T-Cell-Based Universal Dengue Vaccine Design for Robust Protective Response
Abstract
1. Introduction
2. Dengue Vaccine Development
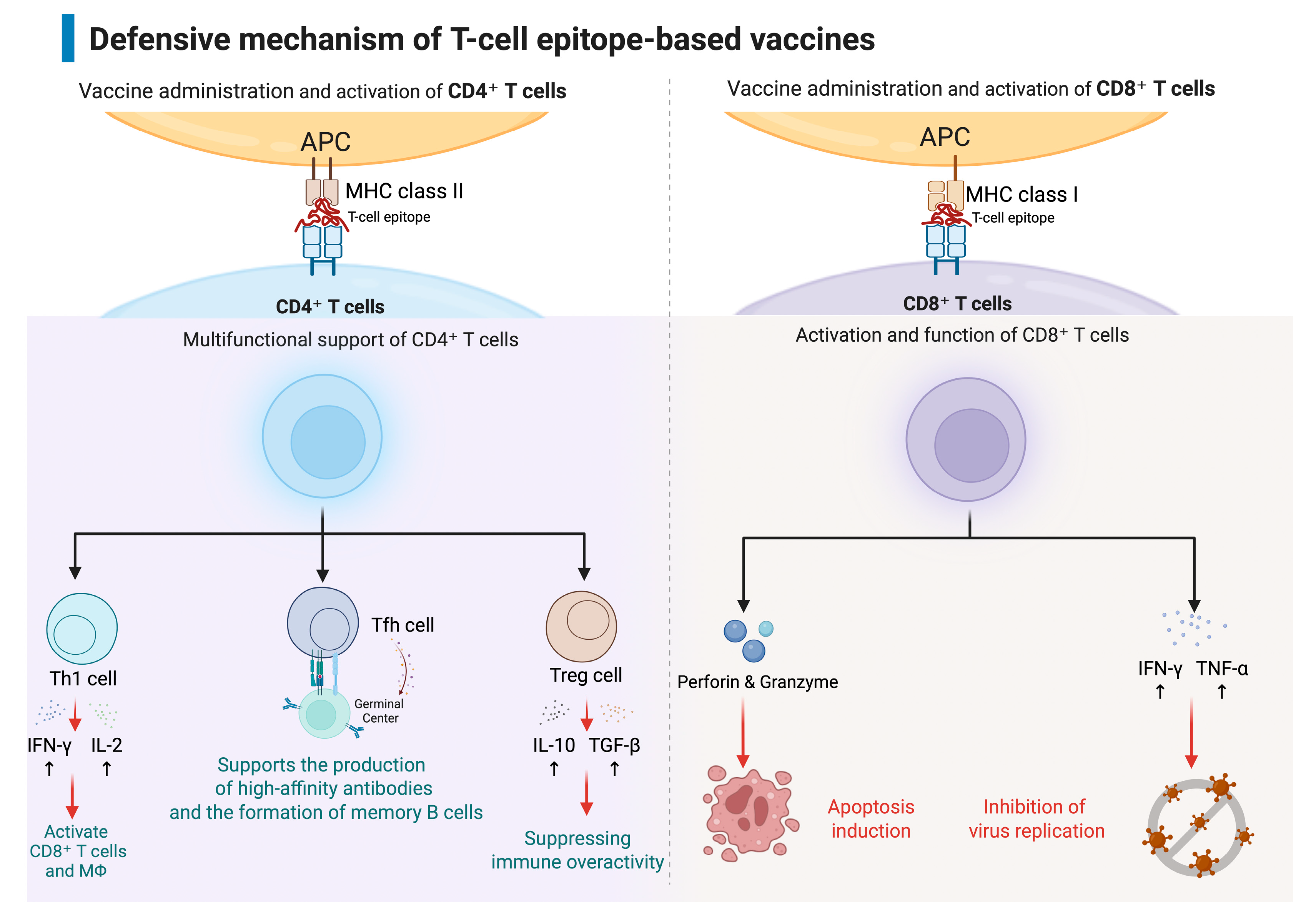
3. T Cell Responses and Protection in Dengue Virus Infection
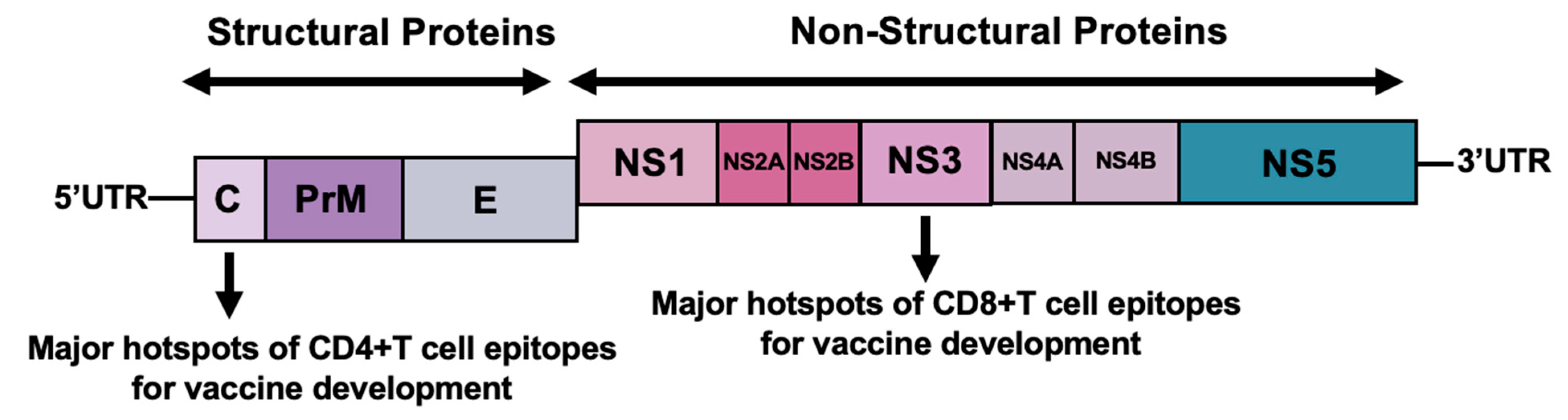
4. Development of T-Cell Epitope-Based Vaccines for Dengue
5. Challenges of Dengue Vaccine Development
6. Vaccine Design and Methods
7. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DENV | Dengue Virus |
| ZIKV | Zika virus |
| JEV | Japanese encephalitis virus |
| ADE | Antibody-dependent enhancement |
| DF | Dengue Fever |
| DHF | Dengue Hemorrhagic fever |
| DSS | Dengue Shock Syndrome |
| FcγR | Fcγ receptor |
| C | Capsid |
| PrM | Pre-membrane |
| E | Envelop |
| NS | Non-structural |
| mRNA-LNP | mRNA-Lipid Nanoparticle |
| HLA | Human Leukocyte Antigen |
| TCRs | T cell Receptors |
| BCR | B cell Receptor |
| IFN-γ | Interferon-gamma |
| TNF-α | Tumor Necrosis Factor-alpha |
| TGF-β | Transforming Growth Factor-beta |
| IL-10 | Interleukin-10 |
| Tfh | T Follicular Helper |
| PRNT | The Plaque Reduction Neutralization Test |
| DDS | Drug Delivery Systems |
References
- Wilder-Smith, A.; Ooi, E.E.; Horstick, O.; Wills, B. Dengue. Lancet 2019, 393, 350–363. [Google Scholar] [CrossRef]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue Infection. Nat. Rev. Dis. Prim. 2016, 2, 16055. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Mandaric, S.; Friberg, H.; Saez-Llorens, X.; Borja-Tabora, C.; Biswal, S.; Escudero, I.; Faccin, A.; Gottardo, R.; Brose, M.; Roubinis, N.; et al. Long term T cell response and safety of a tetravalent dengue vaccine in healthy children. Npj Vaccines 2024, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: A systematic analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.L.; Ennis, F.A. Immunopathogenesis of dengue hemorrhagic fever. Virology 1999, 257, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef]
- Sirohi, D.; Chen, Z.; Sun, L.; Klose, T.; Pierson, T.C.; Rossmann, M.G.; Kuhn, R.J. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 2016, 352, 467–470. [Google Scholar] [CrossRef]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar] [CrossRef]
- Valiant, W.G.; Mattapallil, M.J.; Higgs, S.; Huang, Y.S.; Vanlandingham, D.L.; Lewis, M.G.; Mattapallil, J.J. Simultaneous Coinfection of Macaques with Zika and Dengue Viruses Does not Enhance Acute Plasma Viremia but Leads to Activation of Monocyte Subsets and Biphasic Release of Pro-inflammatory Cytokines. Sci. Rep. 2019, 9, 7877. [Google Scholar] [CrossRef]
- Valiant, W.G.; Lalani, T.; Yun, H.C.; Kunz, A.; Burgess, T.H.; Mattapallil, J.J. Human Serum with High Neutralizing Antibody Titers Against Both Zika and Dengue Virus Shows Delayed In Vitro Antibody-Dependent Enhancement of Dengue Virus Infection. Open Forum Infect. Dis. 2018, 5, ofy151. [Google Scholar] [CrossRef]
- Valiant, W.G.; Huang, Y.S.; Vanlandingham, D.L.; Higgs, S.; Lewis, M.G.; Mattapallil, J.J. Zika convalescent macaques display delayed induction of anamnestic cross-neutralizing antibody responses after dengue infection. Emerg. Microbes Infect. 2018, 7, 130. [Google Scholar] [CrossRef]
- Fowler, A.M.; Tang, W.W.; Young, M.P.; Mamidi, A.; Viramontes, K.M.; McCauley, M.D.; Carlin, A.F.; Schooley, R.T.; Swanstrom, J.; Baric, R.S.; et al. Maternally Acquired Zika Antibodies Enhance Dengue Disease Severity in Mice. Cell Host Microbe 2018, 24, 743–750.e5. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef]
- Pantoja, P.; Pérez-Guzmán, E.X.; Rodríguez, I.V.; White, L.J.; González, O.; Serrano, C.; Giavedoni, L.; Hodara, V.; Cruz, L.; Arana, T.; et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat. Commun. 2017, 8, 15674. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, J.V.; Hasund, C.M.; Aogo, R.A.; Bos, S.; Arguello, S.; Gonzalez, K.; Collado, D.; Miranda, T.; Kuan, G.; Gordon, A.; et al. Primary exposure to Zika virus is linked with increased risk of symptomatic dengue virus infection with serotypes 2, 3, and 4, but not 1. Sci. Transl. Med. 2024, 16, eadn2199. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.J.; Tighe, M.P.; Clark, M.J.; Gromowski, G.D.; Lanthier, P.A.; Travis, K.L.; Bernacki, D.T.; Cookenham, T.S.; Lanzer, K.G.; Szaba, F.M.; et al. Impact of prior dengue virus infection on Zika virus infection during pregnancy in marmosets. Sci. Transl. Med. 2023, 15, eabq6517. [Google Scholar] [CrossRef] [PubMed]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.; Ismail, H.I.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- Guy, B.; Saville, M.; Lang, J. Development of Sanofi Pasteur tetravalent dengue vaccine. Hum. Vaccines 2010, 6, 696–705. [Google Scholar] [CrossRef]
- Guy, B.; Barrere, B.; Malinowski, C.; Saville, M.; Teyssou, R.; Lang, J. From research to phase III: Preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 2011, 29, 7229–7241. [Google Scholar] [CrossRef]
- Durbin, A.P. Historical discourse on the development of the live attenuated tetravalent dengue vaccine candidate TV003/TV005. Curr. Opin. Virol. 2020, 43, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.S. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD. Expert Rev. Vaccines 2016, 15, 509–517. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Whitehead, S.S.; Pierce, K.K.; Tibery, C.M.; Grier, P.L.; Hynes, N.A.; Larsson, C.J.; Sabundayo, B.P.; Talaat, K.R.; Janiak, A.; et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model. Sci. Transl. Med. 2016, 8, 330. [Google Scholar] [CrossRef]
- Biswal, S.; Reynales, H.; Saez-Llorens, X.; Lopez, P.; Borja-Tabora, C.; Kosalaraksa, P.; Sirivichayakul, C.; Watanaveeradej, V.; Rivera, L.; Espinoza, F.; et al. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N. Engl. J. Med. 2019, 381, 2009–2019. [Google Scholar] [CrossRef]
- Rivera, L.; Biswal, S.; Sáez-Llorens, X.; Reynales, H.; López-Medina, E.; Borja-Tabora, C.; Bravo, L.; Sirivichayakul, C.; Kosalaraksa, P.; Vargas, L.M.; et al. Three-year efficacy and safety of Takeda’s dengue vaccine candidate (TAK-003). Clin. Infect. Dis. 2022, 75, 107–117. [Google Scholar] [CrossRef]
- Biswal, S.; Borja-Tabora, C.; Vargas, L.M.; Velásquez, H.; Alera, M.T.; Sierra, V.; Yu, D.; Moreira, E.D.; Fernando, A.D.; Gunasekera, D.; et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: A randomised, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1423–1433. [Google Scholar] [CrossRef]
- López-Medina, E.; Biswal, S.; Saez-Llorens, X.; Borja-Tabora, C.; Bravo, L.; Sirivichayakul, C.; Vargas, L.M.; Alera, M.T.; Velásquez, H.; Reynales, H.; et al. Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents 2 years after vaccination. J. Infect. Dis. 2022, 225, 1521–1532. [Google Scholar] [CrossRef]
- Sáez-Llorens, X.; DeAntonio, R.; Low, J.G.H.; Kosalaraksa, P.; Dean, H.; Sharma, M.; Tricou, V.; Biswal, S. TAK-003: Development of a tetravalent dengue vaccine. Expert Rev. Vaccines 2025, 24, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cunningham, D.; Wasserman, S.S.; Perry, J.; Putnak, J.R.; Eckels, K.H.; Vaughn, D.W.; Thomas, S.J.; Kanesa-Thasan, N.; Innis, B.L.; et al. Phase 2 clinical trial of three formulations of tetravalent live-attenuated dengue vaccine in flavivirus-naïve adults. Hum. Vaccine 2009, 5, 33–40. [Google Scholar] [CrossRef]
- Thomas, S.J.; Eckels, K.H.; Carletti, I.; De La Barrera, R.; Dessy, F.; Fernandez, S.; Putnak, R.; Toussaint, J.-F.; Sun, W.; Bauer, K.; et al. A phase II, randomized, safety and immunogenicity study of a re-derived, live-attenuated dengue virus vaccine in healthy adults. Am. J. Trop. Med. Hyg. 2013, 88, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.-A.; Clements, D.E.; Bett, A.J.; Sagar, S.L.; Ter Meulen, J.H. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine 2011, 29, 7267–7275. [Google Scholar] [CrossRef] [PubMed]
- Manoff, S.B.; Sausser, M.; Falk Russell, A.; Martin, J.; Radley, D.; Hyatt, D.; Roberts, C.C.; Lickliter, J.; Krishnarajah, J.; Bett, A.; et al. Immunogenicity and safety of an investigational tetravalent recombinant subunit vaccine for dengue: Results of a Phase I randomized clinical trial in flavivirus-naïve adults. Hum. Vaccines Immunother. 2019, 15, 2195–2204. [Google Scholar] [CrossRef]
- Raviprakash, K.; Luke, T.; Doukas, J.; Danko, J.; Porter, K.; Burgess, T.; Kochel, T. A dengue DNA vaccine formulated with Vaxfectin is well tolerated and elicits strong neutralizing antibody responses to all four dengue serotypes in New Zealand white rabbits. Hum. Vaccines Immunother. 2012, 8, 1764–1768. [Google Scholar] [CrossRef]
- Danko, J.R.; Kochel, T.; Teneza-Mora, N.; Luke, T.C.; Raviprakash, K.; Sun, P.; Simmons, M.; Moon, J.E.; De La Barrera, R.; Martinez, L.J.; et al. Safety and immunogenicity of a tetravalent Dengue DNA vaccine administered with a cationic lipid-based adjuvant in a phase 1 clinical trial. Am. J. Trop. Med. Hyg. 2018, 98, 849–856. [Google Scholar] [CrossRef]
- Porter, K.R.; Ewing, D.; Chen, L.; Wu, S.-J.; Hayes, C.G.; Ferrari, M.; Teneza-Mora, N.; Raviprakash, K. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine 2012, 30, 336–341. [Google Scholar] [CrossRef]
- Ramasamy, V.; Arora, U.; Shukla, R.; Poddar, A.; Shanmugam, R.K.; White, L.J.; Mattocks, M.M.; Raut, R.; Perween, A.; Tyagi, P.; et al. A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice. PLoS Negl. Trop. Dis. 2018, 12, e0006191. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified mRNA-LNP vaccines confer protection against experimental DENV-2 infection in mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 702–712. [Google Scholar] [CrossRef]
- Waickman, A.T.; Friberg, H.; Gargulak, M.; Kong, A.; Polhemus, M.; Endy, T.; Thomas, S.J.; Jarman, R.G.; Currier, J.R. Assessing the Diversity and Stability of Cellular Immunity Generated in Response to the Candidate Live-Attenuated Dengue Virus Vaccine TAK-003. Front. Immunol. 2019, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. CYD-TDV Dengue Vaccine Working Group. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. CYD15 Study Group. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Shastri, N.; Schwab, S.; Serwold, T. Producing nature’s gene-chips: The generation of peptides for display by MHC class I molecules. Ann. Rev. Immunol. 2002, 20, 463–493. [Google Scholar] [CrossRef]
- Janeway, C.; Travers, P.; Walport, M.; Schlomchik, M. Immunobiology the Immune System in Health and Disease, 5th ed.; Garland Publishing: New York, NY, USA, 2001; Chapter 8 T-Cell-Mediated Immunity; pp. 295–340. [Google Scholar]
- Hatch, S.; Endy, T.P.; Thomas, S.; Mathew, A.; Potts, J.; Pazoles, P.; Libraty, D.H.; Gibbons, R.; Rothman, A.L. Intracellular cytokine production by dengue virus–specific T cells correlates with subclinical secondary infection. J. Infect. Dis. 2011, 203, 1282–1291. [Google Scholar] [CrossRef]
- de Matos, A.M.; Carvalho, K.I.; Rosa, D.S.; Villas-Boas, L.S.; da Silva, W.C.; Rodrigues, C.L.; Oliveira, O.M.; Levi, J.E.; Araújo, E.S.; Pannuti, C.S.; et al. CD8+ T lymphocyte expansion, proliferation and activation in dengue fever. PLoS Negl. Trop. Dis. 2015, 9, e0003520. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Babor, M.; Lane, J.; Seumois, G.; Liang, S.; Goonawardhana, N.D.S.; De Silva, A.D.; Phillips, E.J.; Mallal, S.A.; da Silva, A.R.; et al. Dengue-specific CD8+ T cell subsets display specialized transcriptomic and TCR profiles. J. Clin. Investig. 2019, 130, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Grifoni, A.; Sette, A.; Weiskopf, D. Human T Cell Response to Dengue Virus Infection. Front. Immunol. 2019, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for CD4. (+) T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef]
- Tian, Y.; Zajac, A.J. IL-21 and T cell differentiation: Consider the context. Trends Immunol. 2016, 37, 557–568. [Google Scholar] [CrossRef]
- Weiskopf, D.; Bangs, D.J.; Sidney, J.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; Crotty, S.; Peters, B.; Sette, A. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc. Natl. Acad. Sci. USA 2015, 112, E4256–E4263. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; Barton, J.P.; McKay, M.R. Cross-Serotypically Conserved Epitope Recommendations for a Universal T Cell-Based Dengue Vaccine. PLoS Negl. Trop. Dis. 2020, 14, e0008676. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Zellweger, R.M.; Kotturi, M.F.; Qutubuddin, A.; Sidney, J.; Peters, B.; Prestwood, T.R.; Sette, A.; Shresta, S. A Protective Role for Dengue Virus-Specific CD8+ T Cells. J. Immunol. 2009, 182, 4865–4873. [Google Scholar] [CrossRef]
- Olsen, L.R.; Zhang, G.L.; Keskin, D.B.; Reinherz, E.L.; Brusic, V. Conservation analysis of dengue virus T-cell epitope-based vaccine candidates using Peptide block entropy. Front. Immunol. 2011, 2, 69. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Fuaad, A.A.-H.A.; Azami, N.A.M.; Amin, M.C.I.M.; Azmi, F. Next-generation Dengue Vaccines: Leveraging Peptide-Based Immunogens and Advanced Nanoparticles as Delivery Platforms. J. Pharm. Sci. 2024, 113, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Shresta, S. Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development. Front. Immunol. 2019, 10, 1316. [Google Scholar] [CrossRef]
- Wen, J.; Shresta, S. T Cell Immunity to Zika and Dengue Viral Infections. J. Interferon Cytokine Res. 2017, 37, 475–479. [Google Scholar] [CrossRef]
- Paquin-Proulx, D.; Leal, F.E.; Terrassani Silveira, C.G.; Maestri, A.; Brockmeyer, C.; Kitchen, S.M.; Cabido, V.D.; Kallas, E.G.; Nixon, D.F. T-cell Responses in Individuals Infected with Zika Virus and in Those Vaccinated Against Dengue Virus. Pathog. Immun. 2017, 2, 274–292. [Google Scholar] [CrossRef]
- Herrera, B.B.; Tsai, W.Y.; Chang, C.A.; Hamel, D.J.; Wang, W.K.; Lu, Y.; Mboup, S.; Kanki, P.J. Sustained Specific and Cross-Reactive T Cell Responses to Zika and Dengue Virus NS3 in West Africa. J. Virol. 2018, 92, e01992-17. [Google Scholar] [CrossRef]
- Wen, J.; Elong Ngono, A.; Regla-Nava, J.A.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 2017, 8, 1459. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J. Virol. 2017, 91, e01469-17. [Google Scholar] [CrossRef]
- Hassert, M.; Harris, M.G.; Brien, J.D.; Pinto, A.K. Identification of Protective CD8 T Cell Responses in a Mouse Model of Zika Virus Infection. Front. Immunol. 2019, 10, 1678. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; St John, A.L. Cross-Reactive Immunity Among Flaviviruses. Front. Immunol. 2020, 11, 334. [Google Scholar] [CrossRef]
- Schouest, B.; Grifoni, A.; Pham, J.; Mateus, J.; Sydney, J.; Brien, J.D.; De Silva, A.D.; Balmaseda, A.; Harris, E.; Sette, A.; et al. Pre-existing T Cell Memory against Zika Virus. J. Virol. 2021, 95, e00132-21. [Google Scholar] [CrossRef]
- Subramaniam, K.S.; Lant, S.; Goodwin, L.; Grifoni, A.; Weiskopf, D.; Turtle, L. Two Is Better Than One: Evidence for T-Cell Cross-Protection Between Dengue and Zika and Implications on Vaccine Design. Front. Immunol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Wen, J.; Tang, W.W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat. Microbiol. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Lim, M.Q.; Kumaran, E.A.P.; Tan, H.C.; Lye, D.C.; Leo, Y.S.; Ooi, E.E.; MacAry, P.A.; Bertoletti, A.; Rivino, L. Cross-Reactivity and Anti-viral Function of Dengue Capsid and NS3-Specific Memory T Cells Toward Zika Virus. Front. Immunol. 2018, 9, 2225. [Google Scholar] [CrossRef]
- Rossjohn, J.; Gras, S.; Miles, J.J.; Turner, S.J.; Godfrey, D.I.; McCluskey, J. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 2015, 33, 169–200. [Google Scholar] [CrossRef]
- Yewdell, J.W.; Bennink, J.R. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1999, 17, 51–88. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- Farias, J.P.; Birbrair, A.; Amorim, J.H. Advancing dengue vaccination using a T-cell priming peptide approach. eBioMedicine 2024, 101, 105012. [Google Scholar] [CrossRef]
- Weiskopf, D.; Sette, A. T-cell immunity to infection with dengue virus in humans. Front. Immunol. 2014, 5, 93. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; de Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2050. [Google Scholar] [CrossRef]
- Nemirov, K.; Authié, P.; Souque, P.; Moncoq, F.; Noirat, A.; Blanc, C.; Bourgine, M.; Majlessi, L.; Charneau, P. Preclinical proof of concept of a tetravalent lentiviral T-cell vaccine against dengue viruses. Front. Immunol. 2023, 14, 1208041. [Google Scholar] [CrossRef]
- Kaushik, V.; G, S.K.; Gupta, L.R.; Kalra, U.; Shaikh, A.R.; Cavallo, L.; Chawla, M. Immunoinformatics Aided Design and In-Vivo Validation of a Cross-Reactive Peptide Based Multi-Epitope Vaccine Targeting Multiple Serotypes of Dengue Virus. Front. Immunol. 2022, 13, 865180. [Google Scholar] [CrossRef]
- Pinheiro-Michelsen, J.R.; Souza, R.d.S.O.; Santana, I.V.R.; da Silva, P.d.S.; Mendez, E.C.; Luiz, W.B.; Amorim, J.H. Anti-dengue Vaccines: From Development to Clinical Trials. Front. Immunol. 2020, 11, 1252. [Google Scholar] [CrossRef]
- Morgan, R.N.; Ismail, N.S.; Alshahrani, M.Y.; Aboshanab, K.M. Multi-epitope peptide vaccines targeting dengue virus serotype 2 created via immunoinformatic analysis. Sci. Rep. 2024, 14, 17645. [Google Scholar] [CrossRef] [PubMed]
- G, S.K.; Joshi, A.; Akhtar, N.; Kaushik, V. Immunoinformatics designed T cell multi epitope dengue peptide vaccine derived from non structural proteome. Microb. Pathog. 2021, 150, 104728. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Coloma, J.; Harris, E. Dengue: Knowledge gaps, unmet needs, and research priorities. Lancet Infect. Dis. 2017, 17, e88–e100. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengue vaccine development: A 75% solution? Lancet 2012, 380, 1535–1536. [Google Scholar] [CrossRef]
- Norshidah, H.; Vignesh, R.; Lai, N.S. Updates on Dengue Vaccine and Antiviral: Where Are We Heading? Molecules 2021, 26, 6768. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Rouvinski, A.; Guardado-Calvo, P.; Barba-Spaeth, G.; Duquerroy, S.; Vaney, M.C.; Kikuti, C.M.; Navarro Sanchez, M.E.; Dejnirattisai, W.; Wongwiwat, W.; Haouz, A.; et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015, 520, 109–113. [Google Scholar] [CrossRef]
- Rey, F.A.; Stiasny, K.; Heinz, F.X. Flavivirus structural heterogeneity: Implications for cell entry. Curr. Opin. Virol. 2017, 24, 132–139. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004, 427, 313–319. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Lindow, J.C.; Diehl, S.A.; Whitehead, S.; et al. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J. Virol. 2015, 89, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Adikari, T.N.; Di Giallonardo, F.; Leung, P.; Grifoni, A.; Sette, A.; Weiskopf, D.; Bull, R.A.; Luciani, F. Conserved epitopes with high HLA-I population coverage are targets of CD8+ T cells associated with high IFN-γ responses against all dengue virus serotypes. Sci. Rep. 2020, 10, 20497. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Ye, W.; Chen, J. Current Development and Challenges of Tetravalent Live-Attenuated Dengue Vaccines. Front. Immunol. 2022, 13, 840104. [Google Scholar] [CrossRef]
- Shi, J.; Sun, J.; Wu, M.; Hu, N.; Li, J.; Li, Y.; Wang, H.; Hu, Y. Inferring Protective CD8+ T-Cell Epitopes for NS5 Protein of Four Serotypes of Dengue Virus Chinese Isolates Based on HLA-A, -B and -C Allelic Distribution: Implications for Epitope-Based Universal Vaccine Design. PLoS ONE 2015, 10, e0138729. [Google Scholar] [CrossRef][Green Version]
- Rivino, L.; Kumaran, E.A.; Jovanovic, V.; Nadua, K.; Teo, E.W.; Pang, S.W.; Teo, G.H.; Gan, V.C.; Lye, D.C.; Leo, Y.S.; et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J. Virol. 2013, 87, 2693–2706. [Google Scholar] [CrossRef]
- Simmons, C.P.; Dong, T.; Chau, N.V.; Dung, N.T.; Chau, T.N.; Thao Le, T.T.; Dung, N.T.; Hien, T.T.; Rowland-Jones, S.; Farrar, J. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J. Virol. 2005, 79, 5665–5675. [Google Scholar] [CrossRef]
- Duan, Z.L.; Li, Q.; Wang, Z.B.; Xia, K.D.; Guo, J.L.; Liu, W.Q.; Wen, J.S. HLA-A*0201-restricted CD8+ T-cell epitopes identified in dengue viruses. Virol. J. 2012, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Cerpas, C.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; Sanches, F.P.; Silvera, C.G.; Costa, P.R.; et al. Human CD8+ T-Cell Responses Against the 4 Dengue Virus Serotypes Are Associated with Distinct Patterns of Protein Targets. J. Infect. Dis. 2015, 212, 1743–1751. [Google Scholar] [CrossRef]
- Nascimento, E.J.; Mailliard, R.B.; Khan, A.M.; Sidney, J.; Sette, A.; Guzman, N.; Paulaitis, M.; de Melo, A.B.; Cordeiro, M.T.; Gil, L.V.; et al. Identification of conserved and HLA promiscuous DENV3 T-cell epitopes. PLoS Negl. Trop. Dis. 2013, 7, e2497. [Google Scholar] [CrossRef] [PubMed]
- Rivino, L.; Kumaran, E.A.; Thein, T.-L.; Too, C.T.; Gan, V.C.H.; Hanson, B.J.; Wilder-Smith, A.; Bertoletti, A.; Gascoigne, N.R.J.; Lye, D.C.; et al. Virus-specific T lymphocytes home to the skin during natural dengue infection. Sci. Transl. Med. 2015, 7, 278ra35. [Google Scholar] [CrossRef]
- Friberg, H.; Bashyam, H.; Toyosaki-Maeda, T.; Potts, J.A.; Greenough, T.; Kalayanarooj, S.; Gibbons, R.V.; Nisalak, A.; Srikiatkhachorn, A.; Green, S.; et al. Cross-reactivity and expansion of dengue-specific T cells during acute primary and secondary infections in humans. Sci. Rep. 2011, 1, 51. [Google Scholar] [CrossRef] [PubMed]
- Livingston, P.G.; Kurane, I.; Lai, C.J.; Bray, M.; Ennis, F.A. Recognition of envelope protein by dengue virus serotype-specific human CD4+ CD8- cytotoxic T-cell clones. J. Virol. 1994, 68, 3283–3288. [Google Scholar] [CrossRef]
- Weiskopf, D.; Yauch, L.E.; Angelo, M.A.; John, D.V.; Greenbaum, J.A.; Sidney, J.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; Grey, H.; et al. Insights into HLA-restricted T cell responses in a novel mouse model of dengue virus infection point toward new implications for vaccine design. J. Immunol. 2011, 187, 4268–4279. [Google Scholar] [CrossRef]
- Li, S.; Peng, L.; Zhao, W.; Zhong, H.; Zhang, F.; Yan, Z.; Cao, H. Synthetic peptides containing B- and T-cell epitope of dengue virus-2 E domain III provoked B- and T-cell responses. Vaccine 2011, 29, 3695–3702. [Google Scholar] [CrossRef]
- Hassert, M.; Brien, J.D.; Pinto, A.K. Mouse Models of Heterologous Flavivirus Immunity: A Role for Cross-Reactive T Cells. Front. Immunol. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Duan, Z.L.; Liu, H.F.; Huang, X.; Wang, S.N.; Yang, J.L.; Chen, X.Y.; Li, D.Z.; Zhong, X.Z.; Chen, B.K.; Wen, J.S. Identification of conserved and HLA-A*2402-restricted epitopes in Dengue virus serotype 2. Virus Res. 2015, 196, 5–12. [Google Scholar] [CrossRef]
- de Alwis, R.; Bangs, D.J.; Angelo, M.A.; Cerpas, C.; Fernando, A.; Sidney, J.; Peters, B.; Gresh, L.; Balmaseda, A.; de Silva, A.D. Immunodominant dengue virus-specific CD8+ T cell responses are associated with a memory PD-1+ phenotype. J. Virol. 2016, 90, 4771–4779. [Google Scholar] [CrossRef] [PubMed]
- Turtle, L.; Tatullo, F.; Bali, T.; Ravi, V.; Soni, M.; Chan, S.; Chib, S.; Venkataswamy, M.M.; Fadnis, P.; Yaïch, M.; et al. Cellular Immune Responses to Live Attenuated Japanese Encephalitis (JE) Vaccine SA14-14-2 in Adults in a JE/Dengue Co-Endemic Area. PLoS Negl. Trop. Dis. 2017, 11, e0005263. [Google Scholar] [CrossRef]
- Sierra, B.; García, G.; Pérez, A.B.; Morier, L.; Rodríguez, R.; Alvarez, M.; Guzmán, M.G. Long-term memory cellular immune response to dengue virus after a natural primary infection. Int. J. Infect. Dis. 2002, 6, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, Y.T.; Valentine, K.M.; Dos Santos Alves, R.P.; Xu, Z.; Regla-Nava, J.A.; Ngono, A.E.; Young, M.P.; Ferreira, L.C.S.; Shresta, S. CD4+ T Cells Cross-Reactive with Dengue and Zika Viruses Protect against Zika Virus Infection. Cell Rep. 2020, 31, 107566. [Google Scholar] [CrossRef]
- Turtle, L.; Bali, T.; Buxton, G.; Chib, S.; Chan, S.; Soni, M.; Hussain, M.; Isenman, H.; Fadnis, P.; Venkataswamy, M.M.; et al. Human T cell responses to Japanese encephalitis virus in health and disease. J. Exp. Med. 2016, 213, 1331–1352. [Google Scholar] [CrossRef]
- Kumar, P.; Sulochana, P.; Nirmala, G.; Haridattatreya, M.; Satchidanandam, V. Conserved amino acids 193-324 of non-structural protein 3 are a dominant source of peptide determinants for CD4+ and CD8+ T cells in a healthy Japanese encephalitis virus-endemic cohort. J. Gen. Virol. 2004, 85 Pt 5, 1131–1143. [Google Scholar] [CrossRef]
- Kumar, P.; Sulochana, P.; Nirmala, G.; Chandrashekar, R.; Haridattatreya, M.; Satchidanandam, V. Impaired T helper 1 function of nonstructural protein 3-specific T cells in Japanese patients with encephalitis with neurological sequelae. J. Infect. Dis. 2004, 189, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.P.D.S.; Pereira, L.R.; Fabris, D.L.N.; Salvador, F.S.; Santos, R.A.; Zanotto, P.M.A.; Romano, C.M.; Amorim, J.H.; Ferreira, L.C.S. Production of a Recombinant Dengue Virus 2 NS5 Protein and Potential Use as a Vaccine Antigen. Clin. Vaccine Immunol. 2016, 23, 460–469. [Google Scholar] [CrossRef]
- Roth, C.; Cantaert, T.; Colas, C.; Prot, M.; Casadémont, I.; Levillayer, L.; Thalmensi, J.; Langlade-Demoyen, P.; Gerke, C.; Bahl, K.; et al. A Modified mRNA Vaccine Targeting Immunodominant NS Epitopes Protects Against Dengue Virus Infection in HLA Class I Transgenic Mice. Front. Immunol. 2019, 10, 1424. [Google Scholar] [CrossRef]
- Tarbe, M.; Dong, W.; Hu, G.; Xu, Y.; Sun, J.; Grayo, S.; Chen, X.; Qin, C.; Zhao, J.; Liu, L.; et al. Japanese Encephalitis Virus Vaccination Elicits Cross-Reactive HLA-Class I-Restricted CD8 T Cell Response Against Zika Virus Infection. Front. Immunol. 2020, 11, 577546. [Google Scholar] [CrossRef]
- Verma, M.; Bhatnagar, S.; Kumari, K.; Mittal, N.; Sukhralia, S.; Gopirajan At, S.; Dhanaraj, P.S.; Lal, R. Highly conserved epitopes of DENV structural and non-structural proteins: Candidates for universal vaccine targets. Gene 2019, 695, 18–25. [Google Scholar] [CrossRef]
- Ma, E.; Cheng, G. Host immunity and vaccine development against Dengue virus. Infect. Med. 2022, 1, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.E.; Kalimuddin, S. Insights into dengue immunity from vaccine trials. Sci. Transl. Med. 2023, 15, eadh3067. [Google Scholar] [CrossRef] [PubMed]
- Wilken, L.; Rimmelzwaan, G.F. Adaptive Immunity to Dengue Virus: Slippery Slope or Solid Ground for Rational Vaccine Design? Pathogens 2020, 9, 470. [Google Scholar] [CrossRef]
- Goh, J.Z.H.; De Hayr, L.; Khromykh, A.A.; Slonchak, A. The Flavivirus Non-Structural Protein 5 (NS5): Structure, Functions, and Targeting for Development of Vaccines and Therapeutics. Vaccines 2024, 12, 865. [Google Scholar] [CrossRef]
- Dos Santos Franco, L.; Gushi, L.T.; Luiz, W.B.; Amorim, J.H. Seeking Flavivirus Cross-Protective Immunity. Front. Immunol. 2019, 10, 2260. [Google Scholar] [CrossRef]
- Pinheiro, J.R.; Camilo dos Reis, E.; Souza, R.d.S.O.; Rocha, A.L.S.; Suesdek, L.; Azevedo, V.; Tiwari, S.; Rocha, B.G.S.; Birbrair, A.; Méndez, E.C.; et al. Comparison of Neutralizing Dengue Virus B Cell Epitopes and Protective T Cell Epitopes with Those in Three Main Dengue Virus Vaccines. Front. Immunol. 2021, 12, 715136. [Google Scholar] [CrossRef]
- Lin, T.-H.; Chen, H.-W.; Hsiao, Y.-J.; Yan, J.-Y.; Chiang, C.-Y.; Chen, M.-Y.; Hu, H.-M.; Wu, S.-H.; Pan, C.-H. Immunodomination of Serotype-Specific CD4+ T-Cell Epitopes Contributed to the Biased Immune Responses Induced by a Tetravalent Measles-Vectored Dengue Vaccine. Front. Immunol. 2020, 1, 546. [Google Scholar] [CrossRef]
- Mitchison, N.A. T-Cell–B-Cell Cooperation. Nat. Rev. Immunol. 2004, 4, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- Jerne, N.K. Towards a Network Theory of the Immune System. Ann. Immunol. 1974, 125C, 373–389. [Google Scholar]
- Nakamura, Y.; Moi, M.L.; Shiina, T.; Shin, T.; Suzuki, R. Idiotope-Driven T-Cell/B-Cell Collaboration-Based T-Cell Epitope Prediction Using B-Cell Receptor Repertoire Sequences in Infectious Diseases. Viruses 2023, 15, 1186. [Google Scholar] [CrossRef]
- Liu, X. Opportunities and challenges of mRNA technologies in development of dengue virus vaccine. Front. Immunol. 2025, 16, 1520968. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Beesetti, H.; Brown, J.A.; Ahuja, R.; Ramasamy, V.; Shanmugam, R.K.; Poddar, A.; Batra, G.; Krammer, F.; Lim, J.K.; et al. Dengue and Zika Virus Infections are Enhanced by Live Attenuated Dengue Vaccine But Not by Recombinant DSV4 Vaccine Candidate in Mouse Models. eBioMedicine 2020, 60, 102991. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.R.; Wang, X.; Saron, W.A.A.; Gan, E.S.; Tan, H.C.; Mok, D.Z.L.; Zhang, S.L.-X.; Lee, Y.H.; Liang, C.; Wijaya, L.; et al. Cross-Reactive Antibodies Enhance Live Attenuated Virus Infection for Increased Immunogenicity. Nat. Microbiol. 2016, 1, 116164. [Google Scholar] [CrossRef] [PubMed]
- Montecillo-Aguado, M.R.; Montes-Gómez, A.E.; García-Cordero, J.; Corzo-Gómez, J.; Vivanco-Cid, H.; Mellado-Sánchez, G.; Muñoz-Medina, J.E.; Gutiérrez-Castañeda, B.; Santos-Argumedo, L.; González-Bonilla, C.; et al. Cross-Reaction, Enhancement, and Neutralization Activity of Dengue Virus Antibodies Against Zika Virus: A Study in the Mexican Population. J. Immunol. Res. 2019, 2019, 7239347. [Google Scholar] [CrossRef]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Thomas, S.J.; Nisalak, A.; Anderson, K.B.; Libraty, D.H.; Kalayanarooj, S.; Vaughn, D.W.; Putnak, R.; Gibbons, R.V.; Jarman, R.; Endy, T.P. Dengue Plaque Reduction Neutralization Test (PRNT) in Primary and Secondary Dengue Virus Infections: How Alterations in Assay Conditions Impact Performance. Am. J. Trop. Med. Hyg. 2009, 81, 825. [Google Scholar] [CrossRef]
- Rainwater-Lovett, K.; Rodriguez-Barraquer, I.; Cummings, D.A.T.; Lessler, J. Variation in Dengue Virus Plaque Reduction Neutralization Testing: Systematic Review and Pooled Analysis. BMC Infect. Dis. 2012, 12, 233. [Google Scholar] [CrossRef]
- Sirivichayakul, C.; Sabchareon, A.; Limkittikul, K.; Yoksan, S. Plaque Reduction Neutralization Antibody Test Does Not Accurately Predict Protection Against Dengue Infection in Ratchaburi Cohort, Thailand. Virol. J. 2014, 11, 48. [Google Scholar] [CrossRef]
- Buddhari, D.; Aldstadt, J.; Endy, T.P.; Srikiatkhachorn, A.; Thaisomboonsuk, B.; Klungthong, C.; Nisalak, A.; Khuntirat, B.; Jarman, R.G.; Fernandez, S.; et al. Dengue Virus Neutralizing Antibody Levels Associated with Protection from Infection in Thai Cluster Studies. PLoS Negl. Trop. Dis. 2014, 8, e3230. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Montoya, M.; Gresh, L.; Balmaseda, A.; Harris, E. Neutralizing Antibody Titers Against Dengue Virus Correlate with Protection from Symptomatic Infection in a Longitudinal Cohort. Proc. Natl. Acad. Sci USA 2016, 113, 728–733. [Google Scholar] [CrossRef]
- de Silva, A.M.; Harris, E. Which Dengue Vaccine Approach is the Most Promising, and Should We be Concerned About Enhanced Disease After Vaccination? The Path to a Dengue Vaccine: Learning from Human Natural Dengue Infection Studies and Vaccine Trials. Cold Spring Harb. Perspect. Biol. 2018, 10, a029371. [Google Scholar] [CrossRef] [PubMed]
- Endy, T.P.; Nisalak, A.; Chunsuttitwat, S.; Vaughn, D.W.; Green, S.; Ennis, F.A.; Rothman, A.L.; Libraty, D.H. Relationship of Preexisting Dengue Virus (DV) Neutralizing Antibody Levels to Viremia and Severity of Disease in a Prospective Cohort Study of DV Infection in Thailand. J. Infect. Dis. 2004, 189, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.X.; Lim, J.; Poh, C.L. Identification and selection of immunodominant B and T cell epitopes for dengue multi-epitope-based vaccine. Med. Microbiol. Immunol. 2021, 210, 1–11. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Wu, H.; Qiu, H.-J.; Sun, Y. T-Cell Epitope-Based Vaccines: A Promising Strategy for Prevention of Infectious Diseases. Vaccines 2024, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Miauton, A.; Audran, R.; Besson, J.; Maby-El Hajjami, H.; Karlen, M.; Warpelin-Decrausaz, L.; Sene, L.; Schaufelberger, S.; Faivre, V.; Faouzi, M.; et al. Safety and immunogenicity of a synthetic nanoparticle-based, T cell priming peptide vaccine against dengue in healthy adults in Switzerland: A double- blind, randomized, vehicle-controlled, phase 1 study. eBioMedicine 2024, 99, 104922. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Zhang, J.; Wei, H.; Wang, J.; Hu, C.; Liu, Y.; Cai, S.; Yuan, Q.; Wang, Y.; et al. Lysosome-Associated Membrane Protein Targeting Strategy Improved Immunogenicity of Glycoprotein-Based DNA Vaccine for Marburg Virus. Vaccines 2024, 12, 1013. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Lu, Y.; Raviprakash, K.; Leao, I.C.; Chikhlikar, P.R.; Ewing, D.; Anwar, A.; Chougnet, C.; Murphy, G.; Hayes, C.G.; August, T.J.; et al. Dengue 2 PreM-E/LAMP chimera targeted to the MHC class II compartment elicits long-lasting neutralizing antibodies. Vaccine 2003, 21, 2178–2189. [Google Scholar] [CrossRef]
- Bello, M.B.; Alsaadi, A.; Naeem, A.; Almahboub, S.A.; Bosaeed, M.; Aljedani, S.S. Development of nucleic acid-based vaccines against dengue and other mosquito-borne flaviviruses: The past, present, and future. Front. Immunol. 2025, 15, 1475886. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, J.; Shen, W.; Sun, Y.; Wang, Z.; Wang, J.; Zhang, J.; Zhang, G.; Zhang, G.; Wang, Y.; et al. DNA Vaccines Encoding HTNV GP-Derived Th Epitopes Benefited from a LAMP-Targeting Strategy and Established Cellular Immunoprotection. Vaccines 2024, 12, 928. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah, S.; Li, J.; Yang, F.; Tan, L. An In Silico Design of a Vaccine against All Serotypes of the Dengue Virus Based on Virtual Screening of B-Cell and T-Cell Epitopes. Biology 2024, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef] [PubMed]
- LaMontia-Hankin, E.; Wollner, C.; Pinto, A.; Pinto, A.; Richner, J. mRNA-LNPvaccines encoding for optimized prM-ENVproteins of Dengue virus serotypes 1-4 induce robust protective immunity without ADE. J. Immunol. 2024, 212, 0102_6117. [Google Scholar]
| Name | Strategy | Clinical Trial Phase | Serotypes | Adjuvant | Reference |
|---|---|---|---|---|---|
| Dengvaxia | YFV 17D backbone with the PrM and E proteins of DENV | Licensed | Tetravalent | No | [19,20] |
| TV003/TV005 | One chimeric virus with three attenuated viruses | In vivo (phase IIIB) | Tetravalent | No | [21,22,23] |
| TAK-003 | Live-attenuated. DENV-2 virus genetic backbone for all four serotypes | In vivo (phase III) | Tetravalent | No | [24,25,26,27,28] |
| TDEN | DENV-1 (45AZ5), DENV-2 (S16803), DENV-3 (CH53489) and DENV-4 (341750) | In vivo (phase I–II) | Tetravalent | No | [29,30] |
| V180 | Recombinant prM and a truncated form of the E protein (covering ~80%) from each of the four DENV serotypes. | In vivo (phase I) | Tetravalent | Yes | [31,32] |
| TVDV | DNA vaccine based on prM and E protein coding sequences cloned in VR1012 plasmid and co-administered with VAXFECTIN as an adjuvant | In vivo (animal and phase I) | Tetravalent | Yes | [33,34,35] |
| DSV4 | Virus-like particle vaccine designed to present EDIII of all four DENV serotypes | In vivo (animal) | Tetravalent | No | [36] |
| E80 mRNA vaccine | A modified mRNA-lipid nanoparticle (mRNA-LNP) formulation encoding the DENV E80 protein. | In vivo (animal) | Tetravalent | No | [37] |
| Protein | Epitope (aa) | Conservation (Serotypes) | Class/ Example HLA(s) | Evidence | Reference |
|---|---|---|---|---|---|
| NS5 | DTTPFGQQR | 1–4 | Class I; e.g., B*35/B*07 families | Human datasets & structure mapping | [53] |
| NS5 | TPFGQQRVF | 1–4 | Class I; A*02/B*35 families | Human datasets & structure mapping | [53] |
| NS5 | KTWAYHGSY | 1–4 | Class I; multiple predicted binders | Human datasets & in silico binders | [53] |
| NS5 | GPGHEEPIPM | 1–4 | Class I; multiple | Human datasets; interfaces in NS5 | [53] |
| NS5 | WSIHAHHQW | 1–4 | Class I; multiple | Human datasets; priming loop/motif F | [53] |
| NS5 | TWSIHAHHQW | 1–4 | Class I; multiple | Human datasets; priming loop | [53] |
| NS5 | PTSRTTWSIH | 1–4 | Class I; multiple | Human datasets; priming loop | [53] |
| NS5 | CVYNMMGKREKKLGE | 1–4 | Class I/II (overlapping) | Human datasets; motif F proximity | [53] |
| NS5 | KVRKDIPQW | 1–4 | Class I; multiple | Human datasets; inter-dimer interface | [53] |
| NS3 | KPGTSGSPI | 1–4 | Class I; multiple | Human datasets; includes protease S135 | [53] |
| NS3 | LPAIVREAI | 1–4 | Class I; B*07:02 | Human (Sri Lanka cohort), vaccinology | [99] |
| NS5 (motif) | MYFHRRDLRL | 1–4 | Class I; multiple (predicted) | In silico cross-serotype NS5 motif | [90] |
| Protein | Epitope (aa) | Serotype Specificity/Biased(s) | Class/HLA | Evidence | Reference |
|---|---|---|---|---|---|
| E | QEGAMHTAL vs. QEGAMHSAL | DENV1–3 vs. DENV4 (binary variant) | Class I (various) | Reported conservative (binary E variants) | [53] |
| E (DENV3) | TPTWNRKEL | DENV3 immunogenic | Class I; B*07:02 | Human ex vivo ELISpot | [95] |
| E (DENV3) | RKELLVTFKNAHAKK | DENV3 immunogenic | Class II; DRB1*15:01 (DR2) | Human ex vivo ELISpot | [95] |
| NS3 (DENV3 core) | KLNDWDFVV (399–407) | DENV3 core; DENV2/4 altered ligands | Class I; A*02:01 | Human & Tg mice; variant analysis | [95] |
| NS3 | KPRWLDARI vs. RPKWLDARV | DENV2 vs. DENV3 | Class I; B*07 family | HLA-B*07:02 transgenic model | [101] |
| NS5 | TPRMCTREEF vs. KPRLCTREEF | DENV2 vs. DENV3 | Class I; B*07 family | HLA-B*07:02 transgenic model | [101] |
| NS4A | WYAQIQPHWI (96–105) | DENV2 | Class I;A*24:02 | HLA-A*24:02 transgenic model | [102] |
| NS4B | HPASAWTLYA/RPASAWTLYA | DENV2/1/4 vs. DENV1/3 | Class I; B*35/B*07 | HLA-B*07:02 transgenic model | [101,103] |
| NS3/NS4B | APTRVVAAEM/APTRVVASEM | DENV2/3/4 vs. DENV1 | Class I; B*07:02 | Human, vaccine & cross-flavi data | [104] |
| Epitope Sequence | Position |
|---|---|
| AMTDTTPF | NS5 protein |
| CVYNMMGKREK | NS5 protein |
| FTNMEAQL | NS5 protein |
| LMYFHRRDLRL | NS5 protein |
| MYFHRRDLRL | NS5 protein |
| LMYFHRRDL | NS5 protein |
| WYMWLGAR | NS5 protein |
| LEFEALGF | NS5 protein |
| YFHRRDLR | NS5 protein |
| DTAGWDTR | NS5 protein |
| TFTNMEAQL | NS5 protein |
| VPTSRTTWSI | NS5 protein |
| MYFHRRDLRL | NS5 protein |
| LHKLGYIL | NS5 protein |
| Peptide Sequence | Virus/Protein & Position | Domain (Protease/Helicase/Polymerase/E, etc.) | HLA Restriction(s) | Evidence/Assay (Human Preferred) | Cross-reactivity/Specificity Notes | Reference(s) |
|---|---|---|---|---|---|---|
| “KLNDWDFVV” | DENV NS3 helicase (≈position ~400) | Helicase | HLA-A*11:01 | ICS/ELISpot in DENV-exposed humans | Recognized in both DENV and ZIKV NS3 homologs (cross-reactive) | [60] |
| “ELMRRGDLPV” | DENV NS3 protease (≈position ~100) | Protease | HLA-A*02:01 | Human T cell mapping (conserved-epitope meta sets) | Likely virus-specific, less cross recognition by ZIKV | [57] |
| “APTRVVAAEM” | DENV NS5 | Polymerase | HLA-A*02:01 | CD8 assays in cohorts, IEDB annotation | Highly conserved across DENV, possible partial cross-homology in ZIKV/JEV | [118] |
| “RVIDPRRCL” | DENV NS3 (helicase region) | Helicase | HLA-B*07:02 | Human T cell mapping | Conserved within DENV; homologous positions in ZIKV weaker but possible cross recognition | [57] |
| “LPAIVREAI” | DENV NS5 | Polymerase | HLA-A*02:01 | CD8 response in vaccinated or infected donors | Strongly conserved; cross-homology with ZIKV region moderate | [57] |
| “HYMYLIPGL” | ZIKV (e.g., NS region) | — | HLA-A*24:02 | CD8+ epitope mapped in humans or mice | Described as ZIKV-specific (low DENV homology) | [67] |
| “MTTEDMLQVW” | JEV NS5/NS region | Polymerase/NS5 domain | — (CD8) | Ex vivo ICS in JE-exposed donors | JEV epitope, used to test cross recog with DENV/ZIKV | [107] |
| “P34 (20-mer)” | JEV E protein | Envelope | CD4 (multiple HLA) | ELISpot in JE vaccinees, especially DENV-seronegative | Largely JEV-specific (low cross in DENV/ZIKV) | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.F.; Yeo Syin Lian, A.; Moi, M.L. T-Cell-Based Universal Dengue Vaccine Design for Robust Protective Response. Vaccines 2025, 13, 1118. https://doi.org/10.3390/vaccines13111118
Sun YF, Yeo Syin Lian A, Moi ML. T-Cell-Based Universal Dengue Vaccine Design for Robust Protective Response. Vaccines. 2025; 13(11):1118. https://doi.org/10.3390/vaccines13111118
Chicago/Turabian StyleSun, Yi Fei, Adeline Yeo Syin Lian, and Meng Ling Moi. 2025. "T-Cell-Based Universal Dengue Vaccine Design for Robust Protective Response" Vaccines 13, no. 11: 1118. https://doi.org/10.3390/vaccines13111118
APA StyleSun, Y. F., Yeo Syin Lian, A., & Moi, M. L. (2025). T-Cell-Based Universal Dengue Vaccine Design for Robust Protective Response. Vaccines, 13(11), 1118. https://doi.org/10.3390/vaccines13111118






