Serological Response After the Fourth Dose of COVID-19 Vaccine in Highly Immunosuppressed Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HLI | high-level immunosuppression |
| HSCT | hematopoietic stem cell transplant |
| HIR | humoral immune response |
References
- Interim Public Health Considerations for the Provision of Additional COVID-19 Vaccine Doses. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-public-health-considerations-additional-vaccine-doses (accessed on 1 July 2025).
- Tian, Y.; Qiu, X.; Wang, C.; Zhao, J.; Jiang, X.; Niu, W.; Huang, J.; Zhang, F. Cancer associates with risk and severe events of COVID-19: A systematic review and meta-analysis. Int. J. Cancer 2021, 148, 363–374. [Google Scholar] [CrossRef]
- Ministerio de Sanidad—Áreas—Documentos Técnicos Para Profesionales—Coronavirus. Available online: https://www.sanidad.gob.es/areas/alertasEmergenciasSanitarias/alertasActuales/nCov/documentos.htm (accessed on 12 August 2025).
- Caillard, S.; Thaunat, O.; Benotmane, I.; Masset, C.; Blancho, G. Antibody Response to a Fourth Messenger RNA COVID-19 Vaccine Dose in Kidney Transplant Recipients: A Case Series. Ann. Intern. Med. 2022, 175, 455–456. [Google Scholar] [CrossRef]
- Por qué no es Necesario un Test Para Confirmar la Inmunidad Después de la Vacuna. 2021. Available online: https://www.vacunacovid.gob.es/voces-expertas/por-que-no-es-necesario-un-test-despues-de-la-vacuna (accessed on 18 August 2021).
- Kamar, N.; Abravanel, F.; Marion, O.; Romieu-Mourez, R.; Couat, C.; Del Bello, A.; Izopet, J. Assessment of 4 Doses of SARS-CoV-2 Messenger RNA–Based Vaccine in Recipients of a Solid Organ Transplant. JAMA Netw. Open 2021, 4, e2136030. [Google Scholar] [CrossRef]
- Galmiche, S.; Luong Nguyen, L.B.; Tartour, E.; De Lamballerie, X.; Wittkop, L.; Loubet, P.; Launay, O. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: A systematic review. Clin. Microbiol. Infect. 2022, 28, 163–177. [Google Scholar] [CrossRef]
- Duni, A.; Markopoulos, G.S.; Mallioras, I.; Pappas, H.; Pappas, E.; Koutlas, V.; Tzalavra, E.; Baxevanos, G.; Priska, S.; Gartzonika, K.; et al. The Humoral Immune Response to BNT162b2 Vaccine Is Associated With Circulating CD19+ B Lymphocytes and the Naïve CD45RA to Memory CD45RO CD4+ T Helper Cells Ratio in Hemodialysis Patients and Kidney Transplant Recipients. Front. Immunol. 2021, 12, 760249. [Google Scholar] [CrossRef]
- Caillard, S.; Chavarot, N.; Francois, H.; Matignon, M.; Greze, C.; Kamar, N.; Gatault, P.; Thaunat, O.; Legris, T.; Frimat, L.; et al. Is COVID-19 infection more severe in kidney transplant recipients? Am. J. Transplant. 2021, 21, 1295–1303. [Google Scholar] [CrossRef]
- Cortés, A.; Casado, J.L.; Longo, F.; Serrano, J.J.; Saavedra, C.; Velasco, H.; Martin, A.; Chamorro, J.; Rosero, D.; Fernández, M.; et al. Limited T cell response to SARS-CoV-2 mRNA vaccine among patients with cancer receiving different cancer treatments. Eur. J. Cancer 2022, 166, 229–239. [Google Scholar] [CrossRef]
- H-Vázquez, J.; Cal-Sabater, P.; Arribas-Rodríguez, E.; Fiz-López, A.; Perez-Segurado, C.; Martín-Muñoz, Á.; De Prado, Á.; Perez Mazzali, M.; De Castro, C.G.; Del Hierro, A.G.; et al. Unbiased immunome characterisation correlates with COVID-19 mRNA vaccine failure in immunocompromised adults. Front. Immunol. 2024, 15, 1405217. [Google Scholar] [CrossRef]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Stein, N.; Saliba, W. Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score–Matched Analysis. Clin. Infect. Dis. 2023, 76, 1067–1073. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Esposito, G.L.; Fassio, F.; Girardi, D.; Picasso, E.; Meloni, F.; Montini, S.; Codullo, V.; Pattonieri, E.F.; Defrancesco, I.; Bianchessi, A.; et al. “REAl LIfe” observational study on the effectiveness of Evusheld prophylaxis against SARS-CoV-2 omicron variants in vaccine non-responder immunocompromised patients (REALISE). Vaccine 2024, 42, 126208. [Google Scholar] [CrossRef]
- Parker, E.P.K.; Desai, S.; Marti, M.; Nohynek, H.; Kaslow, D.C.; Kochhar, S.; O’Brien, K.L.; Hombach, J.; Wilder-Smith, A. Response to additional COVID-19 vaccine doses in people who are immunocompromised: A rapid review. Lancet Glob. Health 2022, 10, e326–e328. [Google Scholar] [CrossRef]
- Medeiros Figueiredo, A.; Daponte-Codina, A.; Moreira Marculino Figueiredo, D.C.; Toledo Vianna, R.P.; Costa De Lima, K.; Gil-García, E. Factores asociados a la incidencia y la mortalidad por COVID-19 en las comunidades autónomas. Gac. Sanit. 2021, 35, 445–452. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Martino, G.; Gangemi, S. Impact of Immunosenescence on Viral Infections with an Emphasis on COVID-19. Front. Biosci.-Landmark 2023, 28, 225. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef]
- Desai, M.K.; Brinton, R.D. Autoimmune Disease in Women: Endocrine Transition and Risk Across the Lifespan. Front. Endocrinol. 2019, 10, 265. [Google Scholar] [CrossRef]
- Juarez, I.; Pérez-Flores, I.; Aiffil Meneses, A.S.; Lopez-Gomez, A.; Calvo Romero, N.; Rodríguez-Cubillo, B.; Moreno de la Higuera, M.A.; Peix-Jiménez, B.; Gonzalez-Garcia, R.; Amorós-Pérez, B.; et al. Immunosuppressive Therapy Modifies Anti-Spike IgG Subclasses Distribution After Four Doses of mRNA Vaccination in a Cohort of Kidney Transplant Recipients. Vaccines 2025, 13, 123. [Google Scholar] [CrossRef]
- Thomson, T.; Prendecki, M.; Gleeson, S.; Martin, P.; Spensley, K.; De Aguiar, R.C.; Sandhu, B.; Seneschall, C.; Gan, J.; Clarke, C.L.; et al. Immune responses following 3rd and 4th doses of heterologous and homologous COVID-19 vaccines in kidney transplant recipients. eClinicalMedicine 2022, 53, 101642. [Google Scholar] [CrossRef]
- Gleeson, S.; Martin, P.; Thomson, T.; Spensley, K.J.; Goodall, D.; Bedi, R.; Thind, A.K.; Seneschall, C.; Gan, J.; McAdoo, S.; et al. Lack of seroresponse to SARS-CoV-2 booster vaccines given early post-transplant in patients primed pre-transplantation. Front. Immunol. 2023, 13, 1083167. [Google Scholar] [CrossRef]
- Mu, Y.; Wu, H.; Jiang, Z.; Liu, K.; Xue, X.; Zhang, W.; Chen, Z. Serological Responses after a Fourth Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 1130. [Google Scholar] [CrossRef]
- Ward, H.; Whitaker, M.; Flower, B.; Tang, S.N.; Atchison, C.; Darzi, A.; Donnelly, C.A.; Cann, A.; Diggle, P.J.; Ashby, D.; et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat. Commun. 2022, 13, 907. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef]
- Sparks, J.A.; Wallace, Z.S.; Seet, A.M.; Gianfrancesco, M.A.; Izadi, Z.; Hyrich, K.L.; Strangfeld, A.; Gossec, L.; Carmona, L.; Mateus, E.F.; et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: Results from the COVID-19 Global Rheumatology Alliance physician registry. Ann. Rheum. Dis. 2021, 80, 1137–1146. [Google Scholar] [CrossRef]
- Gurion, R.; Rozovski, U.; Itchaki, G.; Gafter-Gvili, A.; Leibovitch, C.; Raanani, P.; Ben-Zvi, H.; Szwarcwort, M.; Taylor-Abigadol, M.; Dann, E.J.; et al. Humoral serological response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica 2021, 107, 715–720. [Google Scholar] [CrossRef]
- Tvito, A.; Ronson, A.; Ghosheh, R.; Kharit, M.; Ashkenazi, J.; Magen, S.; Broide, E.; Benayoun, E.; Rowe, J.M.; Ofran, Y.; et al. Anti-CD20 monoclonal antibodies inhibit seropositive response to COVID-19 vaccination in non-Hodgkin lymphoma patients within 6 months after treatment. Exp. Hematol. 2022, 107, 20–23. [Google Scholar] [CrossRef]
- Martinelli, S.; Pascucci, D.; Laurenti, P. Humoral response after a fourth dose of SARS-CoV-2 vaccine in immunocompromised patients. Results of a systematic review. Front. Public Health 2023, 11, 1108546. [Google Scholar] [CrossRef]
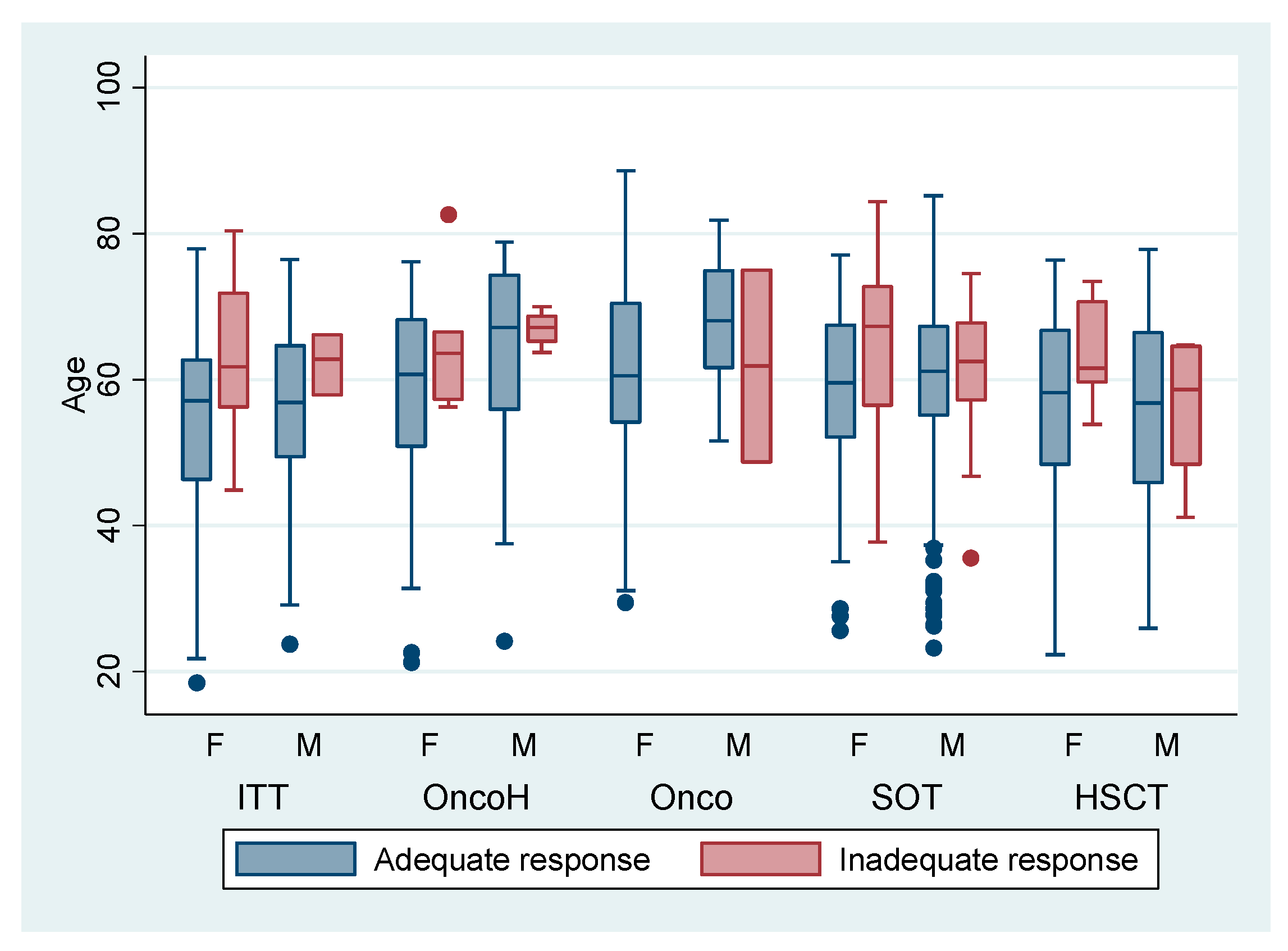
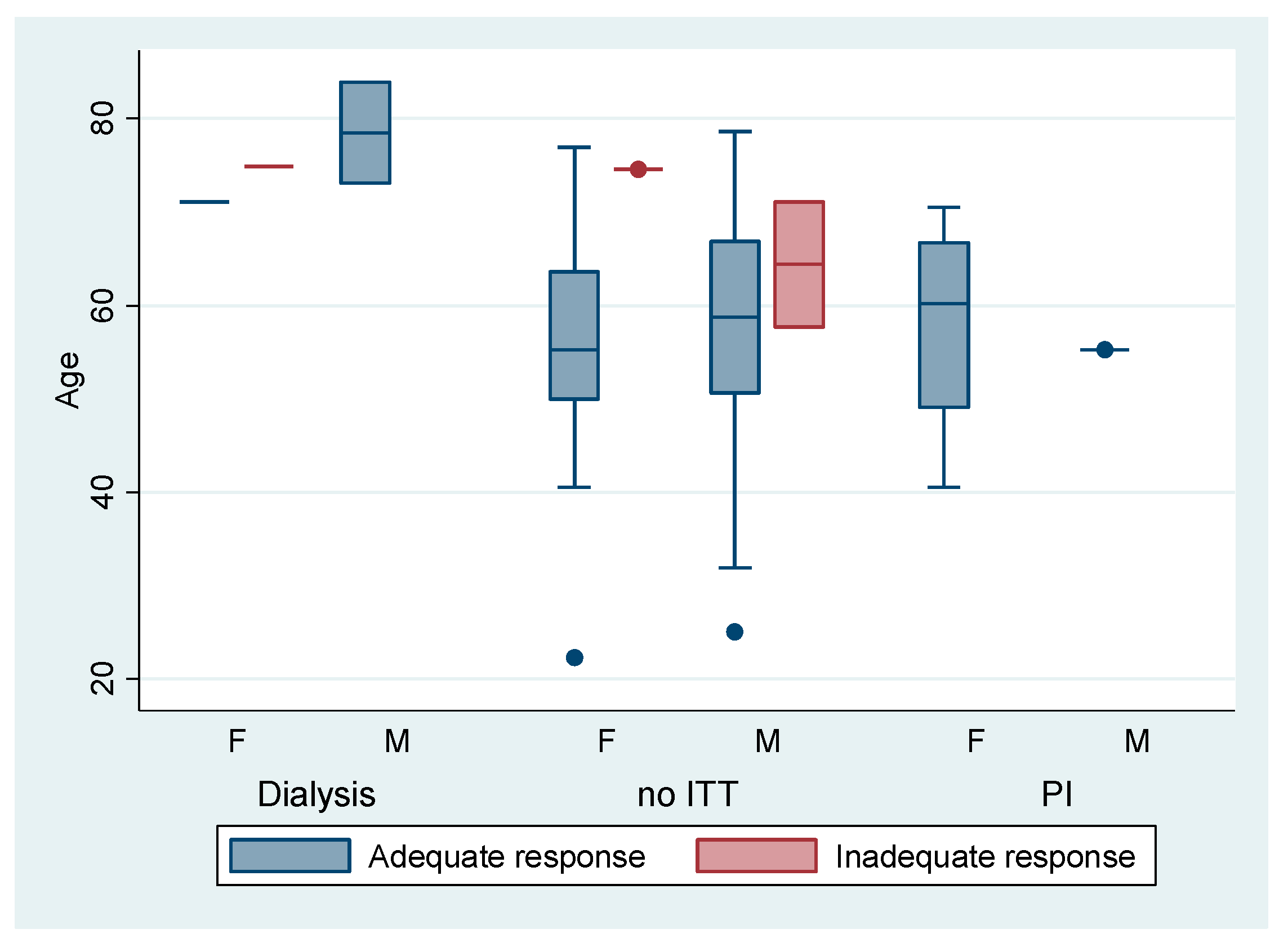
| Variable | Adequate HIR Anti-S BAU/mL ≥260 (n = 846) | Inadequate HIR Anti-S BAU/mL <260 (n = 97) |
|---|---|---|
| Age groups | ||
| 18/39 | 64 (7.57%) | 2 (2.06%) |
| 40/59 | 347 (41.02%) | 30 (30.93%) |
| 60/74 | 371 (43.85%) | 57 (58.76%) |
| ≥75 | 64 (7.57%) | 8 (8.25%) |
| Sex | ||
| Female | 323 (323/370) | 47 (47/370) |
| Male | 523 (523/573) | 50 (50/573) |
| Level of immunosuppression | ||
| Without high-level | 58 (6.86%) | 4 (4.12%) |
| High-level | 788 (93.14%) | 93 (95.88%) |
| Clinical conditions | ||
| SOT 1 | 392 (46.34%) | 54 (55.67%) |
| Immuno-targeted therapy | 110 (13.00%) | 17 (17.53%) |
| HSCT 2 | 108 (12.77%) | 10 (10.31%) |
| Hematologic Malignancies | 105 (12.41%) | 10 (10.31%) |
| Oncological | 73 (8.93%) | 2 (2.06%) |
| Non-immuno-targeted therapy | 28 (3.31%) | 3 (3.09%) |
| Dialysis | 3 (0.35%) | 1 (1.03%) |
| HIV | 22 (2.60%) | 0 (0.00%) |
| Primary immunodeficiencies | 5 (0.59%) | 0 (0.00%) |
| Fourth dose–serology gap 3 | 18 (RI 16–26) | 18 (RI 16–23) |
| Variable | Unadjusted OR (95% CI) | p | Adjusted OR (95% CI) | p |
|---|---|---|---|---|
| High level of immunosuppression | 1.711 (0.612–6.634) | 0.303 | 1.705 (0.600–4.845) | 0.316 |
| Age 40–59 years | 0.643 (0.411–1.008) | 0.054 | 2.924 (0.680–12.575) | 0.149 |
| Age 60–74 years | 1.824 (1.193–2.788) | 0.005 | 5.289 (1.255–22.293) | 0.023 |
| Age ≥ 75 years | 1.098 (0.518–2.329) | 0.810 | 4.236 (0.862–20.809) | 0.075 |
| Sex | 0.657 (0.421–1.025) | 0.049 | 0.611 (0.399–0.936) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández Chávez, A.C.; Navarro López, P.; De Andrés Martín, A.; Sánchez Carmona, D.L.; Ordoñez León, G.Y.; Aranaz Andrés, J.M. Serological Response After the Fourth Dose of COVID-19 Vaccine in Highly Immunosuppressed Patients. Vaccines 2025, 13, 994. https://doi.org/10.3390/vaccines13100994
Fernández Chávez AC, Navarro López P, De Andrés Martín A, Sánchez Carmona DL, Ordoñez León GY, Aranaz Andrés JM. Serological Response After the Fourth Dose of COVID-19 Vaccine in Highly Immunosuppressed Patients. Vaccines. 2025; 13(10):994. https://doi.org/10.3390/vaccines13100994
Chicago/Turabian StyleFernández Chávez, Abelardo Claudio, Paula Navarro López, Ana De Andrés Martín, Daniel Leonardo Sánchez Carmona, Guillermo Yovany Ordoñez León, and Jesús María Aranaz Andrés. 2025. "Serological Response After the Fourth Dose of COVID-19 Vaccine in Highly Immunosuppressed Patients" Vaccines 13, no. 10: 994. https://doi.org/10.3390/vaccines13100994
APA StyleFernández Chávez, A. C., Navarro López, P., De Andrés Martín, A., Sánchez Carmona, D. L., Ordoñez León, G. Y., & Aranaz Andrés, J. M. (2025). Serological Response After the Fourth Dose of COVID-19 Vaccine in Highly Immunosuppressed Patients. Vaccines, 13(10), 994. https://doi.org/10.3390/vaccines13100994






