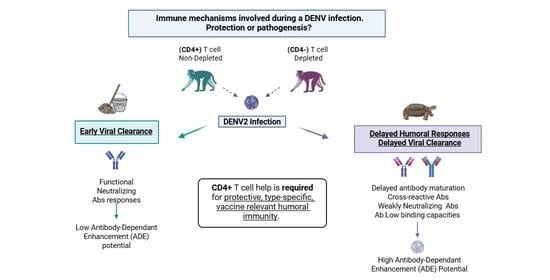
CD4+ T Cells Are Key to Shaping a Protective Humoral Immunity in Primary Dengue 2 Virus Infection: Implications for Rational Vaccine Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study Design
2.2. Viral Stock
2.3. Immunophenotyping
2.4. qRT-PCR
2.5. ELISAs for Detection of DENV NS1 Antigen and DENV Anti-NS1 IgG
2.6. ELISAs for DENV and ZIKV Antibodies
2.7. Endpoint Dilution Binding Assay
2.8. EDIII and NS1 Antigen Production
2.9. Coupling of EDIII Antigens to Beads via Avidin-Biotin Interaction
2.10. Coupling of Biotinylated NS1 Antigens to Beads via Avidin-His-Tag Ab Interaction
2.11. EDIII and NS1 Multiplex Assay
2.12. In Vitro ADE Assay in K562 Cells
2.13. DENV Neutralization Assays
2.14. ZIKV Neutralization Assays
2.15. BAFF ELISA
2.16. Serum Cytokines Assay
2.17. Quantification and Statistical Analysis
3. Results
3.1. CD4+ T Cell Depletion Impairs Viral Clearance Following Primary DENV2 Infection
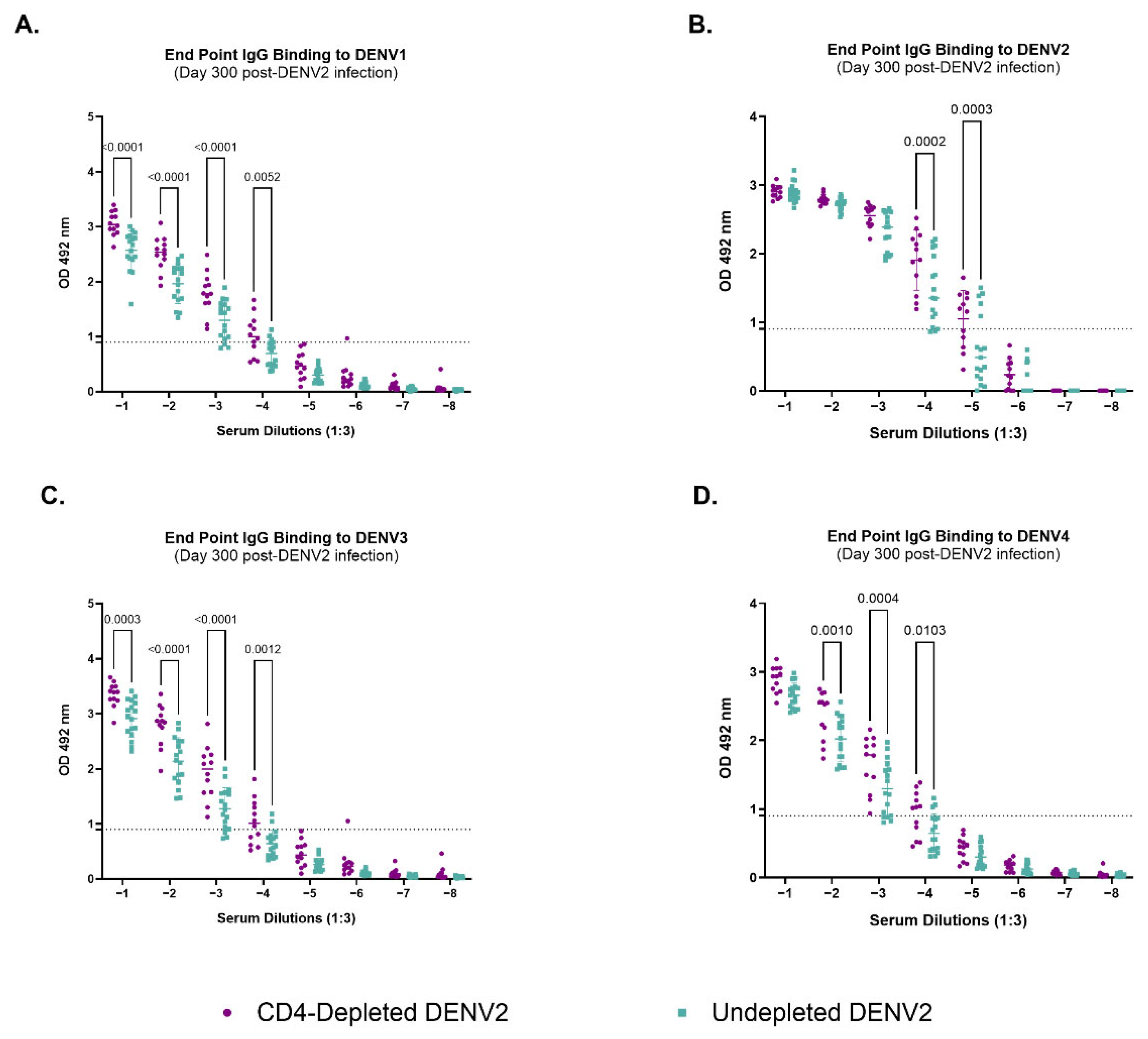
3.2. Lack of CD4+ T Cells Constrains Anti-DENV2 Antibody Induction
3.3. DENV Anti-NS1 Abs Are Modified by a Lack of CD4+ T Cells
3.4. Depletion of CD4+ T Cells Modifies IgG-Binding Capacities of Antibodies Against Heterologous Dengue After Primary DENV2 Infection
3.5. Lack of CD4+ T Cells During a Primary DENV2 Infection Reshapes Abs Binding to the Envelope EDIII Domain and to the NS1 Protein
3.6. Cross-Reactivity Is Increased by Lack of CD4 T Cells
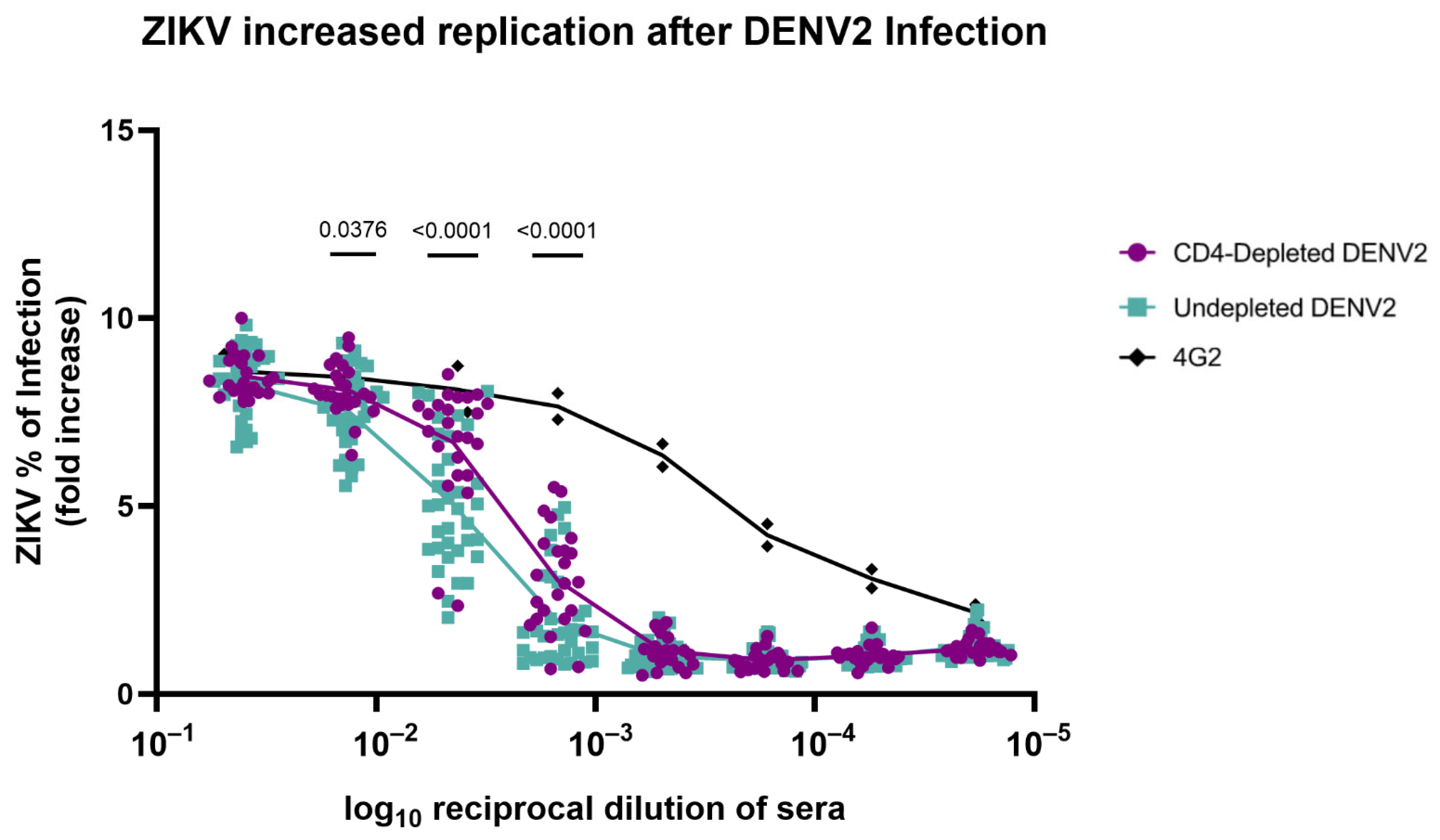
3.7. Absence of CD4+ T Cells Modifies Antibody Repertoire Properties Increasing ZIKV Replication
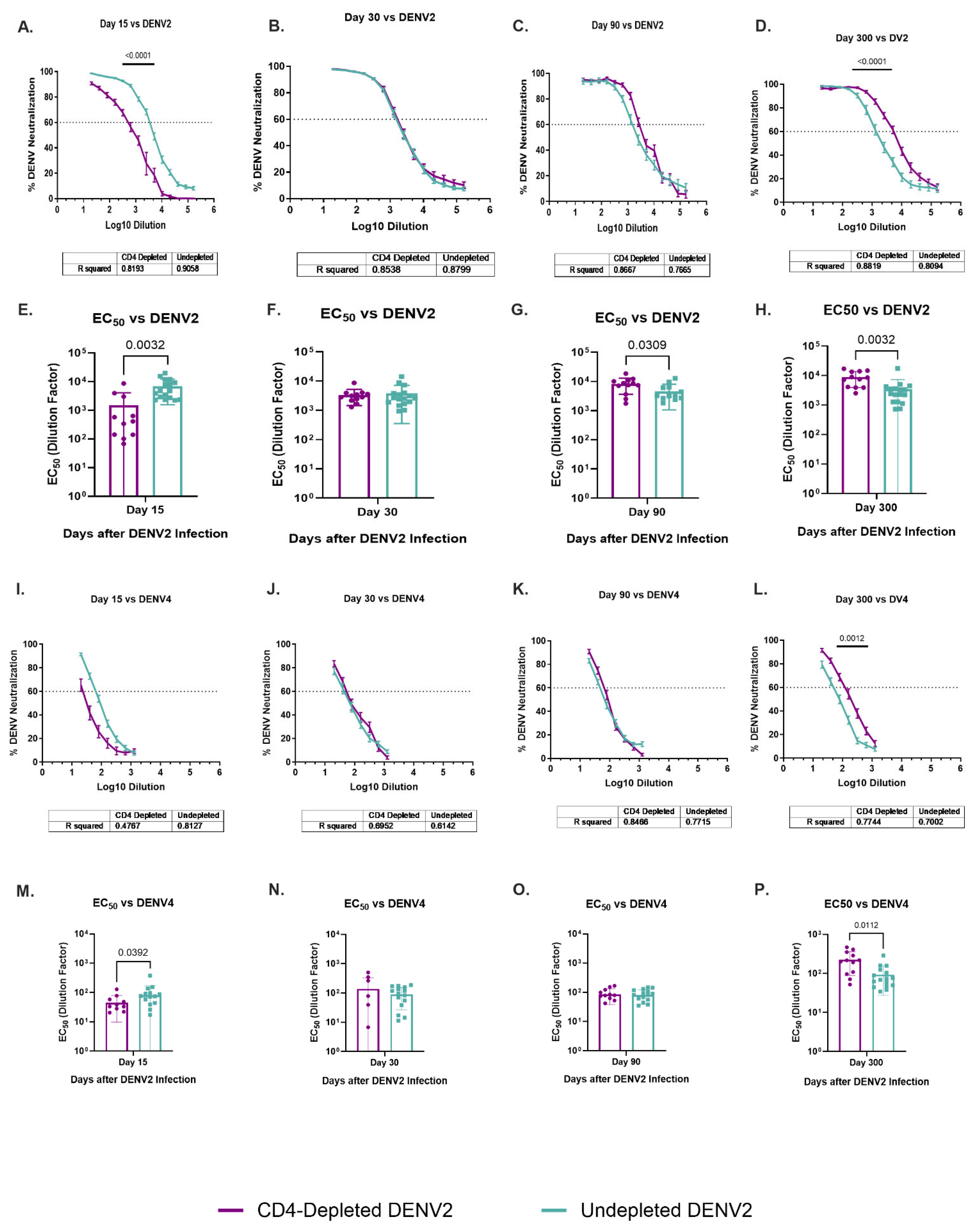
3.8. Dynamic of Specific DENV2 and Heterologous DENV4 Neutralizations Are Modulated by a Lack of CD4+ T Cells
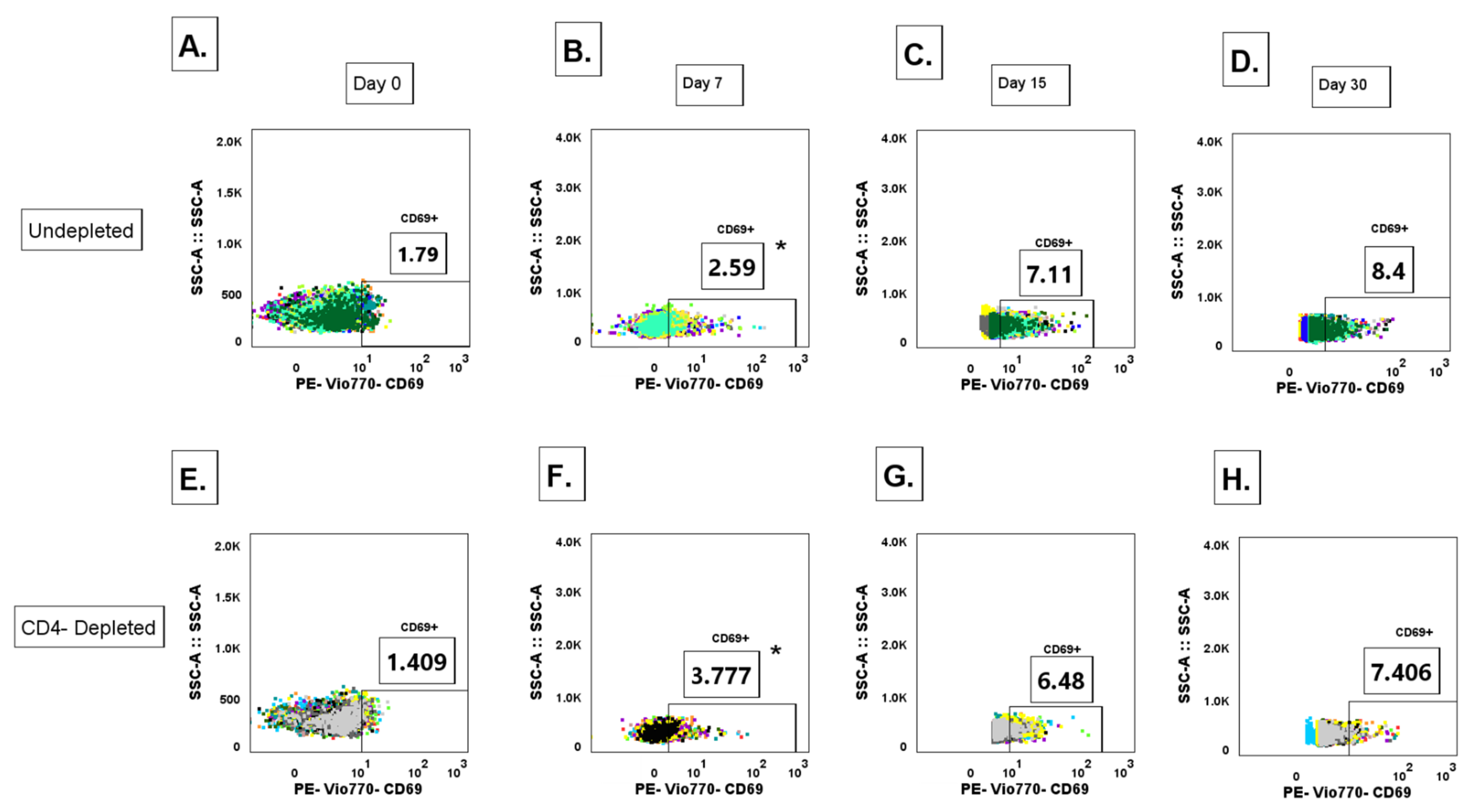
3.9. Lack of CD4+ T Cell Priming Promotes Increased IgG+ MBC Polyclonal Activation During a Primary DENV2 Infection
3.10. CD4 T Cell Depletion Before DENV2 Infection Results in a Delay of Cytokine Profiles in Rhesus Macaques
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guirakhoo, F.; Pugachev, K.; Zhang, Z.; Myers, G.; Levenbook, I.; Draper, K.; Lang, J.; Ocran, S.; Mitchell, F.; Parsons, M.; et al. Safety and efficacy of chimeric yellow Fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J. Virol. 2004, 78, 4761–4775. [Google Scholar] [CrossRef]
- Guy, B.; Barban, V.; Mantel, N.; Aguirre, M.; Gulia, S.; Pontvianne, J.; Jourdier, T.-M.; Ramirez, L.; Gregoire, V.; Charnay, C.; et al. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am. J. Trop. Med. Hyg. 2009, 80, 302–311. [Google Scholar] [CrossRef]
- Guy, B.; Briand, O.; Lang, J.; Saville, M.; Jackson, N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine 2015, 33, 7100–7111. [Google Scholar] [CrossRef] [PubMed]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.; Ismail, H.I.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.; Dayan, G.H.; Arredondo-Garcia, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.E.; Brewoo, J.N.; Silengo, S.J.; Arguello, J.; Moldovan, I.R.; Tary-Lehmann, M.; Powell, T.D.; Livengood, J.A.; Kinney, R.M.; Huang, C.Y.-H.; et al. Efficacy of a tetravalent chimeric dengue vaccine (DENVax) in Cynomolgus macaques. Am. J. Trop. Med. Hyg. 2011, 84, 978–987. [Google Scholar] [CrossRef]
- Biswal, S.; Borja-Tabora, C.; Martinez Vargas, L.; Velásquez, H.; Theresa Alera, M.; Sierra, V.; Yu, D.; Moreira, E.D.; Fernando, A.D.; Gunasekera, D.; et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: A randomised, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1423–1433. [Google Scholar] [CrossRef]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell. Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef]
- Mandaric, S.; Friberg, H.; Saez-Llorens, X.; Borja-Tabora, C.; Biswal, S.; Escudero, I.; Faccin, A.; Gottardo, R.; Brose, M.; Roubinis, N.; et al. Long term T cell response and safety of a tetravalent dengue vaccine in healthy children. npj Vaccines 2024, 9, 192. [Google Scholar] [CrossRef]
- Wilder-Smith, A. TAK-003 dengue vaccine as a new tool to mitigate dengue in countries with a high disease burden. Lancet Glob. Health 2024, 12, e179–e180. [Google Scholar] [CrossRef]
- Tian, N.; Zheng, J.X.; Guo, Z.Y.; Li, L.H.; Xia, S.; Lv, S.; Zhou, X.-N. Dengue Incidence Trends and Its Burden in Major Endemic Regions from 1990 to 2019. Trop. Med. Infect. Dis. 2022, 7, 180. [Google Scholar] [CrossRef]
- Wong, J.M.; Adams, L.E.; Durbin, A.P.; Muñoz-Jordán, J.L.; Poehling, K.A.; Sánchez-González, L.M.; Volkman, H.R.; Paz-Bailey, G. Dengue: A Growing Problem with New Interventions. Pediatrics 2022, 149, e2021055522. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. Cell Rep. 2022, 39, 110829. [Google Scholar] [CrossRef] [PubMed]
- Paixao, E.S.; Cardim, L.L.; Costa, M.C.N.; Brickley, E.B.; de Carvalho-Sauer, R.C.O.; Carmo, E.H.; Andrade, R.F.; Rodrigues, M.S.; Veiga, R.V.; Costa, L.C.; et al. Mortality from Congenital Zika Syndrome—Nationwide Cohort Study in Brazil. N. Engl. J. Med. 2022, 386, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Martelli, C.M.T.; Raja, A.I.; Sanchez Clemente, N.; de Araùjo, T.V.B.; de Alencar Ximenes, R.A.; Miranda-Filho, D.d.B.; Ramond, A.; Brickley, E.B. Epidemic preparedness: Prenatal Zika virus screening during the next epidemic. BMJ Glob. Health 2021, 6, e005332. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Frieden, T.R. Six Components Necessary for Effective Public Health Program Implementation. Am. J. Public Health 2014, 104, 17–22. [Google Scholar] [CrossRef]
- Akinsulie, O.C.; Idris, I. Global re-emergence of dengue fever: The need for a rapid response and surveillance. Microbe 2024, 4, 100107. [Google Scholar] [CrossRef]
- Guzman, M.G.; Alvarez, M.; Halstead, S.B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013, 158, 1445–1459. [Google Scholar] [CrossRef]
- Halstead, S.B. Neutralization and Antibody-Dependent Enhancement of Dengue Viruses. Adv. Viruses Res. 2003, 60, 421–467. [Google Scholar] [CrossRef]
- Halstead, S.B.; Rojanasuphot, S.; Sangkawibha, N. Original antigenic sin in dengue. Am. J. Trop. Med. Hyg. 1983, 32, 154–156. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.J.; Magnani, D.M.; Grifoni, A.; Kwon, Y.C.; Gutman, M.J.; Grubaugh, N.D.; Gangavarapu, K.; Sharkey, M.; Silveira, C.G.T.; Bailey, V.K.; et al. Ontogeny of the B- and T-cell response in a primary Zika virus infection of a dengue-naive individual during the 2016 outbreak in Miami, FL. PLoS Negl. Trop. Dis. 2017, 11, e0006000. [Google Scholar] [CrossRef] [PubMed]
- Wahala, W.M.; Silva, A.M. The human antibody response to dengue virus infection. Viruses 2011, 3, 2374–2395. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.R.; Metz, S.W.; Baric, R.S. Dengue Vaccines: The Promise and Pitfalls of Antibody-Mediated Protection. Cell Host Microbe 2021, 29, 13–22. [Google Scholar] [CrossRef]
- Gallichotte, E.N.; Widman, D.G.; Yount, B.L.; Wahala, M.W.; Durbin, A.; Whitehead, S.; Sariol, C.A.; Crowe, J.E.; de Silva, A.M.; Baric, R.S. A New Quaternary Structure Epitope on Dengue Virus Serotype 2 Is the Target of Durable Type-Specific Neutralizing Antibodies. mBio 2015, 6, 8. [Google Scholar] [CrossRef]
- Rouvinski, A.; Guardado-Calvo, P.; Barba-Spaeth, G.; Duquerroy, S.; Vaney, M.C.; Kikuti, C.M.; Sanchez, M.E.N.; Dejnirattisai, W.; Wongwiwat, W.; Haouz, A.; et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015, 520, 109–113. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef]
- Fibriansah, G.; Tan, J.L.; Smith, S.A.; de Alwis, R.; Ng, T.S.; Kostyuchenko, V.A.; Jadi, R.S.; Kukkaro, P.; de Silva, A.M.; Crowe, J.E.; et al. A highly potent human antibody neutralizes dengue virus serotype 3 by binding across three surface proteins. Nat. Commun. 2015, 6, 6341. [Google Scholar] [CrossRef]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vargas, L.A.; Mathew, A.; Salje, H.; Sousa, D.; Casale, N.A.; Farmer, A.; Buddhari, D.; Anderson, K.; Iamsirithaworn, S.; Kaewhiran, S.; et al. Protective Role of NS1-Specific Antibodies in the Immune Response to Dengue Virus Through Antibody-Dependent Cellular Cytotoxicity. J. Infect. Dis. 2024, 230, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Helmold Hait, S.; Vargas-Inchaustegui, D.A.; Musich, T.; Mohanram, V.; Tuero, I.; Venzon, D.J.; Bear, J.; Rosati, M.; Vaccari, M.; Franchini, G.; et al. Early T Follicular Helper Cell Responses and Germinal Center Reactions Are Associated with Viremia Control in Immunized Rhesus Macaques. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. A brief history of T cell help to B cells. Nat. Rev. Immunol. 2015, 15, 185–189. [Google Scholar] [CrossRef]
- Ma, C.S.; Deenick, E.K.; Batten, M.; Tangye, S.G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 2012, 209, 1241–1253. [Google Scholar] [CrossRef]
- Karnell, J.; Ettinger, R. The Interplay of IL-21 and BAFF in the Formation and Maintenance of Human B Cell Memory. Front. Immunol. 2012, 3, 17089. [Google Scholar] [CrossRef]
- Avery, D.T.; Kalled, S.L.; Ellyard, J.I.; Ambrose, C.; Bixler, S.A.; Thien, M.; Brink, R.; Mackay, F.; Hodgkin, P.D.; Tangye, S.G. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Investig. 2003, 112, 286–297. [Google Scholar] [CrossRef]
- O’Connor, B.P.; Raman, V.S.; Erickson, L.D.; Cook, W.J.; Weaver, L.K.; Ahonen, C.; Lin, L.-L.; Mantchev, G.T.; Bram, R.J.; Noelle, R.J. BCMA Is Essential for the Survival of Long-lived Bone Marrow Plasma Cells. J. Exp. Med. 2004, 199, 91–98. [Google Scholar] [CrossRef]
- Lee, W.S.; Amengual, O. B cells targeting therapy in the management of systemic lupus erythematosus. Immunol. Med. 2020, 43, 16–35. [Google Scholar] [CrossRef]
- Jackson, S.W.; Davidson, A. BAFF inhibition in SLE—Is tolerance restored? Immunol. Rev. 2019, 292, 102–119. [Google Scholar] [CrossRef]
- Asao, H. Interleukin-21 in Viral Infections. Int. J. Mol. Sci. 2021, 22, 9521. [Google Scholar] [CrossRef] [PubMed]
- Dvorscek, A.R.; McKenzie, C.I.; Robinson, M.J.; Ding, Z.; Pitt, C.; O’Donnell, K.; Zotos, D.; Brink, R.; Tarlinton, D.M.; Quast, I. IL-21 has a critical role in establishing germinal centers by amplifying early B cell proliferation. EMBO Rep. 2022, 23, e54677. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, T.; Bela-Ong, D.B.; Toh, Y.X.; Flamand, M.; Devi, S.; Koh, M.B.; Hibberd, M.L.; Ooi, E.E.; Low, J.G.; Leo, Y.S.; et al. Dengue virus activates polyreactive, natural IgG B cells after primary and secondary infection. PLoS ONE 2011, 6, e29430. [Google Scholar] [CrossRef] [PubMed]
- Fink, K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front. Immunol. 2012, 3, 78. [Google Scholar] [CrossRef]
- Bernasconi, N.L.; Onai, N.; Lanzavecchia, A. A role for Toll-like receptors in acquired immunity: Up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood 2003, 101, 4500–4504. [Google Scholar] [CrossRef]
- Pantoja, P.; Pérez-Guzmán, E.X.; Rodríguez, I.V.; White, L.J.; González, O.; Serrano, C.; Giavedoni, L.; Hodara, V.; Cruz, L.; Arana, T.; et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat. Commun. 2017, 8, 15674. [Google Scholar] [CrossRef]
- Perez-Guzman, E.X.; Pantoja, P.; Serrano-Collazo, C.; Hassert, M.A.; Ortiz-Rosa, A.; Rodriguez, I.V.; Giavedoni, L.; Hodara, V.; Parodi, L.; Cruz, L.; et al. Time elapsed between Zika and dengue virus infections affects antibody and T cell responses. Nat. Commun. 2019, 10, 4316. [Google Scholar] [CrossRef]
- Serrano-Collazo, C.; Pérez-Guzmán, E.X.; Pantoja, P.; Hassert, M.A.; Rodríguez, I.V.; Giavedoni, L.; Hodara, V.; Parodi, L.; Cruz, L.; Arana, T.; et al. Effective control of early Zika virus replication by Dengue immunity is associated to the length of time between the 2 infections but not mediated by antibodies. PLoS Negl. Trop. Dis. 2020, 14, e0008285. [Google Scholar] [CrossRef]
- Adams, C.; Jadi, R.; Segovia-Chumbez, B.; Daag, J.; Ylade, M.; Medina, F.A.; Sharp, T.M.; Munoz-Jordan, J.L.; Yoon, I.-K.; Deen, J.; et al. Novel Assay to Measure Seroprevalence of Zika Virus in the Philippines. Emerg. Infect. Dis. 2021, 27, 3073–3081. [Google Scholar] [CrossRef]
- Duong, V.; Ly, S.; Lorn Try, P.; Tuiskunen, A.; Ong, S.; Chroeung, N.; Lundkvist, A.; Leparc-Goffart, I.; Deubel, V.; Vong, S.; et al. Clinical and virological factors influencing the performance of a NS1 antigen-capture assay and potential use as a marker of dengue disease severity. PLoS Negl. Trop. Dis. 2011, 5, e1244. [Google Scholar] [CrossRef]
- Chau, T.N.; Anders, K.L.; Lien, L.B.; Hung, N.T.; Hieu, L.T.; Tuan, N.M.; Thuy, T.T.; Phuong, L.T.; Tham, N.T.H.; Lanh, M.N.; et al. Clinical and virological features of Dengue in Vietnamese infants. PLoS Negl. Trop. Dis. 2010, 4, e657. [Google Scholar] [CrossRef]
- Avirutnan, P.; Punyadee, N.; Noisakran, S.; Komoltri, C.; Thiemmeca, S.; Auethavornanan, K.; Jairungsri, A.; Kanlaya, R.; Tangthawornchaikul, N.; Puttikhunt, C.; et al. Vascular Leakage in Severe Dengue Virus Infections: A Potential Role for the Nonstructural Viral Protein NS1 and Complement. J. Infect. Dis. 2006, 193, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.R.; Ranadeva, N.D.; Sirisena, K.; Wijesinghe, K.J. Roles of NS1 Protein in Flavivirus Pathogenesis. ACS Infect. Dis. 2024, 10, 20–56. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Moutaftsi, M.; Moyron-Quiroz, J.; McCausland, M.M.; Davies, D.H.; Johnston, R.J.; Peters, B.; Benhnia, M.R.-E.; Hoffmann, J.; Su, H.-P.; et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: Deterministic linkage of specificities. Immunity 2008, 28, 847–858. [Google Scholar] [CrossRef]
- Graham, N.; Eisenhauer, P.; Diehl, S.A.; Pierce, K.K.; Whitehead, S.S.; Durbin, A.P.; Kirkpatrick, B.D.; Sette, A.; Weiskopf, D.; Boyson, J.E.; et al. Rapid Induction and Maintenance of Virus-Specific CD8+ TEMRA and CD4+ TEM Cells Following Protective Vaccination Against Dengue Virus Challenge in Humans. Front. Immunol. 2020, 11, 479. [Google Scholar] [CrossRef]
- Weiskopf, D.; Bangs, D.J.; Sidney, J.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; Crotty, S.; Peters, B.; Sette, A. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc. Natl. Acad. Sci. USA 2015, 112, E4256–E4263. [Google Scholar] [CrossRef]
- Scinicariello, F.; Engleman, C.N.; Jayashankar, L.; McClure, H.M.; Attanasio, R. Rhesus macaque antibody molecules: Sequences and heterogeneity of alpha and gamma constant regions. Immunology 2004, 111, 66–74. [Google Scholar] [CrossRef]
- Teo, A.; Tan, H.D.; Loy, T.; Chia, P.Y.; Chua, C.L.L. Understanding antibody-dependent enhancement in dengue: Are afucosylated IgG1s a concern? PLoS Pathog. 2023, 19, e1011223. [Google Scholar] [CrossRef]
- Rodrigo, W.W.; Block, O.K.; Lane, C.; Sukupolvi-Petty, S.; Goncalvez, A.P.; Johnson, S.; Diamond, M.S.; Lai, C.-J.; Rose, R.C.; Jin, X.; et al. Dengue virus neutralization is modulated by IgG antibody subclass and Fcgamma receptor subtype. Virology 2009, 394, 175–182. [Google Scholar] [CrossRef]
- Koraka, P.; Suharti, C.; Setiati, T.E.; Mairuhu, A.T.; Van Gorp, E.; Hack, C.E.; Juffrie, M.; Sutaryo, J.; Van Der Meer, G.M.; Groen, J.; et al. Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. J. Clin. Microbiol. 2001, 39, 4332–4338. [Google Scholar] [CrossRef]
- Boesch, A.W.; Osei-Owusu, N.Y.; Crowley, A.R.; Chu, T.H.; Chan, Y.N.; Weiner, J.A.; Bharadwaj, P.; Hards, R.; Adamo, M.E.; Gerber, S.A.; et al. Biophysical and Functional Characterization of Rhesus Macaque IgG Subclasses. Front. Immunol. 2016, 7, 589. [Google Scholar] [CrossRef] [PubMed]
- Blancher, A.; Roubinet, F.; Reid, M.E.; Socha, W.W.; Bailly, P.; Bénard, P. Characterization of a Macaque Anti-Rh17-Like Monoclonal Antibody. Vox Sang. 1998, 75, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Calvas, P.; Apoil, P.; Fortenfant, F.; Roubinet, F.; Andris, J.; Capra, D.; Blancher, A. Characterization of the Three Immunoglobulin G Subclasses of Macaques. Scand. J. Immunol. 1999, 49, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ewing, D.; Subramanian, H.; Block, K.; Rayner, J.; Alterson, K.D.; Sedegah, M.; Hayes, C.; Porter, K.; Raviprakash, K. A heterologous DNA prime-Venezuelan equine encephalitis virus replicon particle boost dengue vaccine regimen affords complete protection from virus challenge in cynomolgus macaques. J. Virol. 2007, 81, 11634–11639. [Google Scholar] [CrossRef]
- Yauch, L.E.; Prestwood, T.R.; May, M.M.; Morar, M.M.; Zellweger, R.M.; Peters, B.; Sette, A.; Shresta, S. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J. Immunol. 2010, 185, 5405–5416. [Google Scholar] [CrossRef]
- Henriques, H.R.; Rampazo, E.V.; Gonçalves, A.J.; Vicentin, E.C.; Amorim, J.H.; Panatieri, R.H.; Amorim, K.N.S.; Yamamoto, M.M.; Ferreira, L.C.S.; Alves, A.M.B.; et al. Targeting the non-structural protein 1 from dengue virus to a dendritic cell population confers protective immunity to lethal virus challenge. PLoS Negl. Trop. Dis. 2013, 7, e2330. [Google Scholar] [CrossRef]
- Pinto, P.B.A.; Assis, M.L.; Vallochi, A.L.; Pacheco, A.R.; Lima, L.M.; Quaresma, K.R.L.; Pereira, B.A.S.; Costa, S.M.; Alves, A.M.B. T Cell Responses Induced by DNA Vaccines Based on the DENV2 E and NS1 Proteins in Mice: Importance in Protection and Immunodominant Epitope Identification. Front. Immunol. 2019, 10, 1522. [Google Scholar] [CrossRef]
- Goncalves, A.J.; Oliveira, E.R.; Costa, S.M.; Paes, M.V.; Silva, J.F.; Azevedo, A.S.; Mantuano-Barradas, M.; Nogueira, A.C.M.A.; Almeida, C.J.; Alves, A.M.B. Cooperation between CD4+ T Cells and Humoral Immunity Is Critical for Protection against Dengue Using a DNA Vaccine Based on the NS1 Antigen. PLoS Negl. Trop. Dis. 2015, 9, e0004277. [Google Scholar] [CrossRef]
- Dias, A.G.; Jr Atyeo, C.; Loos, C.; Montoya, M.; Roy, V.; Bos, S.; Narvekar, P.; Singh, T.; Katzelnick, L.C.; Kuan, G.; et al. Antibody Fc characteristics and effector functions correlate with protection from symptomatic dengue virus type 3 infection. Sci. Transl. Med. 2022, 14, eabm3151. [Google Scholar] [CrossRef]
- Mladinich, K.M.; Piaskowski, S.M.; Rudersdorf, R.; Eernisse, C.M.; Weisgrau, K.L.; Martins, M.A.; Furlott, J.R.; Partidos, C.D.; Brewoo, J.N.; Osorio, J.E.; et al. Dengue virus-specific CD4+ and CD8+ T lymphocytes target NS1, NS3 and NS5 in infected Indian rhesus macaques. Immunogenetics 2012, 64, 111–121. [Google Scholar] [CrossRef]
- Rivino, L.; Kumaran, E.A.; Jovanovic, V.; Nadua, K.; Teo, E.W.; Pang, S.W.; Teo, G.H.; Gan, V.C.H.; Lye, D.C.; Leo, Y.S.; et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J. Virol. 2013, 87, 2693–2706. [Google Scholar] [CrossRef]
- Nivarthi, U.K.; Kose, N.; Sapparapu, G.; Widman, D.; Gallichotte, E.; Pfaff, J.M.; Doranz, B.J.; Weiskopf, D.; Sette, A.; Durbin, A.P.; et al. Mapping the Human Memory B Cell and Serum Neutralizing Antibody Responses to Dengue Virus Serotype 4 Infection and Vaccination. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Priyamvada, L.; Cho, A.; Onlamoon, N.; Zheng, N.Y.; Huang, M.; Kovalenkov, Y.; Chokephaibulkit, K.; Angkasekwinai, N.; Pattanapanyasat, K.; Ahmed, R.; et al. B Cell Responses during Secondary Dengue Virus Infection Are Dominated by Highly Cross-Reactive, Memory-Derived Plasmablasts. J. Virol. 2016, 90, 5574–5585. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; Graber, A.L.; Cardona-Ospina, J.A.; Duarte, E.M.; Zambrana, J.V.; Ruíz Salinas, J.A.; Mercado-Hernandez, R.; Singh, T.; Katzelnick, L.C.; de Silva, A.; et al. Protection against symptomatic dengue infection by neutralizing antibodies varies by infection history and infecting serotype. Nat. Commun. 2024, 15, 382. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Bos, S.; Harris, E. Protective and enhancing interactions among dengue viruses 1-4 and Zika virus. Curr. Opin. Virol. 2020, 43, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, J.V.; Hasund, C.M.; Aogo, R.A.; Bos, S.; Arguello, S.; Gonzalez, K.; Collado, D.; Miranda, T.; Kuan, G.; Gordon, A.; et al. Primary exposure to Zika virus is linked with increased risk of symptomatic dengue virus infection with serotypes 2, 3, and 4, but not 1. Sci. Transl. Med. 2024, 16, eadn2199. [Google Scholar] [CrossRef]
- Crooks, C.M.; Weiler, A.M.; Rybarczyk, S.L.; Bliss, M.I.; Jaeger, A.S.; Murphy, M.E.; Simmons, H.A.; Mejia, A.; Fritsch, M.K.; Hayes, J.M.; et al. Previous exposure to dengue virus is associated with increased Zika virus burden at the maternal-fet al interface in rhesus macaques. PLoS Negl. Trop. Dis. 2021, 15, e0009641. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, R.; Freyn, A.W.; Wang, J.; Strohmeier, S.; Lederer, K.; Locci, M.; Zhao, H.; Angeletti, D.; O’connor, K.C.; et al. CD4+ follicular regulatory T cells optimize the influenza virus-specific B cell response. J. Exp. Med. 2021, 218, e20200547. [Google Scholar] [CrossRef]
- Elong Ngono, A.; Young, M.P.; Bunz, M.; Xu, Z.; Hattakam, S.; Vizcarra, E.; Regla-Nava, J.A.; Tang, W.W.; Yamabhai, M.; Wen, J.; et al. CD4+ T cells promote humoral immunity and viral control during Zika virus infection. PLoS Pathog. 2019, 15, e1007474. [Google Scholar] [CrossRef]
- Elsner, R.A.; Shlomchik, M.J. Coordinated Regulation of Extrafollicular B Cell Responses by IL-12 and IFNγ. Immunol. Rev. 2025, 331, e70027. [Google Scholar] [CrossRef]
- Provine, N.M.; Badamchi-Zadeh, A.; Bricault, C.A.; Penaloza-MacMaster, P.; Larocca, R.A.; Borducchi, E.N.; Seaman, M.S.; Barouch, D.H. Transient CD4+ T Cell Depletion Results in Delayed Development of Functional Vaccine-Elicited Antibody Responses. J. Virol. 2016, 90, 4278–4288. [Google Scholar] [CrossRef]
- Correa, A.R.; Berbel, A.C.; Papa, M.P.; Morais, A.T.; Peçanha, L.M.; Arruda, L.B. Dengue Virus Directly Stimulates Polyclonal B Cell Activation. PLoS ONE 2015, 10, e0143391. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, N.; Thang, P.H.; Rethi, B.; Nilsson, A.; Chiodi, F. The impact of inflammation and immune activation on B cell differentiation during HIV-1 infection. Front. Immunol. 2011, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Kwissa, M.; Nakaya, H.I.; Onlamoon, N.; Wrammert, J.; Villinger, F.; Perng, G.C.; Yoksan, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Ahmed, R.; et al. Dengue virus infection induces expansion of a CD14+CD16+ monocyte population that stimulates plasmablast differentiation. Cell Host Microbe 2014, 16, 115–127. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Qiao, X.; Klasse, P.J.; Chiu, A.; Chadburn, A.; Knowles, D.M.; Moore, J.P.; Cerutti, A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 2006, 176, 3931–3941. [Google Scholar] [CrossRef]
- Tian, Y.; Grifoni, A.; Sette, A.; Weiskopf, D. Human T Cell Response to Dengue Virus Infection. Front. Immunol. 2019, 10, 2125. [Google Scholar] [CrossRef]
- Beaumier, C.M.; Rothman, A.L. Cross-reactive memory CD4+ T cells alter the CD8+ T-cell response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. Viral Immunol. 2009, 22, 215–219. [Google Scholar] [CrossRef]
- Landry, S.J.; Moss, D.L.; Cui, D.; Ferrie, R.P.; Fullerton, M.L.; Wells, E.A.; Yang, L.; Zhou, N.; Dougherty, T.; Mettu, R.R. Structural Basis for CD4+ T Cell Epitope Dominance in Arbo-Flavivirus Envelope Proteins: A Meta-Analysis. Viral Immunol. 2017, 30, 479–489. [Google Scholar] [CrossRef]
- Saron, W.A.A.; Rathore, A.P.S.; Ting, L.; Ooi, E.E.; Low, J.; Abraham, S.N.; John, A.L.S. Flavivirus serocomplex cross-reactive immunity is protective by activating heterologous memory CD4 T cells. Sci. Adv. 2018, 4, eaar4297. [Google Scholar] [CrossRef]
- Angelo, M.A.; Grifoni, A.; O’Rourke, P.H.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Phillips, E.; Mallal, S.; Diehl, S.A.; et al. Human CD4+ T Cell Responses to an Attenuated Tetravalent Dengue Vaccine Parallel Those Induced by Natural Infection in Magnitude, HLA Restriction, and Antigen Specificity. J. Virol. 2017, 91, e02147-16. [Google Scholar] [CrossRef]
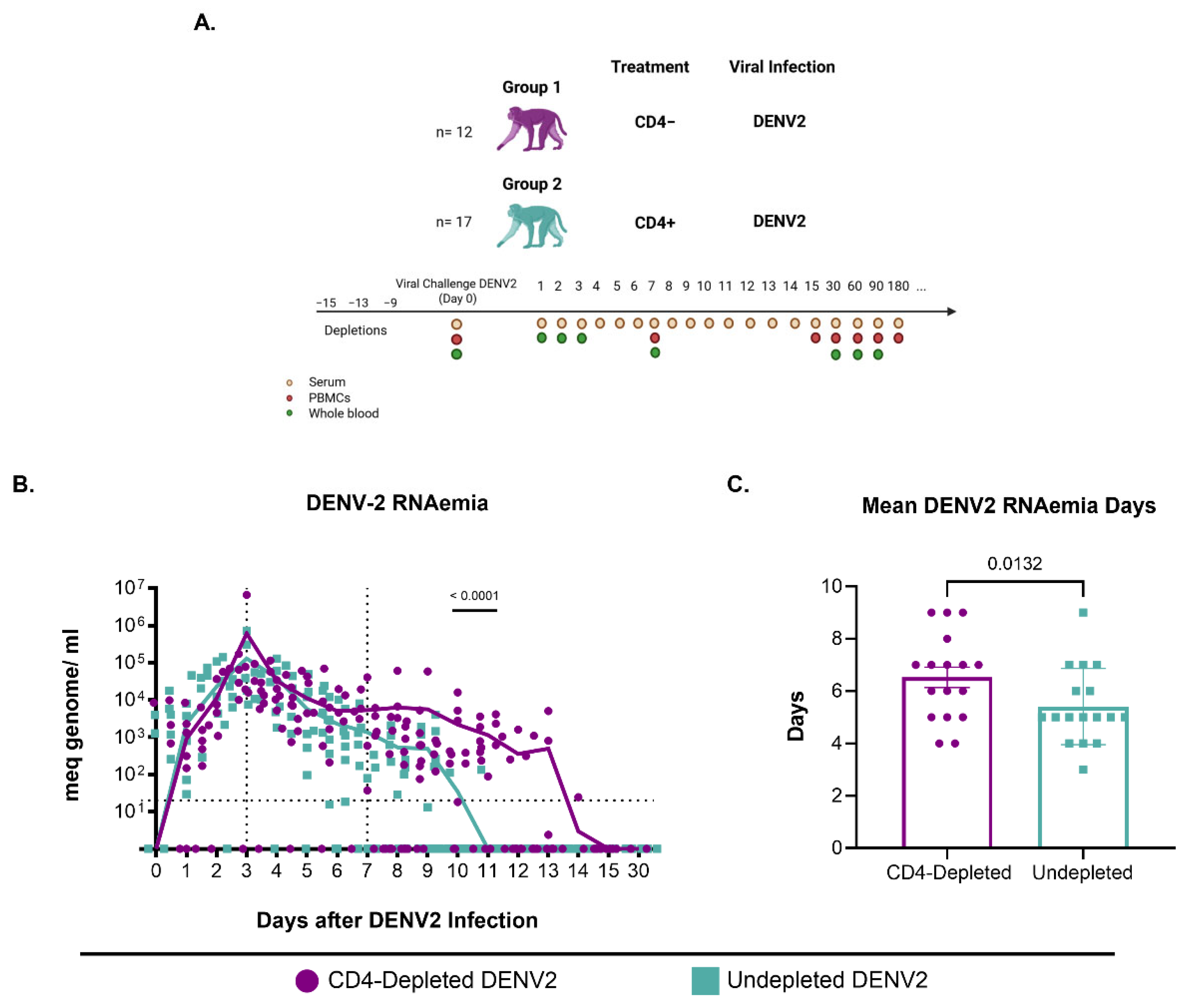

| ID | Immune History | RNAemia (Log10 Genome Copies/mL) Post-DENV Infection | Days | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 30 | Total | Mean | ||
| 0Z6 | CD4-/DV2 | ND | 2.905 | 3.924 | 4.505 | 4.245 | 3.875 | 3.799 | 3.040 | ND | 2.259 | 3.446 | 3.615 | 3.039 | ND | ND | ND | ND | 117 | 9.75 |
| AE9 | ND | 2.834 | 4.204 | 4.759 | 4.059 | 3.758 | 3.164 | 3.545 | 2.957 | 2.540 | 2.623 | 3.272 | 3.202 | ND | ND | ND | ND | |||
| 1Z3 | ND | 3.120 | 4.123 | 4.672 | 4.528 | 3.641 | 3.768 | 3.566 | 3.828 | 2.786 | 2.720 | 2.892 | ND | ND | ND | ND | ND | |||
| MA317 | ND | 2.682 | 4.557 | 5.235 | 4.888 | 4.291 | 3.176 | ND | 2.356 | 2.083 | 2.293 | ND | 2.492 | ND | ND | ND | ND | |||
| MA312 | ND | 2.493 | 3.908 | 5.060 | 3.718 | 3.961 | 3.217 | 3.425 | 3.285 | 3.107 | 3.713 | ND | ND | ND | ND | ND | ND | |||
| MA209 | ND | 2.167 | 3.323 | 4.003 | 3.844 | 2.765 | 4.283 | 4.608 | 4.784 | 4.765 | 4.202 | 3.555 | 3.018 | ND | ND | ND | ND | |||
| 5Z1 | ND | 3.357 | 3.655 | 4.967 | 4.298 | 2.869 | 2.325 | ND | ND | 1.876 | ND | ND | ND | ND | ND | ND | ND | |||
| 0Z7 | ND | 3.326 | 3.991 | 6.821 | 4.282 | 3.663 | 3.494 | 3.490 | 2.549 | 3.720 | 2.578 | 3.362 | 2.403 | ND | ND | ND | ND | |||
| MA333 | ND | ND | 4.111 | 4.812 | 4.456 | 3.692 | 3.147 | 3.429 | 2.831 | 2.604 | 2.368 | 2.600 | ND | ND | ND | ND | ND | |||
| MA321 | ND | 2.226 | 3.873 | 4.753 | 4.844 | 4.016 | 3.659 | 2.770 | 2.786 | ND | 2.294 | 1.945 | ND | ND | ND | ND | ND | |||
| MA267 | ND | 3.030 | 3.805 | 4.459 | 4.467 | 4.334 | 3.236 | 1.572 | ND | 2.916 | 1.257 | 2.786 | ND | ND | ND | ND | ND | |||
| MA316 | ND | 3.192 | 3.928 | 4.785 | 4.840 | 4.632 | 4.127 | 3.785 | 3.515 | 2.220 | 2.540 | ND | ND | ND | ND | ND | ND | |||
| 9X5 | CD4+/DV2 | ND | 3.076 | 3.879 | 4.741 | 4.379 | 3.663 | 2.551 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 126 | 7.4 |
| MA341 | ND | 1.473 | 5.037 | 5.855 | 5.111 | 4.006 | 3.174 | 3.054 | 3.114 | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 4Z0 | ND | 2.466 | 4.092 | 4.865 | 4.364 | 3.085 | 3.052 | 3.474 | 2.524 | 2.003 | ND | ND | ND | ND | ND | ND | ND | |||
| MA320 | ND | 3.566 | 4.598 | 4.650 | 4.641 | 3.674 | 2.390 | ND | 2.401 | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 8X2 | ND | 3.598 | 4.078 | 4.505 | 3.255 | 1.983 | 3.257 | 2.227 | ND | 1.119 | ND | ND | ND | ND | ND | ND | ND | |||
| MA258 | ND | 1.853 | 3.330 | 4.924 | 4.692 | 4.249 | 3.768 | 3.313 | 1.457 | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 9X7 | ND | 2.895 | 4.247 | 5.148 | 4.305 | 2.716 | 1.266 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 5Z8 | ND | 3.901 | 4.555 | 5.487 | 4.782 | 3.123 | 1.193 | 1.898 | 2.797 | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 9X2 | ND | 3.638 | 4.313 | 4.662 | 4.704 | 4.079 | 3.456 | 2.340 | 3.067 | ND | ND | ND | ND | ND | ND | ND | ND | |||
| 9X1 | ND | 2.408 | 3.804 | 4.242 | 4.009 | 3.552 | 2.940 | 2.190 | 2.190 | 2.421 | ND | ND | ND | ND | ND | ND | ND | |||
| 2Z4 | ND | 3.604 | 3.827 | 4.477 | 4.215 | 3.089 | 2.754 | 3.162 | 2.792 | 3.159 | ND | ND | ND | ND | ND | ND | ND | |||
| MA163 | ND | 3.103 | 3.762 | 4.026 | 4.581 | 4.030 | 4.078 | 3.911 | 3.282 | 3.605 | ND | ND | ND | ND | ND | ND | ND | |||
| MA254 | ND | 2.470 | 4.443 | 5.228 | 5.012 | 3.087 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| MA264 | ND | 2.087 | 4.210 | 3.857 | 5.160 | 4.333 | 3.097 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| MA311 | ND | 3.243 | 4.216 | 4.511 | 4.387 | 2.845 | 2.551 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| MA350 | ND | 1.630 | 4.534 | 5.385 | 5.190 | 3.504 | 1.688 | 1.183 | 2.971 | ND | ND | ND | ND | ND | ND | ND | ND | |||
| MA364 | ND | 2.779 | 4.229 | 5.033 | 5.035 | 4.128 | 3.654 | ND | ND | 1.884 | ND | ND | ND | ND | ND | ND | ND | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Santiago, A.E.; Serrano-Collazo, C.; Cruz, L.A.; Henein, S.; Alvarez, L.; Arana, T.; Sánchez-Bibiloni, J.L.; Martinez, M.I.; Roman, C.; Burgos, A.G.; et al. CD4+ T Cells Are Key to Shaping a Protective Humoral Immunity in Primary Dengue 2 Virus Infection: Implications for Rational Vaccine Design. Vaccines 2025, 13, 1103. https://doi.org/10.3390/vaccines13111103
Miranda-Santiago AE, Serrano-Collazo C, Cruz LA, Henein S, Alvarez L, Arana T, Sánchez-Bibiloni JL, Martinez MI, Roman C, Burgos AG, et al. CD4+ T Cells Are Key to Shaping a Protective Humoral Immunity in Primary Dengue 2 Virus Infection: Implications for Rational Vaccine Design. Vaccines. 2025; 13(11):1103. https://doi.org/10.3390/vaccines13111103
Chicago/Turabian StyleMiranda-Santiago, Angel E., Crisanta Serrano-Collazo, Lorna A. Cruz, Sandra Henein, Laura Alvarez, Teresa Arana, Jorge L. Sánchez-Bibiloni, Melween I. Martinez, Chiara Roman, Armando G. Burgos, and et al. 2025. "CD4+ T Cells Are Key to Shaping a Protective Humoral Immunity in Primary Dengue 2 Virus Infection: Implications for Rational Vaccine Design" Vaccines 13, no. 11: 1103. https://doi.org/10.3390/vaccines13111103
APA StyleMiranda-Santiago, A. E., Serrano-Collazo, C., Cruz, L. A., Henein, S., Alvarez, L., Arana, T., Sánchez-Bibiloni, J. L., Martinez, M. I., Roman, C., Burgos, A. G., Ramos-Benitez, M. J., Caro-Rivera, L. M., Brien, J. D., Pinto, A. K., de Silva, A. M., & Sariol, C. A. (2025). CD4+ T Cells Are Key to Shaping a Protective Humoral Immunity in Primary Dengue 2 Virus Infection: Implications for Rational Vaccine Design. Vaccines, 13(11), 1103. https://doi.org/10.3390/vaccines13111103