Rapid Quantification of Bluetongue Virus-Neutralizing Antibodies Using Bioluminescent Reporter-Expressing Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell and Viruses
2.2. Microneutralization Assay
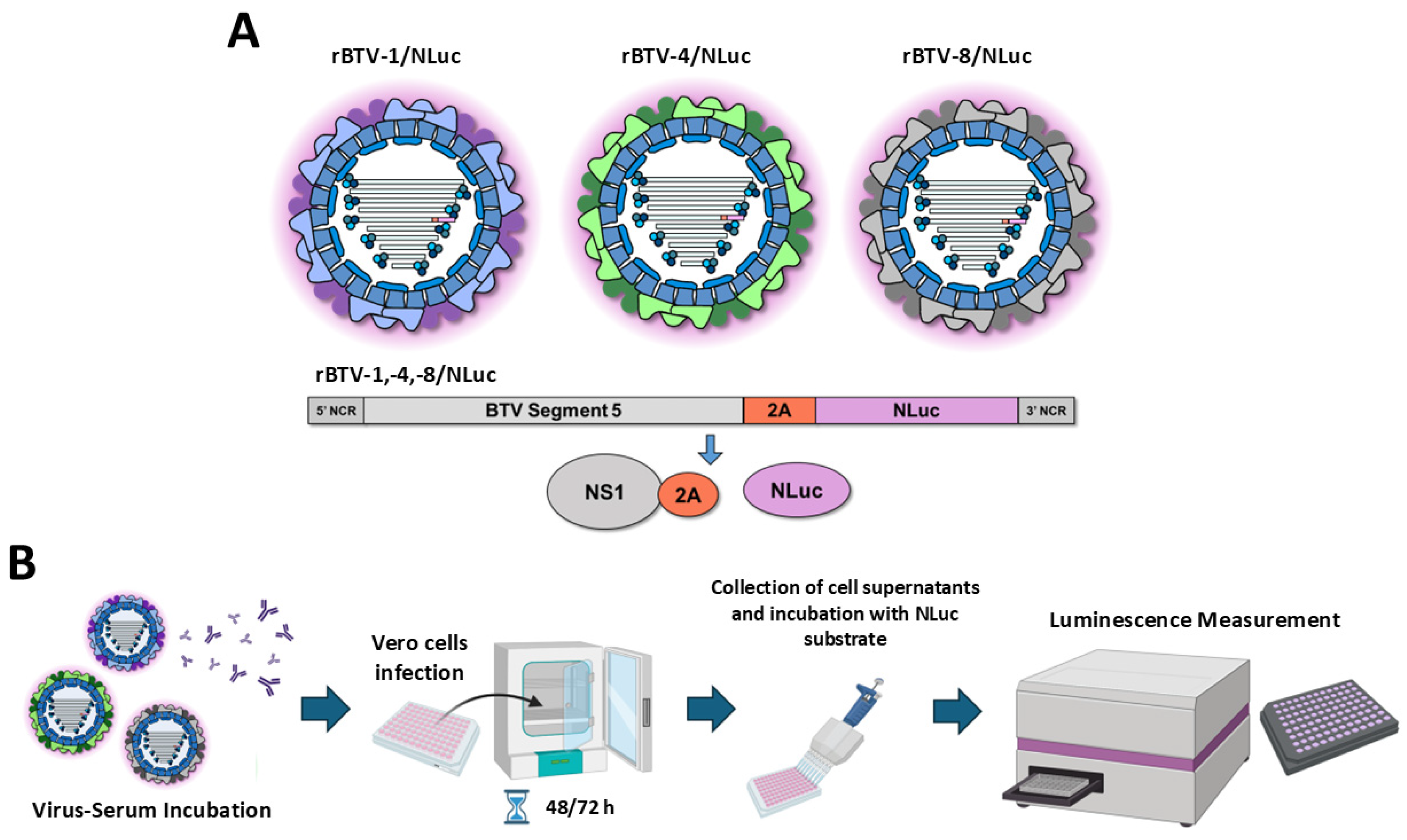
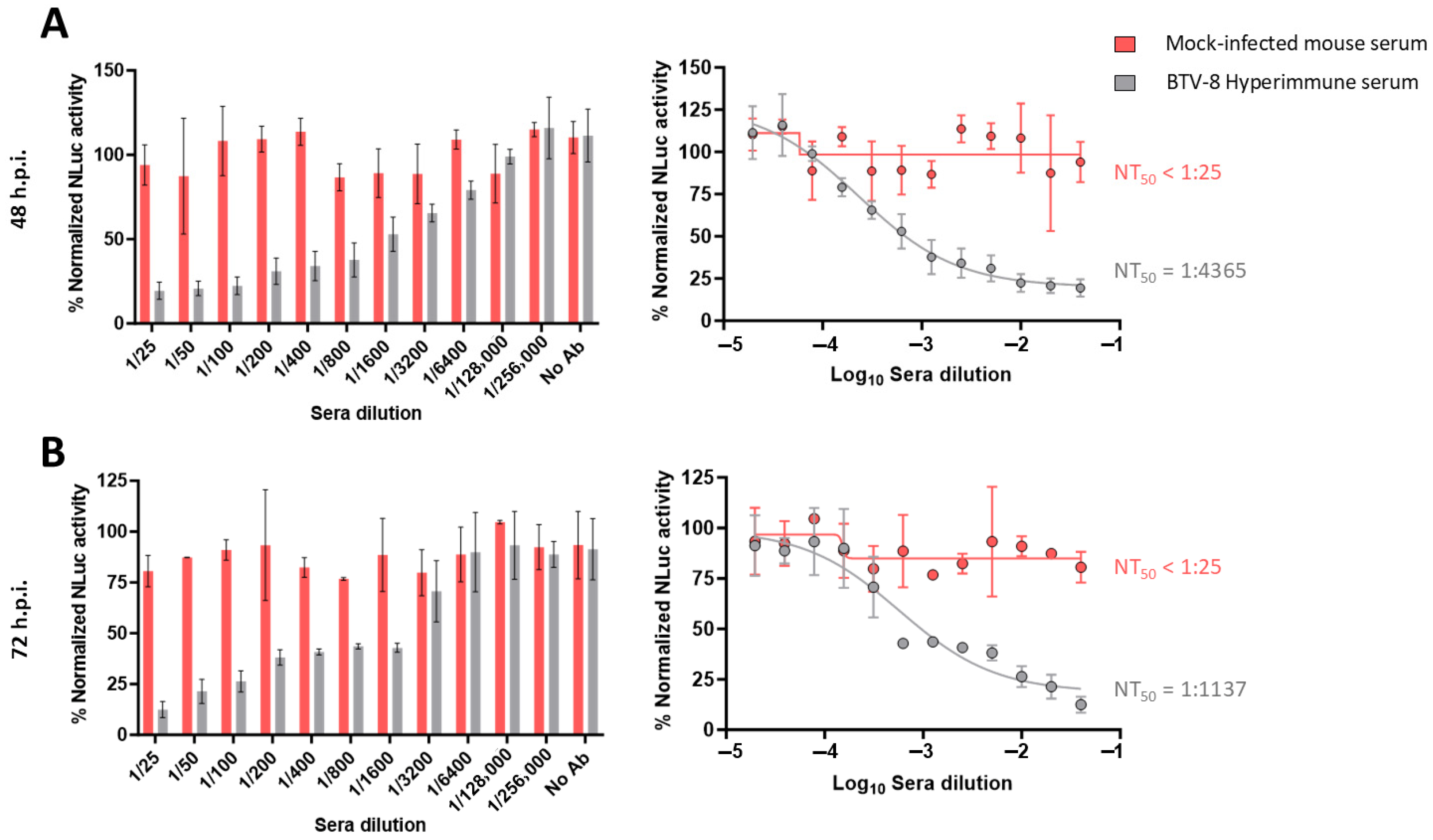
2.3. NLuc-Based Microneutralization Assays
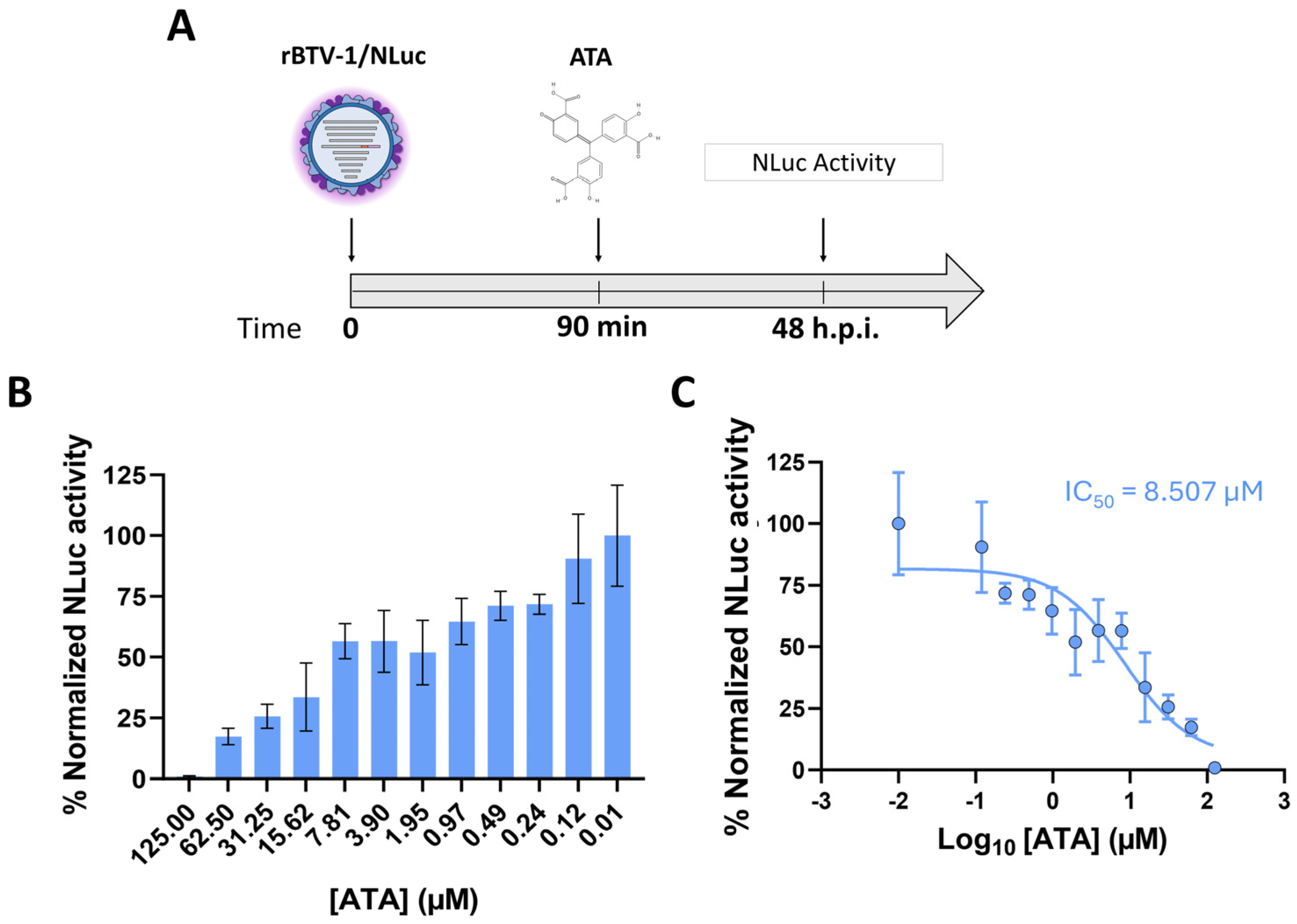
2.4. Antiviral Assays
3. Results
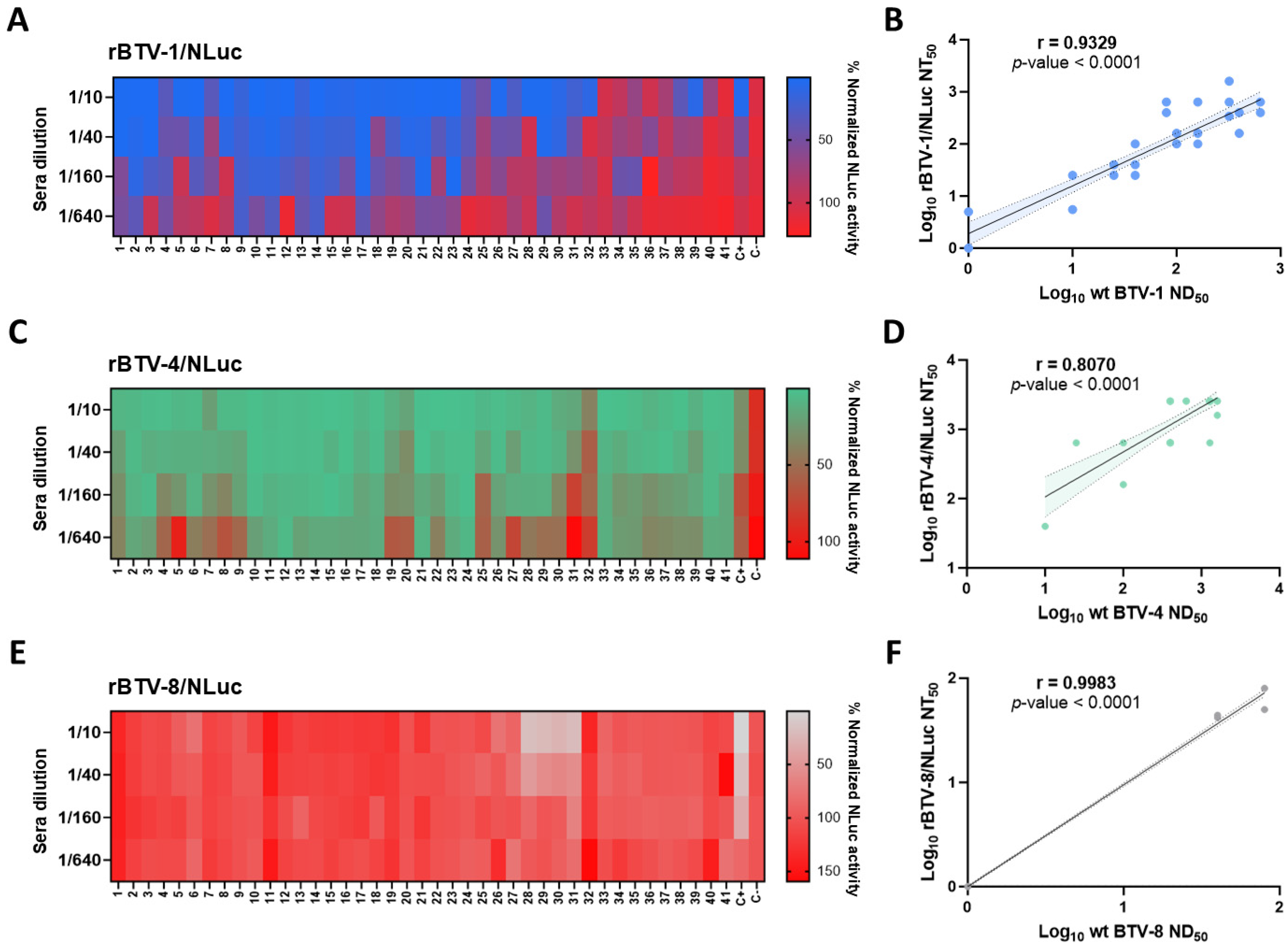
3.1. Use of Luminescent BTVs for Determination of BTV-NAb Titers
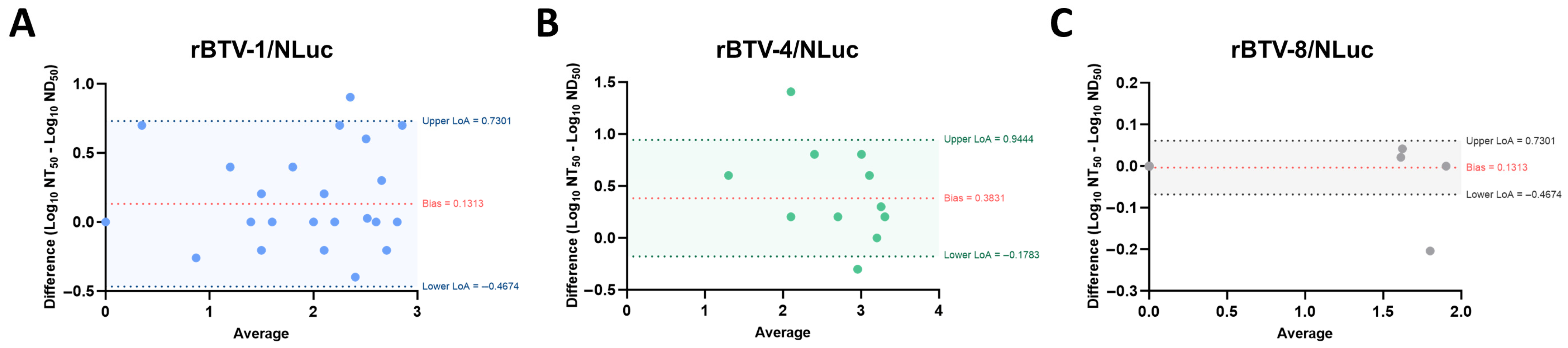
3.2. NAbs Quantification by Luminescence Activity Correlates with NAbs Titer Determined via Classic SNT
3.3. NLuc-Based Microneutralization Assay for Assessment of Antiviral Efficacy Against BTV
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erasmus, B.J. Bluetongue in Sheep and Goats. Aust. Vet. J. 1975, 51, 165–170. [Google Scholar] [CrossRef]
- Howerth, E.W.; Stallknecht, D.E.; Kirkland, P.D. Bluetongue, Epizootic Hemorrhagic Disease, and Other Orbivirus-Related Diseases. In Infectious Diseases of Wild Mammals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001; pp. 77–97. ISBN 978-0-470-34488-0. [Google Scholar]
- Du Toit, R.M. The Transmission of Blue-Tongue and Horse-Sickness by Culicoides. J. Vet. Sci. Anim. Ind. 1944, 19, 7–16. [Google Scholar]
- Kampen, H.; Werner, D. Biting Midges (Diptera: Ceratopogonidae) as Vectors of Viruses. Microorganisms 2023, 11, 2706. [Google Scholar] [CrossRef]
- Grimes, J.M.; Burroughs, J.N.; Gouet, P.; Diprose, J.M.; Malby, R.; Ziéntara, S.; Mertens, P.P.; Stuart, D.I. The Atomic Structure of the Bluetongue Virus Core. Nature 1998, 395, 470–478. [Google Scholar] [CrossRef]
- Verwoerd, D.W.; Louw, H.; Oellermann, R.A. Characterization of Bluetongue Virus Ribonucleic Acid. J. Virol. 1970, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huismans, H.; Erasmus, B.J. Identification of the Serotype-Specific and Group-Specific Antigens of Bluetongue Virus. Onderstepoort J. Vet. Res. 1981, 48, 51–58. [Google Scholar] [PubMed]
- Boyce, M.; Wehrfritz, J.; Noad, R.; Roy, P. Purified Recombinant Bluetongue Virus VP1 Exhibits RNA Replicase Activity. J. Virol. 2004, 78, 3994–4002. [Google Scholar] [CrossRef]
- Ramadevi, N.; Burroughs, N.J.; Mertens, P.P.C.; Jones, I.M.; Roy, P. Capping and Methylation of mRNA by Purified Recombinant VP4 Protein of Bluetongue Virus. Proc. Natl. Acad. Sci. USA 1998, 95, 13537–13542. [Google Scholar] [CrossRef] [PubMed]
- Stäuber, N.; Martinez-Costas, J.; Sutton, G.; Monastyrskaya, K.; Roy, P. Bluetongue Virus VP6 Protein Binds ATP and Exhibits an RNA-Dependent ATPase Function and a Helicase Activity That Catalyze the Unwinding of Double-Stranded RNA Substrates. J. Virol. 1997, 71, 7220–7226. [Google Scholar] [CrossRef]
- Ratinier, M.; Caporale, M.; Golder, M.; Franzoni, G.; Allan, K.; Nunes, S.F.; Armezzani, A.; Bayoumy, A.; Rixon, F.; Shaw, A.; et al. Identification and Characterization of a Novel Non-Structural Protein of Bluetongue Virus. PLoS Pathog. 2011, 7, e1002477. [Google Scholar] [CrossRef]
- Stewart, M.; Hardy, A.; Barry, G.; Pinto, R.M.; Caporale, M.; Melzi, E.; Hughes, J.; Taggart, A.; Janowicz, A.; Varela, M.; et al. Characterization of a Second Open Reading Frame in Genome Segment 10 of Bluetongue Virus. J. Gen. Virol. 2015, 96, 3280–3293. [Google Scholar] [CrossRef]
- Forzan, M.; Marsh, M.; Roy, P. Bluetongue Virus Entry into Cells. J. Virol. 2007, 81, 4819–4827. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Roy, P. Sialic Acid Binding Sites in VP2 of Bluetongue Virus and Their Use during Virus Entry. J. Virol. 2022, 96, e0167721. [Google Scholar] [CrossRef]
- Zhang, X.; Boyce, M.; Bhattacharya, B.; Zhang, X.; Schein, S.; Roy, P.; Zhou, Z.H. Bluetongue Virus Coat Protein VP2 Contains Sialic Acid-Binding Domains, and VP5 Resembles Enveloped Virus Fusion Proteins. Proc. Natl. Acad. Sci. USA 2010, 107, 6292–6297. [Google Scholar] [CrossRef] [PubMed]
- French, T.J.; Marshall, J.J.; Roy, P. Assembly of Double-Shelled, Viruslike Particles of Bluetongue Virus by the Simultaneous Expression of Four Structural Proteins. J. Virol. 1990, 64, 5695–5700. [Google Scholar] [CrossRef]
- Jeggo, M.H.; Wardley, R.C.; Taylor, W.P. Role of Neutralising Antibody in Passive Immunity to Bluetongue Infection. Res. Vet. Sci. 1984, 36, 81–86. [Google Scholar] [CrossRef]
- Jiménez-Cabello, L.; Utrilla-Trigo, S.; Barreiro-Piñeiro, N.; Pose-Boirazian, T.; Martínez-Costas, J.; Marín-López, A.; Ortego, J. Nanoparticle- and Microparticle-Based Vaccines against Orbiviruses of Veterinary Importance. Vaccines 2022, 10, 1124. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Samuel, A.R.; Rao, S.; Attoui, H.; Mertens, P.P.C. Analysis and Phylogenetic Comparisons of Full-Length VP2 Genes of the 24 Bluetongue Virus Serotypes. J. Gen. Virol. 2007, 88, 621–630. [Google Scholar] [CrossRef]
- Bissett, S.L.; Roy, P. Impact of VP2 Structure on Antigenicity: Comparison of BTV1 and the Highly Virulent BTV8 Serotype. J. Virol. 2024, 98, e00953-24. [Google Scholar] [CrossRef]
- Roy, P.; French, T.; Erasmus, B.J. Protective Efficacy of Virus-like Particles for Bluetongue Disease. Vaccine 1992, 10, 28–32. [Google Scholar] [CrossRef]
- Fay, P.C.; Mohd Jaafar, F.; Batten, C.; Attoui, H.; Saunders, K.; Lomonossoff, G.P.; Reid, E.; Horton, D.; Maan, S.; Haig, D.; et al. Serological Cross-Reactions between Expressed VP2 Proteins from Different Bluetongue Virus Serotypes. Viruses 2021, 13, 1455. [Google Scholar] [CrossRef]
- Schwartz-Cornil, I.; Mertens, P.P.C.; Contreras, V.; Hemati, B.; Pascale, F.; Bréard, E.; Mellor, P.S.; MacLachlan, N.J.; Zientara, S. Bluetongue Virus: Virology, Pathogenesis and Immunity. Vet. Res. 2008, 39, 46. [Google Scholar] [CrossRef]
- van Rijn, P.A. Prospects of Next-Generation Vaccines for Bluetongue. Front. Vet. Sci. 2019, 6, 407. [Google Scholar] [CrossRef]
- Savini, G.; MacLachlan, N.J.; Sánchez-Vizcaino, J.-M.; Zientara, S. Vaccines against Bluetongue in Europe. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 101–120. [Google Scholar] [CrossRef]
- Hamblin, C. Bluetongue Virus Antigen and Antibody Detection, and the Application of Laboratory Diagnostic Techniques. Vet. Ital. 2004, 40, 538–545. [Google Scholar] [PubMed]
- Haga, K.; Chen, Z.; Himeno, M.; Majima, R.; Moi, M.L. Utility of an In-Vitro Micro-Neutralizing Test in Comparison to a Plaque Reduction Neutralization Test for Dengue Virus, Japanese Encephalitis Virus, and Zika Virus Serology and Drug Screening. Pathogens 2023, 13, 8. [Google Scholar] [CrossRef]
- Zou, J.; Xia, H.; Shi, P.-Y.; Xie, X.; Ren, P. A Single-Round Infection Fluorescent SARS-CoV-2 Neutralization Test for COVID-19 Serological Testing at a Biosafety Level-2 Laboratory. Viruses 2022, 14, 1211. [Google Scholar] [CrossRef]
- Tetsuo, M.; Matsuno, K.; Tamura, T.; Fukuhara, T.; Kim, T.; Okamatsu, M.; Tautz, N.; Matsuura, Y.; Sakoda, Y. Development of a High-Throughput Serum Neutralization Test Using Recombinant Pestiviruses Possessing a Small Reporter Tag. Pathogens 2020, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, M.V.; Pulkina, A.A.; Romanovskaya-Romanko, E.A.; Mustafaeva, A.S.; Egorov, A.Y.; Stukova, M.A. Rapid Assessment of Neutralizing Antibodies Using Influenza Viruses with a Luciferase Reporter. Appl. Biochem. Microbiol. 2022, 58, 878–886. [Google Scholar] [CrossRef]
- Sanz-Muñoz, I.; Sánchez-Martínez, J.; Rodríguez-Crespo, C.; Concha-Santos, C.S.; Hernández, M.; Rojo-Rello, S.; Domínguez-Gil, M.; Mostafa, A.; Martinez-Sobrido, L.; Eiros, J.M.; et al. Are We Serologically Prepared against an Avian Influenza Pandemic and Could Seasonal Flu Vaccines Help Us? mBio 2025, 16, e0372124. [Google Scholar] [CrossRef] [PubMed]
- Nogales, A.; Alonso, C.; Moreno, S.; Lorenzo, G.; Borrego, B.; Martinez-Sobrido, L.; Brun, A. Novel Replication-Competent Reporter-Expressing Rift Valley Fever Viruses for Molecular Studies. J. Virol. 2025, 99, e0178224. [Google Scholar] [CrossRef]
- Li, L.-H.; Chiu, W.; Huang, Y.-A.; Rasulova, M.; Vercruysse, T.; Thibaut, H.J.; ter Horst, S.; Rocha-Pereira, J.; Vanhoof, G.; Borrenberghs, D.; et al. Multiplexed Multicolor Antiviral Assay Amenable for High-Throughput Research. Nat. Commun. 2024, 15, 42. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, C.; Khalil, A.M.; Mahmoud, S.H.; Sobolik, E.B.; Greninger, A.L.; Castro, E.; Jackson, N.; Bayoumi, M.; Plemper, R.K.; et al. A Luminescent Attenuated SARS-CoV-2 for the Identification and Validation of Drug-Resistant Mutants. bioRxiv 2025. [Google Scholar] [CrossRef]
- Madani, A.; Alvarez, N.; Park, S.; Murugan, M.; Perlin, D.S. Rapid Luminescence-Based Screening Method for SARS-CoV-2 Inhibitors Discovery. SLAS Discov. 2025, 31, 100211. [Google Scholar] [CrossRef]
- Diefenbacher, M.V.; Baric, T.J.; Martinez, D.R.; Baric, R.S.; Catanzaro, N.J.; Sheahan, T.P. A Nano-Luciferase Expressing Human Coronavirus OC43 for Countermeasure Development. Virus Res. 2024, 339, 199286. [Google Scholar] [CrossRef]
- Cherkashchenko, L.; Gros, N.; Trausch, A.; Neyret, A.; Hénaut, M.; Dubois, G.; Villeneuve, M.; Chable-Bessia, C.; Lyonnais, S.; Merits, A.; et al. Validation of Flavivirus Infectious Clones Carrying Fluorescent Markers for Antiviral Drug Screening and Replication Studies. Front. Microbiol. 2023, 14, 1201640. [Google Scholar] [CrossRef]
- Calvo-Pinilla, E.; Marín-López, A.; Utrilla-Trigo, S.; Jiménez-Cabello, L.; Ortego, J. Reverse Genetics Approaches: A Novel Strategy for African Horse Sickness Virus Vaccine Design. Curr. Opin. Virol. 2020, 44, 49–56. [Google Scholar] [CrossRef]
- Boyce, M.; Celma, C.C.P.; Roy, P. Development of Reverse Genetics Systems for Bluetongue Virus: Recovery of Infectious Virus from Synthetic RNA Transcripts. J. Virol. 2008, 82, 8339–8348. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, J.; Xu, Q.; Sun, E.; Li, J.; Lv, S.; Feng, Y.; Zhang, Q.; Wang, H.; Wang, H.; et al. Development of a Reverse Genetics System for Epizootic Hemorrhagic Disease Virus and Evaluation of Novel Strains Containing Duplicative Gene Rearrangements. J. Gen. Virol. 2015, 96, 2714–2720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiménez-Cabello, L.; Utrilla-Trigo, S.; Lorenzo, G.; Ortego, J.; Calvo-Pinilla, E. Epizootic Hemorrhagic Disease Virus: Current Knowledge and Emerging Perspectives. Microorganisms 2023, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Utrilla-Trigo, S.; Jiménez-Cabello, L.; Marín-López, A.; Illescas-Amo, M.; Andrés, G.; Calvo-Pinilla, E.; Lorenzo, G.; van Rijn, P.A.; Ortego, J.; Nogales, A. Engineering Recombinant Replication-Competent Bluetongue Viruses Expressing Reporter Genes for in Vitro and Non-Invasive in Vivo Studies. Microbiol. Spectr. 2024, 12, e02493-23. [Google Scholar] [CrossRef]
- Nogales, A.; Ávila-Pérez, G.; Rangel-Moreno, J.; Chiem, K.; DeDiego, M.L.; Martínez-Sobrido, L. A Novel Fluorescent and Bioluminescent Bireporter Influenza A Virus to Evaluate Viral Infections. J. Virol. 2019, 93, e00032-19. [Google Scholar] [CrossRef]
- Chiem, K.; Nogales, A.; Lorenzo, M.; Morales Vasquez, D.; Xiang, Y.; Gupta, Y.K.; Blasco, R.; de la Torre, J.C.; Martínez-Sobrido, L. Identification of In Vitro Inhibitors of Monkeypox Replication. Microbiol. Spectr. 2023, 11, e0474522. [Google Scholar] [CrossRef]
- Jiménez-Cabello, L.; Utrilla-Trigo, S.; Illescas-Amo, M.; Rodríguez-Sabando, K.; Benavides-Silván, J.; Calvo-Pinilla, E.; Ortego, J. The MVA-VP2-NS1-2A-NS2-Nt Vaccine Candidate Provides Heterologous Protection in Sheep against Bluetongue Virus. Front. Immunol. 2025, 16, 1566225. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación. Actualización de la Situación Epidemiológica de la Lengua Azul (2/11/2024); Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2024.
- Alonso, C.; Utrilla-Trigo, S.; Calvo-Pinilla, E.; Jiménez-Cabello, L.; Ortego, J.; Nogales, A. Inhibition of Orbivirus Replication by Aurintricarboxylic Acid. Int. J. Mol. Sci. 2020, 21, 7294. [Google Scholar] [CrossRef]
- Purse, B.V.; Mellor, P.S.; Rogers, D.J.; Samuel, A.R.; Mertens, P.P.C.; Baylis, M. Climate Change and the Recent Emergence of Bluetongue in Europe. Nat. Rev. Microbiol. 2005, 3, 171–181. [Google Scholar] [CrossRef] [PubMed]
- van den Brom, R.; Santman-Berends, I.; van der Heijden, M.G.; Harders, F.; Engelsma, M.; van Gennip, R.G.P.; Maris-Veldhuis, M.A.; Feddema, A.-J.; Peterson, K.; Golender, N.; et al. Bluetongue Virus Serotype 12 in Sheep and Cattle in the Netherlands in 2024—A BTV Serotype Reported in Europe for the First Time. Vet. Microbiol. 2025, 301, 110365. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.C.; Henriques, A.M.; Ramos, F.; Luís, T.; Fagulha, T.; Magalhães, A.; Caetano, I.; Abade dos Santos, F.; Correia, F.O.; Santana, C.C.; et al. Emergence of Bluetongue Virus Serotype 3 in Portugal (2024). Viruses 2024, 16, 1845. [Google Scholar] [CrossRef]
- van Gennip, R.G.P.; van de Water, S.G.P.; Potgieter, C.A.; Wright, I.M.; Veldman, D.; van Rijn, P.A. Rescue of Recent Virulent and Avirulent Field Strains of Bluetongue Virus by Reverse Genetics. PLoS ONE 2012, 7, e30540. [Google Scholar] [CrossRef]
- Matsuo, E.; Roy, P. Bluetongue Virus VP6 Acts Early in the Replication Cycle and Can Form the Basis of Chimeric Virus Formation. J. Virol. 2009, 83, 8842–8848. [Google Scholar] [CrossRef]
- van Rijn, P.A.; van de Water, S.G.P.; Feenstra, F.; van Gennip, R.G.P. Requirements and Comparative Analysis of Reverse Genetics for Bluetongue Virus (BTV) and African Horse Sickness Virus (AHSV). Virol. J. 2016, 13, 119. [Google Scholar] [CrossRef]
- Pretorius, J.M.; Huismans, H.; Theron, J. Establishment of an Entirely Plasmid-Based Reverse Genetics System for Bluetongue Virus. Virology 2015, 486, 71–77. [Google Scholar] [CrossRef]
- Xu, Q.; Ge, J.; Li, M.; Sun, E.; Zhou, Y.; Guo, Y.; Wu, D.; Bu, Z. PCR-Based Reverse Genetics Strategy for Bluetongue Virus Recovery. Virol. J. 2019, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- van Gennip, R.G.P.; van de Water, S.G.P.; Maris-Veldhuis, M.; van Rijn, P.A. Bluetongue Viruses Based on Modified-Live Vaccine Serotype 6 with Exchanged Outer Shell Proteins Confer Full Protection in Sheep against Virulent BTV8. PLoS ONE 2012, 7, e44619. [Google Scholar] [CrossRef][Green Version]
- Hund, A.; Gollnick, N.; Sauter-Louis, C.; Neubauer-Juric, A.; Lahm, H.; Büttner, M. A Two Year BTV-8 Vaccination Follow up: Molecular Diagnostics and Assessment of Humoral and Cellular Immune Reactions. Vet. Microbiol. 2012, 154, 247–256. [Google Scholar] [CrossRef]
- Martinelle, L.; Dal Pozzo, F.; Thys, C.; De Leeuw, I.; Van Campe, W.; De Clercq, K.; Thiry, E.; Saegerman, C. Assessment of Cross-Protection Induced by a Bluetongue Virus (BTV) Serotype 8 Vaccine towards Other BTV Serotypes in Experimental Conditions. Vet. Res. 2018, 49, 63. [Google Scholar] [CrossRef]
- Mahapatra, C.S.; Sharma, P.; Biswas, S.K.; Chand, K. Development of ELISA for the Detection of Antibodies against VP2 Protein of Bluetongue Virus Serotype-1. J. Immunol. Methods 2022, 511, 113386. [Google Scholar] [CrossRef]
- Bréard, E.; Turpaud, M.; Beaud, G.; Postic, L.; Fablet, A.; Beer, M.; Sailleau, C.; Caignard, G.; Viarouge, C.; Hoffmann, B.; et al. Development and Validation of an ELISA for the Detection of Bluetongue Virus Serotype 4-Specific Antibodies. Viruses 2021, 13, 1741. [Google Scholar] [CrossRef]
- Anderson, J.; Hägglund, S.; Bréard, E.; Riou, M.; Zohari, S.; Comtet, L.; Olofson, A.-S.; Gélineau, R.; Martin, G.; Elvander, M.; et al. Strong Protection Induced by an Experimental DIVA Subunit Vaccine against Bluetongue Virus Serotype 8 in Cattle. Vaccine 2014, 32, 6614–6621. [Google Scholar] [CrossRef]
- Matsuo, E.; Celma, C.C.P.; Boyce, M.; Viarouge, C.; Sailleau, C.; Dubois, E.; Bréard, E.; Thiéry, R.; Zientara, S.; Roy, P. Generation of Replication-Defective Virus-Based Vaccines That Confer Full Protection in Sheep against Virulent Bluetongue Virus Challenge. J. Virol. 2011, 85, 10213–10221. [Google Scholar] [CrossRef]
- Jiménez-Cabello, L.; Utrilla-Trigo, S.; Calvo-Pinilla, E.; Lorenzo, G.; Illescas-Amo, M.; Benavides, J.; Moreno, S.; Marín-López, A.; Nogales, A.; Ortego, J. Co-Expression of VP2, NS1 and NS2-Nt Proteins by an MVA Viral Vector Induces Complete Protection against Bluetongue Virus. Front. Immunol. 2024, 15, 1440407. [Google Scholar] [CrossRef]
- Mohd Jaafar, F.; Monsion, B.; Belhouchet, M.; Mertens, P.P.C.; Attoui, H. Inhibition of Orbivirus Replication by Fluvastatin and Identification of the Key Elements of the Mevalonate Pathway Involved. Viruses 2021, 13, 1437. [Google Scholar] [CrossRef]
- John, L.; Vernersson, C.; Kwon, H.; Elling, U.; Penninger, J.M.; Mirazimi, A. Redirecting Imipramine against Bluetongue Virus Infection: Insights from a Genome-Wide Haploid Screening Study. Pathogens 2022, 11, 602. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, W.; Huang, R. High-throughput Screening Assays for SARS-CoV-2 Drug Development: Current Status and Future Directions. Drug Discov. Today 2021, 26, 2439–2444. [Google Scholar] [CrossRef]
- Li, Q.; Maddox, C.; Rasmussen, L.; Hobrath, J.V.; White, L.E. Assay Development and High-Throughput Antiviral Drug Screening against Bluetongue Virus. Antivir. Res. 2009, 83, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, K.; Chen, C.Z.; Xu, M.; Carlos de la Torre, J.; Martinez-Sobrido, L.; Moran, T.; Zheng, W. Development of a High-Throughput Homogeneous AlphaLISA Drug Screening Assay for the Detection of SARS-CoV-2 Nucleocapsid. ACS Pharmacol. Transl. Sci. 2020, 3, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.-F.; Yu, S.; Wang, X.; Zhang, L.; Yu, J.; Xie, L.; Li, W.; Ali, R.; Qiu, H.-J. Applications of Replicating-Competent Reporter-Expressing Viruses in Diagnostic and Molecular Virology. Viruses 2016, 8, 127. [Google Scholar] [CrossRef]
- Davies, K.A.; Welch, S.R.; Jain, S.; Sorvillo, T.E.; Coleman-McCray, J.D.; Montgomery, J.M.; Spiropoulou, C.F.; Albariño, C.; Spengler, J.R. Fluorescent and Bioluminescent Reporter Mouse-Adapted Ebola Viruses Maintain Pathogenicity and Can Be Visualized In Vivo. J. Infect. Dis. 2023, 228, S536–S547. [Google Scholar] [CrossRef]
- Nogales, A.; Baker, S.F.; Martínez-Sobrido, L. Replication-Competent Influenza A Viruses Expressing a Red Fluorescent Protein. Virology 2015, 476, 206–216. [Google Scholar] [CrossRef]
- Shaw, A.E.; Veronesi, E.; Maurin, G.; Ftaich, N.; Guiguen, F.; Rixon, F.; Ratinier, M.; Mertens, P.; Carpenter, S.; Palmarini, M.; et al. Drosophila Melanogaster as a Model Organism for Bluetongue Virus Replication and Tropism. J. Virol. 2012, 86, 9015–9024. [Google Scholar] [CrossRef]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, E.; Saeki, K.; Roy, P.; Kawano, J. Development of Reverse Genetics for Ibaraki Virus to Produce Viable VP6-Tagged IBAV. FEBS Open Bio 2015, 5, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pretorius, J.M.; Xu, Q.; Wu, D.; Bu, Z.; Theron, J.; Sun, E. Development and Optimization of a DNA-Based Reverse Genetics Systems for Epizootic Hemorrhagic Disease Virus. Arch. Virol. 2020, 165, 1079–1087. [Google Scholar] [CrossRef]


| Sheep ID | BTV-1 (ND50) | rBTV-1/NLuc (NT50) | Sheep ID | BTV-1 (ND50) | rBTV-1/NLuc (NT50) |
|---|---|---|---|---|---|
| 1 | 100 | 100 | 22 | 320 | 340 |
| 2 | 320 | 640 | 23 | 160 | 160 |
| 3 | 100 | 160 | 24 | 100 | 100 |
| 4 | 320 | 1600 | 25 | 25 | 25 |
| 5 | 25 | 40 | 26 | 25 | 25 |
| 6 | 100 | 100 | 27 | 25 | 25 |
| 7 | 400 | 160 | 28 | 40 | 25 |
| 8 | 160 | 100 | 29 | 100 | 100 |
| 9 | 80 | 640 | 30 | 100 | 100 |
| 10 | 640 | 400 | 31 | 10 | 25 |
| 11 | 320 | 640 | 32 | 25 | 40 |
| 12 | 640 | 400 | 33 | 0 | 0 |
| 13 | 320 | 640 | 34 | 10 | 25 |
| 14 | 160 | 640 | 35 | 0 | 5.5 |
| 15 | 80 | 400 | 36 | 0 | 0 |
| 16 | 160 | 160 | 37 | 0 | 5.5 |
| 17 | 640 | 640 | 38 | 320 | 340 |
| 18 | 40 | 100 | 39 | 40 | 40 |
| 19 | 40 | 40 | 40 | 10 | 5.5 |
| 20 | 400 | 400 | 41 | 0 | 0 |
| 21 | 640 | 400 |
| Sheep ID | BTV-4 (ND50) | rBTV-4/NLuc (NT50) | Sheep ID | BTV-4 (ND50) | rBTV-4/NLuc (NT50) |
|---|---|---|---|---|---|
| 1 | 1600 | 1600 | 22 | 400 | 2560 |
| 2 | 1280 | 2560 | 23 | 1600 | 2560 |
| 3 | 1280 | 2560 | 24 | 1600 | 2560 |
| 4 | 400 | 640 | 25 | 100 | 640 |
| 5 | 400 | 640 | 26 | 400 | 2560 |
| 6 | 640 | 2560 | 27 | 400 | 640 |
| 7 | 400 | 2560 | 28 | 640 | 2560 |
| 8 | 400 | 640 | 29 | 400 | 2560 |
| 9 | 400 | 640 | 30 | 400 | 2560 |
| 10 | 1280 | 2560 | 31 | 100 | 160 |
| 11 | 1280 | 2560 | 32 | 10 | 40 |
| 12 | 1280 | 2560 | 33 | 1280 | 2560 |
| 13 | 1280 | 2560 | 34 | 1280 | 2560 |
| 14 | 1280 | 2560 | 35 | 1280 | 2560 |
| 15 | 1280 | 2560 | 36 | 1280 | 2560 |
| 16 | 1280 | 2560 | 37 | 1280 | 2560 |
| 17 | 1280 | 2560 | 38 | 1280 | 2560 |
| 18 | 1280 | 2560 | 39 | 1280 | 2560 |
| 19 | 1280 | 640 | 40 | 1280 | 2560 |
| 20 | 25 | 640 | 41 | 1280 | 2560 |
| 21 | 1280 | 2560 |
| Sheep ID | BTV-8 (ND50) | rBTV-8/NLuc (NT50) | Sheep ID | BTV-8 (ND50) | rBTV-8/NLuc (NT50) |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 22 | 0 | 0 |
| 2 | 0 | 0 | 23 | 0 | 0 |
| 3 | 0 | 0 | 24 | 0 | 0 |
| 4 | 0 | 0 | 25 | 0 | 0 |
| 5 | 0 | 0 | 26 | 0 | 0 |
| 6 | 0 | 0 | 27 | 0 | 0 |
| 7 | 0 | 0 | 28 | 80 | 50 |
| 8 | 0 | 0 | 29 | 40 | 42 |
| 9 | 0 | 0 | 30 | 80 | 80 |
| 10 | 0 | 0 | 31 | 40 | 44 |
| 11 | 0 | 0 | 32 | 0 | 0 |
| 12 | 0 | 0 | 33 | 0 | 0 |
| 13 | 0 | 0 | 34 | 0 | 0 |
| 14 | 0 | 0 | 35 | 0 | 0 |
| 15 | 0 | 0 | 36 | 0 | 0 |
| 16 | 0 | 0 | 37 | 0 | 0 |
| 17 | 0 | 0 | 38 | 0 | 0 |
| 18 | 0 | 0 | 39 | 0 | 0 |
| 19 | 0 | 0 | 40 | 0 | 0 |
| 20 | 0 | 0 | 41 | 0 | 0 |
| 21 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Cabello, L.; Utrilla-Trigo, S.; Calvo-Pinilla, E.; Nogales, A.; Ortego, J. Rapid Quantification of Bluetongue Virus-Neutralizing Antibodies Using Bioluminescent Reporter-Expressing Viruses. Vaccines 2025, 13, 1102. https://doi.org/10.3390/vaccines13111102
Jiménez-Cabello L, Utrilla-Trigo S, Calvo-Pinilla E, Nogales A, Ortego J. Rapid Quantification of Bluetongue Virus-Neutralizing Antibodies Using Bioluminescent Reporter-Expressing Viruses. Vaccines. 2025; 13(11):1102. https://doi.org/10.3390/vaccines13111102
Chicago/Turabian StyleJiménez-Cabello, Luis, Sergio Utrilla-Trigo, Eva Calvo-Pinilla, Aitor Nogales, and Javier Ortego. 2025. "Rapid Quantification of Bluetongue Virus-Neutralizing Antibodies Using Bioluminescent Reporter-Expressing Viruses" Vaccines 13, no. 11: 1102. https://doi.org/10.3390/vaccines13111102
APA StyleJiménez-Cabello, L., Utrilla-Trigo, S., Calvo-Pinilla, E., Nogales, A., & Ortego, J. (2025). Rapid Quantification of Bluetongue Virus-Neutralizing Antibodies Using Bioluminescent Reporter-Expressing Viruses. Vaccines, 13(11), 1102. https://doi.org/10.3390/vaccines13111102






