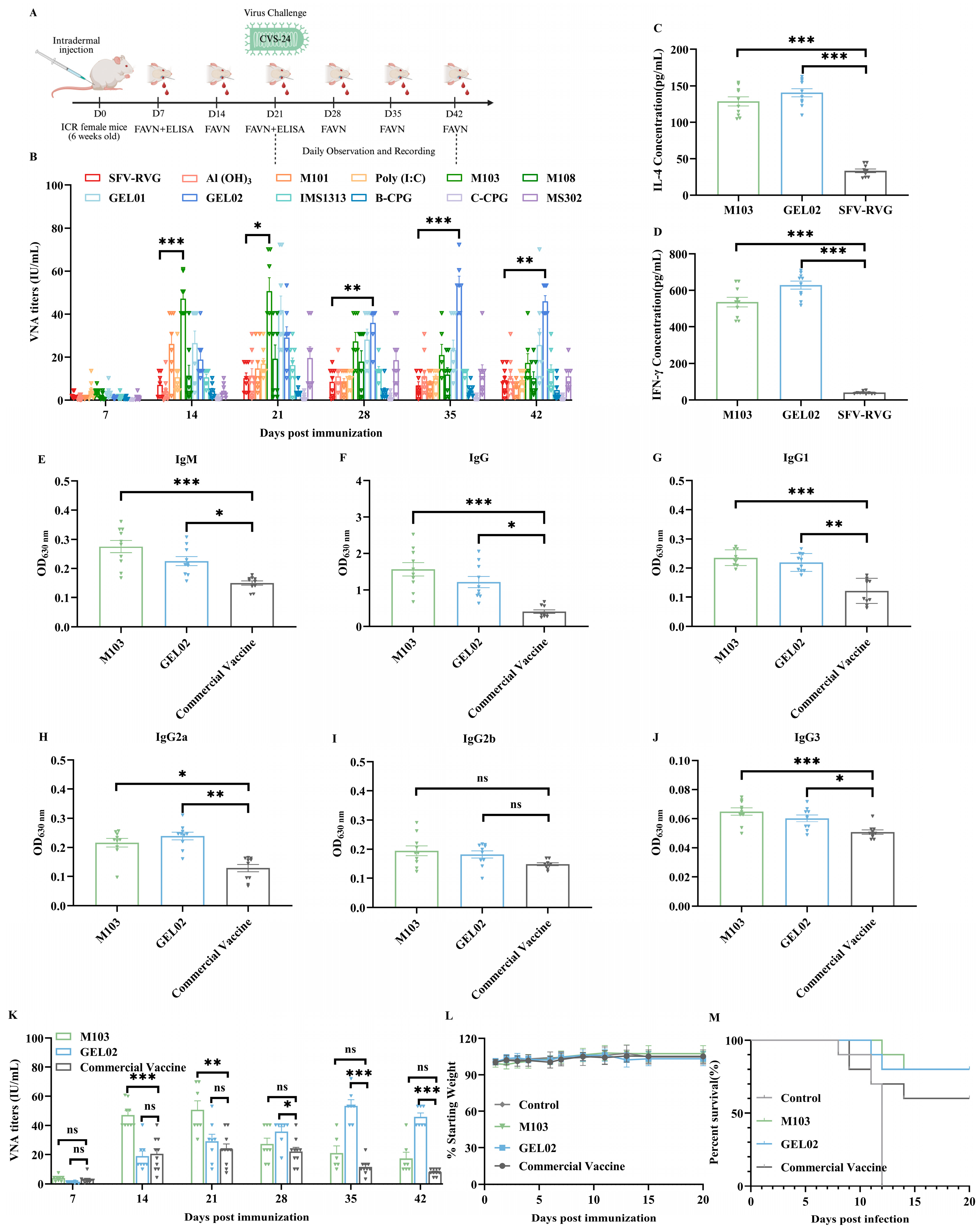
3.4. Adjuvant Selection and Immunogenicity Evaluation of Adjuvanted SFV-RVG Vaccine in Mice
3.5. Immunogenicity Evaluation of Adjuvanted SFV-RVG Vaccines in Dogs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RABV | rabies virus |
| MOI | multiplicity of infection |
| VNAs | virus-neutralizing antibodies |
| VSV | vesicular stomatitis virus |
| TOI | time of infection |
| CCI | cell concentration at infection |
| MAb | monoclonal antibody |
| IFA | immunofluorescence assays |
| WB | Western blot |
| TFF | tangential flow filtration |
| DO | dissolved oxygen |
| Lac | lactate |
| Glc | glucose |
| Amm | ammonium |
| Gln | glutamine |
References
- Fisher, C.R.; Streicker, D.G.; Schnell, M.J. The spread and evolution of rabies virus: Conquering new frontiers. Nat. Rev. Microbiol. 2018, 16, 241–255. [Google Scholar] [CrossRef]
- Fujisawa, M.; Onodera, T.; Kuroda, D.; Kewcharoenwong, C.; Sasaki, M.; Itakura, Y.; Yumoto, K.; Nithichanon, A.; Ito, N.; Takeoka, S.; et al. Molecular convergence of neutralizing antibodies in human revealed by repeated rabies vaccination. NPJ Vaccines 2025, 10, 39. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, Y.; Chen, C.; Wang, Z.; Pei, J.; Lin, C.; Zhou, M.; Fu, Z.F.; Zhao, L. Virus-Like Vesicles Based on Semliki Forest Virus-Containing Rabies Virus Glycoprotein Make a Safe and Efficacious Rabies Vaccine Candidate in a Mouse Model. J. Virol. 2021, 95, e0079021. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Dahiya, S.S.; Sonwane, A.A.; Patel, C.L.; Saini, M.; Rai, A.; Gupta, P.K. A sindbis virus replicon-based DNA vaccine encoding the rabies virus glycoprotein elicits immune responses and complete protection in mice from lethal challenge. Vaccine 2008, 26, 6592–6601. [Google Scholar] [CrossRef]
- Shi, C.; Tian, L.; Zheng, W.; Zhu, Y.; Sun, P.; Liu, L.; Liu, W.; Song, Y.; Xia, X.; Xue, X.; et al. Recombinant adeno-associated virus serotype 9 AAV-RABVG expressing a Rabies Virus G protein confers long-lasting immune responses in mice and non-human primates. Emerg. Microbes Infect. 2022, 11, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Espino, A.M.; Armina-Rodriguez, A.; Alvarez, L.; Ocasio-Malavé, C.; Ramos-Nieves, R.; Rodriguez Martinó, E.I.; López-Marte, P.; Torres, E.A.; Sariol, C.A. The Anti-SARS-CoV-2 IgG1 and IgG3 Antibody Isotypes with Limited Neutralizing Capacity against Omicron Elicited in a Latin Population a Switch toward IgG4 after Multiple Doses with the mRNA Pfizer-BioNTech Vaccine. Viruses 2024, 16, 187. [Google Scholar] [CrossRef]
- Longhi, M.P.; Trumpfheller, C.; Idoyaga, J.; Caskey, M.; Matos, I.; Kluger, C.; Salazar, A.M.; Colonna, M.; Steinman, R.M. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 2009, 206, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Tabynov, K.; Sansyzbay, A.; Tulemissova, Z.; Tabynov, K.; Dhakal, S.; Samoltyrova, A.; Renukaradhya, G.J.; Mambetaliyev, M. Inactivated porcine reproductive and respiratory syndrome virus vaccine adjuvanted with Montanide™ Gel 01 ST elicits virus-specific cross-protective inter-genotypic response in piglets. Vet. Microbiol. 2016, 192, 81–89. [Google Scholar] [CrossRef]
- Wang, B.; Gao, F.; Hu, R.; Huyan, H.; Wang, G.; Cao, Z.; Zhao, Y.; Lu, H.; Song, D.; Gao, F.; et al. Development and evaluation of inactivated vaccines incorporating a novel Senecavirus A strain-based Immunogen and various adjuvants in mice. Front. Vet. Sci. 2024, 11, 1376678. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Li, B.; Sun, X.; Pan, Q.; Zheng, Y.; Liu, J.; Zhao, Y.; Wang, J.; Liu, L.; et al. A novel CpG ODN compound adjuvant enhances immune response to spike subunit vaccines of porcine epidemic diarrhea virus. Front. Immunol. 2024, 15, 1336239. [Google Scholar] [CrossRef]
- Silva, C.A.T.; Kamen, A.A.; Henry, O. Intensified Influenza Virus Production in Suspension HEK293SF Cell Cultures Operated in Fed-Batch or Perfusion with Continuous Harvest. Vaccines 2023, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, S.; Kim, G.N.; Shen, C.F.; Kang, C.Y.; Kamen, A.A. Bioreactor production of rVSV-based vectors in Vero cell suspension cultures. Biotechnol. Bioeng. 2021, 118, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bissinger, T.; Genzel, Y.; Liu, X.; Reichl, U.; Tan, W.S. High cell density perfusion process for high yield of influenza A virus production using MDCK suspension cells. Appl. Microbiol. Biotechnol. 2021, 105, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Chen, S.; Zhang, M.; Cheng, Z.; Zhang, W.; Liu, D.; Shan, Y.; Du, G.; Li, W.; et al. Evaluation of immune effect to recombinant potential protective antigens of Mycoplasma ovipneumoniae in mice. Microb. Pathog. 2025, 204, 107555. [Google Scholar] [CrossRef]
- Zhao, H.; Li, P.; Bian, L.; Zhang, W.; Jiang, C.; Chen, Y.; Kong, W.; Zhang, Y. Immune Response of Inactivated Rabies Vaccine Inoculated via Intraperitoneal, Intramuscular, Subcutaneous and Needle-Free Injection Technology-Based Intradermal Routes in Mice. Int. J. Mol. Sci. 2023, 24, 13587. [Google Scholar] [CrossRef]
- Cliquet, F.; Aubert, M.; Sagné, L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J. Immunol. Methods 1998, 212, 79–87. [Google Scholar] [CrossRef]
- Vicente, T.; Roldão, A.; Peixoto, C.; Carrondo, M.J.; Alves, P.M. Large-scale production and purification of VLP-based vaccines. J. Invertebr. Pathol. 2011, 107, S42–S48. [Google Scholar] [CrossRef]
- Grigoryan, L.; Lee, A.; Walls, A.C.; Lai, L.; Franco, B.; Arunachalam, P.S.; Feng, Y.; Luo, W.; Vanderheiden, A.; Floyd, K.; et al. Adjuvanting a subunit SARS-CoV-2 vaccine with clinically relevant adjuvants induces durable protection in mice. NPJ Vaccines 2022, 7, 55. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Fang, R.; Li, X.; Xing, J.; Li, Z.; Song, N. Enhancing TB Vaccine Efficacy: Current Progress on Vaccines, Adjuvants and Immunization Strategies. Vaccines 2023, 12, 38. [Google Scholar] [CrossRef]
- Bryant, C.E.; Monie, T.P. Mice, men and the relatives: Cross-species studies underpin innate immunity. Open Biol. 2012, 2, 120015. [Google Scholar] [CrossRef]
- Shakya, A.K.; Nandakumar, K.S. Applications of polymeric adjuvants in studying autoimmune responses and vaccination against infectious diseases. J. R. Soc. Interface 2013, 10, 20120536. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.; Rayat, A. Lentiviral Vector Bioprocessing. Viruses 2021, 13, 268. [Google Scholar] [CrossRef]
- Kaplonek, P.; Deng, Y.; Shih-Lu Lee, J.; Zar, H.J.; Zavadska, D.; Johnson, M.; Lauffenburger, D.A.; Goldblatt, D.; Alter, G. Hybrid immunity expands the functional humoral footprint of both mRNA and vector-based SARS-CoV-2 vaccines. Cell Rep. Med. 2023, 4, 101048. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, T.; Liang, B.; Deng, H.; Wang, H.; Feng, X.; Quan, X.; Wang, X.; Li, S.; Lu, S.; et al. Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting. Front. Immunol. 2021, 12, 802858. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, X.; Wu, Y.; Fu, Z.; Zhao, L.; Zhou, M. Production Optimization, Adjuvant Screening and Immunogenicity Evaluation of a Virus-like Vesicle Rabies Vaccine. Vaccines 2025, 13, 1122. https://doi.org/10.3390/vaccines13111122
Zhang X, Liu X, Wu Y, Fu Z, Zhao L, Zhou M. Production Optimization, Adjuvant Screening and Immunogenicity Evaluation of a Virus-like Vesicle Rabies Vaccine. Vaccines. 2025; 13(11):1122. https://doi.org/10.3390/vaccines13111122
Chicago/Turabian StyleZhang, Xiaoyu, Xin Liu, Ying Wu, Zhenfang Fu, Ling Zhao, and Ming Zhou. 2025. "Production Optimization, Adjuvant Screening and Immunogenicity Evaluation of a Virus-like Vesicle Rabies Vaccine" Vaccines 13, no. 11: 1122. https://doi.org/10.3390/vaccines13111122
APA StyleZhang, X., Liu, X., Wu, Y., Fu, Z., Zhao, L., & Zhou, M. (2025). Production Optimization, Adjuvant Screening and Immunogenicity Evaluation of a Virus-like Vesicle Rabies Vaccine. Vaccines, 13(11), 1122. https://doi.org/10.3390/vaccines13111122





