Efficacy Evaluation of an E2 Subunit Vaccine Against Highly Virulent Classical Swine Fever Virus Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Recombinant CSFV E2 Antigen Subsection
2.2. Vaccine Preparation
2.3. Animal Trials
2.4. Examinations of E2-CpG Vaccines Vaccination Effectiveness
2.5. Statistical Analysis
3. Results
3.1. Development of E2-Specific Antibodies in Animal Trial I
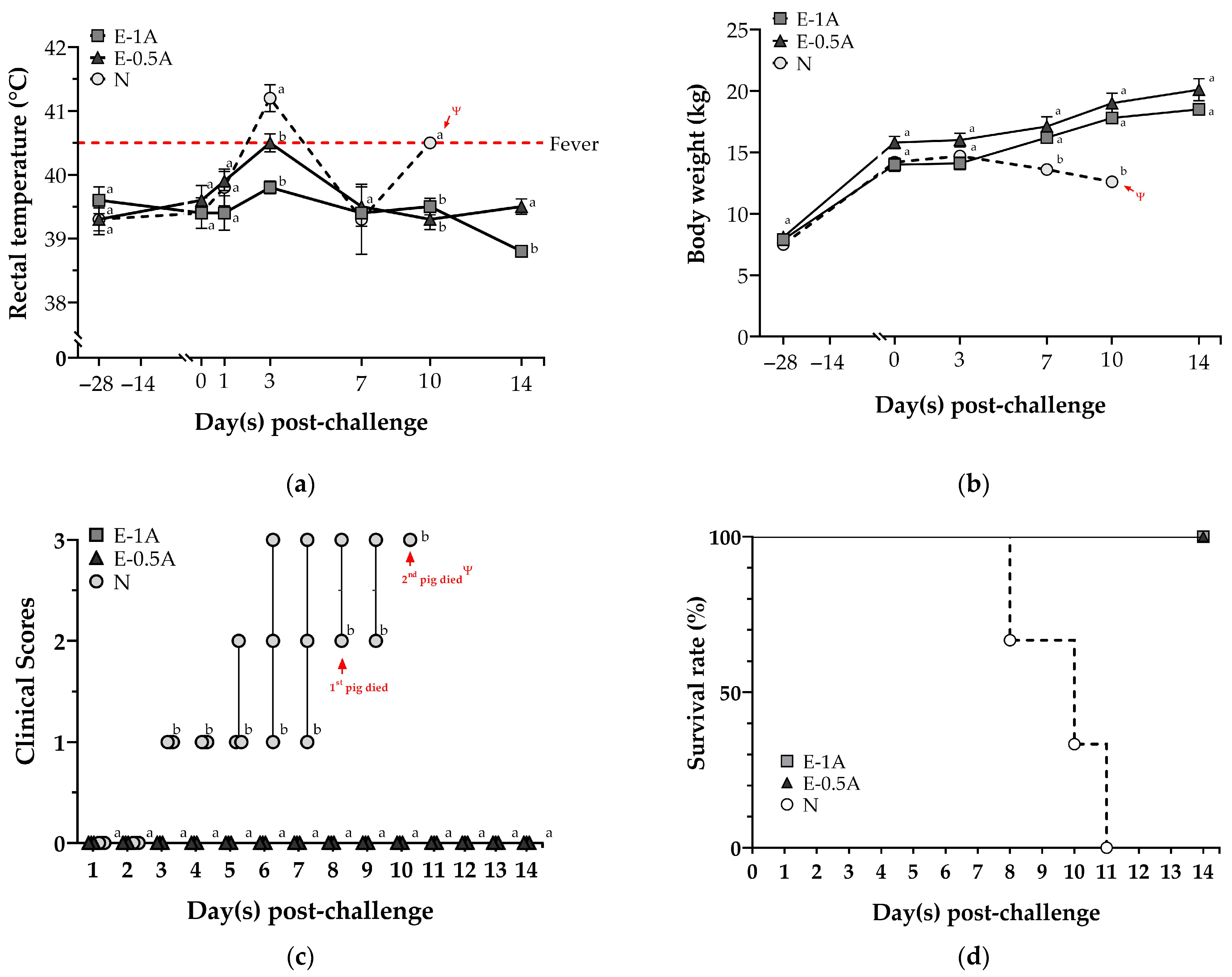
3.2. Assessment of the Clinical Signs and Mortality After CSFV Challenge in Animal Trial II
3.3. Protection of the Pigs with E2-CpG Vaccination from Highly Virulent CSFV Challenge
3.4. Histopathological Analysis of Evaluated Vaccine Protection Against CSFV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSF | Classical swine fever |
| CSFV | Classical swine fever virus |
| SPF | Specific-pathogen-free |
| WOAH | World Organisation for Animal Health |
| DIVA | Differentiate between infected and vaccinated animals |
| AHRI | Veterinary Research Institute, Ministry of Agriculture (Miaoli, Taiwan) |
| dpc | Days post-challenge |
| mAbs | Monoclonal antibodies |
| ABSLs | Animal Biosafety Levels |
| ELISA | Enzyme-linked immunosorbent assay |
| H&E | Hematoxylin and eosin |
| SDS-PAGE | Sodium dodecylsulfate-polyacrylamide gel electrophoresis |
| β-ME | β-mercaptoethanol |
| FAID50 | 50% Fluorescent antibody infective dose |
| IACUC | Institutional Animal Care and Use Committee |
References
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever-An Updated Review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef]
- Edwards, S.; Fukusho, A.; Lefevre, P.C.; Lipowski, A.; Pejsak, Z.; Roehe, P.; Westergaard, J. Classical swine fever: The global situation. Vet. Microbiol. 2000, 73, 103–119. [Google Scholar] [CrossRef]
- Lowings, P.; Ibata, G.; Needham, J.; Paton, D. Classical swine fever virus diversity and evolution. J. Gen. Virol. 1996, 77, 1311–1321. [Google Scholar] [CrossRef]
- Paton, D.J.; McGoldrick, A.; Greiser-Wilke, I.; Parchariyanon, S.; Song, J.Y.; Liou, P.P.; Stadejek, T.; Lowings, J.P.; Bjorklund, H.; Belak, S. Genetic typing of classical swine fever virus. Vet. Microbiol. 2000, 73, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Schmeiser, S.; Bernau, J.; Meindl-Boehmer, A.; Pridotkas, G.; Dirbakova, Z.; Mojzis, M.; Becher, P. Improved strategy for phylogenetic analysis of classical swine fever virus based on full-length E2 encoding sequences. Vet. Res. 2012, 43, 50. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Nishi, T.; Kameyama, K.I.; Meyer, D.; Suckstorff, O.; Fukai, K.; Becher, P. Reemergence of Classical Swine Fever, Japan, 2018. Emerg. Infect. Dis. 2019, 25, 1228–1231. [Google Scholar] [CrossRef]
- Postel, A.; Austermann-Busch, S.; Petrov, A.; Moennig, V.; Becher, P. Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 248–261. [Google Scholar] [CrossRef]
- Coronado, L.; Perera, C.L.; Rios, L.; Frias, M.T.; Perez, L.J. A Critical Review about Different Vaccines against Classical Swine Fever Virus and Their Repercussions in Endemic Regions. Vaccines 2021, 9, 154. [Google Scholar] [CrossRef]
- Narita, M.; Kawashima, K.; Kimura, K.; Mikami, O.; Shibahara, T.; Yamada, S.; Sakoda, Y. Comparative immunohistopathology in pigs infected with highly virulent or less virulent strains of hog cholera virus. Vet. Pathol. 2000, 37, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Leifer, I.; Hoeper, D.; Blome, S.; Beer, M.; Ruggli, N. Clustering of classical swine fever virus isolates by codon pair bias. BMC Res. Notes. 2011, 4, 521. [Google Scholar] [CrossRef]
- Hoffmann, B.; Beer, M.; Schelp, C.; Schirrmeier, H.; Depner, K. Validation of a real-time RT-PCR assay for sensitive and specific detection of classical swine fever. J. Virol. Methods 2005, 130, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Mittelholzer, C.; Moser, C.; Tratschin, J.D.; Hofmann, M.A. Analysis of classical swine fever virus replication kinetics allows differentiation of highly virulent from avirulent strains. Vet. Microbiol. 2000, 74, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Floegel-Niesmann, G.; Bunzenthal, C.; Fischer, S.; Moennig, V.; Kaaden, O. Virulence of recent and former classical swine fever virus isolates evaluated by their clinical and pathological signs. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 214–220. [Google Scholar] [CrossRef]
- Malswamkima, D.; Rajkhowa, T.K.; Chandra, R.; Dutta, T.K. Pathology and molecular diagnosis of classical swine fever in Mizoram. Vet. World 2015, 8, 76–81. [Google Scholar] [CrossRef][Green Version]
- de Smit, A.J.; Bouma, A.; de Kluijver, E.P.; Terpstra, C.; Moormann, R.J. Duration of the protection of an E2 subunit marker vaccine against classical swine fever after a single vaccination. Vet. Microbiol. 2001, 78, 307–317. [Google Scholar] [CrossRef]
- Huang, Y.L.; Deng, M.C.; Wang, F.I.; Huang, C.C.; Chang, C.Y. The challenges of classical swine fever control: Modified live and E2 subunit vaccines. Virus Res. 2014, 179, 1–11. [Google Scholar] [CrossRef]
- Suradhat, S.; Damrongwatanapokin, S.; Thanawongnuwech, R. Factors critical for successful vaccination against classical swine fever in endemic areas. Vet. Microbiol. 2007, 119, 1–9. [Google Scholar] [CrossRef]
- Moormann, R.J.; Bouma, A.; Kramps, J.A.; Terpstra, C.; De Smit, H.J. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet. Microbiol. 2000, 73, 209–219. [Google Scholar] [CrossRef]
- Bouma, A.; de Smit, A.J.; de Kluijver, E.P.; Terpstra, C.; Moormann, R.J. Efficacy and stability of a subunit vaccine based on glycoprotein E2 of classical swine fever virus. Vet. Microbiol. 1999, 66, 101–114. [Google Scholar] [CrossRef]
- Li, F.; Li, B.; Niu, X.; Chen, W.; Li, Y.; Wu, K.; Li, X.; Ding, H.; Zhao, M.; Chen, J.; et al. The Development of Classical Swine Fever Marker Vaccines in Recent Years. Vaccines 2022, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Hayama, Y.; Murato, Y.; Sawai, K.; Yamaguchi, E.; Yamamoto, T. Epidemiological analysis of classical swine fever in wild boars in Japan. BMC Vet. Res. 2021, 17, 188. [Google Scholar] [CrossRef]
- Jang, G.; Kim, E.J.; Cho, S.C.; Moon, S.U.; Kim, B.S.; Kim, J.; Jeong, K.J.; Song, K.; Mun, S.H.; Kang, W.M.; et al. Field evaluation of the safety and immunogenicity of a classical swine fever virus E2 subunit vaccine in breeding and nursery animals on Jeju Island, South Korea. Clin. Exp. Vaccine Res. 2022, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wu, C.M.; Chen, Z.W.; Liao, C.M.; Deng, M.C.; Chia, M.Y.; Huang, C.; Chien, M.S. Evaluation of classical swine fever E2 (CSF-E2) subunit vaccine efficacy in the prevention of virus transmission and impact of maternal derived antibody interference in field farm applications. Porc. Health Manag. 2021, 7, 9. [Google Scholar] [CrossRef]
- Sordo-Puga, Y.; Perez-Perez, D.; Montero-Espinosa, C.; Oliva-Cardenas, A.; Sosa-Teste, I.; Duarte, C.A.; Rodriguez-Molto, M.P.; Sardina-Gonzalez, T.; Santana-Rodriguez, E.; Vargas-Hernandez, M.; et al. Immunogenicity of E2CD154 Subunit Vaccine Candidate against Classical Swine Fever in Piglets with Different Levels of Maternally Derived Antibodies. Vaccines 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Uttenthal, A.; Le Potier, M.F.; Romero, L.; De Mia, G.M.; Floegel-Niesmann, G. Classical swine fever (CSF) marker vaccine Trial, I. Challenge studies in weaner pigs. Vet. Microbiol. 2001, 83, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Bouma, A.; De Smit, A.J.; De Jong, M.C.; De Kluijver, E.P.; Moormann, R.J. Determination of the onset of the herd-immunity induced by the E2 subunit vaccine against classical swine fever virus. Vaccine 2000, 18, 1374–1381. [Google Scholar] [CrossRef]
- Martens, M.; Rosales, C.; Morilla, A. Evaluation of the use of a subunit classical swine fever marker vaccine under field conditions in Mexico. J. Swine Health Prod. 2003, 11, 81–85. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Wang, Z.; Sun, J.; Mi, S.; Xu, J.; Cao, J.; Hou, Y.; Wang, D.; Huo, X.; et al. Commercial E2 subunit vaccine provides full protection to pigs against lethal challenge with 4 strains of classical swine fever virus genotype 2. Vet. Microbiol. 2019, 237, 108403. [Google Scholar] [CrossRef]
- Zhang, H.; Wen, W.; Zhao, Z.; Wang, J.; Chen, H.; Qian, P.; Li, X. Enhanced protective immunity to CSFV E2 subunit vaccine by using IFN-gamma as immunoadjuvant in weaning piglets. Vaccine 2018, 36, 7353–7360. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ma, H.; Ren, X.; Hao, G.; Zhang, H.; Zhao, Z.; Fang, K.; Li, X.; Rong, Z.; et al. Efficient mucosal vaccination of a novel classical swine fever virus E2-Fc fusion protein mediated by neonatal Fc receptor. Vaccine 2020, 38, 4574–4583. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Truong, D.A.; Ly, V.D.; Vu, H.T.; Hoang, T.V.; Nguyen, C.T.; Chu, N.T.; Nguyen, V.T.; Nguyen, D.T.; Miyazawa, K.; et al. The potential efficacy of the E2-subunit vaccine to protect pigs against different genotypes of classical swine fever virus circulating in Vietnam. Clin. Exp. Vaccine Res. 2020, 9, 26–39. [Google Scholar] [CrossRef]
- Madera, R.; Gong, W.; Wang, L.; Burakova, Y.; Lleellish, K.; Galliher-Beckley, A.; Nietfeld, J.; Henningson, J.; Jia, K.; Li, P.; et al. Pigs immunized with a novel E2 subunit vaccine are protected from subgenotype heterologous classical swine fever virus challenge. BMC Vet. Res. 2016, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Huang, J.; Li, Y.; Chen, H.; Wu, Y.; Fu, X.; Hao, X.; Li, Q.; Zeng, R.; Zhang, G. Efficiency Comparison of a Novel E2 Subunit Vaccine and a Classic C-Strain Vaccine against Classical Swine Fever. Vet. Sci. 2021, 8, 148. [Google Scholar] [CrossRef]
- Linghua, Z.; Xingshan, T.; Fengzhen, Z. The efficacy of CpG oligodinucleotides, in combination with conventional adjuvants, as immunological adjuvants to swine streptococcic septicemia vaccine in piglets in vivo. Int. Immunopharmacol. 2006, 6, 1267–1276. [Google Scholar] [CrossRef]
- Kayraklioglu, N.; Horuluoglu, B.; Klinman, D.M. CpG Oligonucleotides as Vaccine Adjuvants. Methods Mol. Biol. 2021, 2197, 51–85. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, P.; Deng, H.; Li, Y.; Ren, J.; Fu, X.; Zhang, L.; Huang, J. Immunological effects of different types of synthetic CpG oligodeoxynucleotides on porcine cells. RSC Adv. 2017, 7, 43289–43299. [Google Scholar] [CrossRef]
- Laughlin, R.C.; Madera, R.; Peres, Y.; Berquist, B.R.; Wang, L.; Buist, S.; Burakova, Y.; Palle, S.; Chung, C.J.; Rasmussen, M.V.; et al. Plant-made E2 glycoprotein single-dose vaccine protects pigs against classical swine fever. Plant Biotechnol. J. 2019, 17, 410–420. [Google Scholar] [CrossRef]
- Wei, Q.; Bai, Y.; Song, Y.; Liu, Y.; Yu, W.; Sun, Y.; Wang, L.; Deng, R.; Xing, G.; Zhang, G. Generation and immunogenicity analysis of recombinant classical swine fever virus glycoprotein E2 and E(rns) expressed in baculovirus expression system. Virol. J. 2021, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.J.; Liu, T.Y.; Tseng, Y.Y.; Chen, Z.W.; You, C.C.; Hsuan, S.L.; Chien, M.S.; Huang, C. Yeast-expressed classical swine fever virus glycoprotein E2 induces a protective immune response. Vet. Microbiol. 2009, 139, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.J.; Deng, M.C.; Chen, Z.W.; Liu, T.Y.; Wu, C.W.; Cheng, C.Y.; Chien, M.S.; Huang, C. Yeast expressed classical swine fever E2 subunit vaccine candidate provides complete protection against lethal challenge infection and prevents horizontal virus transmission. Vaccine 2012, 30, 2336–2341. [Google Scholar] [CrossRef] [PubMed]
- Madera, R.F.; Wang, L.; Gong, W.; Burakova, Y.; Buist, S.; Nietfeld, J.; Henningson, J.; Cino-Ozuna, A.G.; Tu, C.; Shi, J. Toward the development of a one-dose classical swine fever subunit vaccine: Antigen titration, immunity onset, and duration of immunity. J. Vet. Sci. 2018, 19, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Liu, Y.; Zhang, G. Research Progress and Challenges in Vaccine Development against Classical Swine Fever Virus. Viruses 2021, 13, 445. [Google Scholar] [CrossRef]
- Hasan, N.H.; Ignjatovic, J.; Peaston, A.; Hemmatzadeh, F. Avian Influenza Virus and DIVA Strategies. Viral Immunol. 2016, 29, 198–211. [Google Scholar] [CrossRef]
- Rose, N.; Andraud, M. The use of vaccines to control pathogen spread in pig populations. Porc. Health Manag. 2017, 3, 8. [Google Scholar] [CrossRef]
- Erdem, A.E.; SareyyÜPoĞLu, B. DIVA (Differentiating Infected from Vaccinated Animals) Vaccines and Strategies. Etlik Vet. Mikrobiyoloji Derg. 2022, 33, 102–109. [Google Scholar] [CrossRef]
- van Gennip, H.G.; van Rijn, P.A.; Widjojoatmodjo, M.N.; de Smit, A.J.; Moormann, R.J. Chimeric classical swine fever viruses containing envelope protein E(RNS) or E2 of bovine viral diarrhoea virus protect pigs against challenge with CSFV and induce a distinguishable antibody response. Vaccine 2000, 19, 447–459. [Google Scholar] [CrossRef]
- Wang, L.; Mi, S.; Madera, R.; Ganges, L.; Borca, M.V.; Ren, J.; Cunningham, C.; Cino-Ozuna, A.G.; Li, H.; Tu, C.; et al. A neutralizing monoclonal antibody-based competitive ELISA for classical swine fever C-strain post-vaccination monitoring. BMC Vet. Res. 2020, 16, 14. [Google Scholar] [CrossRef]
- Wang, L.; Madera, R.; Li, Y.; McVey, D.S.; Drolet, B.S.; Shi, J. Recent Advances in the Diagnosis of Classical Swine Fever and Future Perspectives. Pathogens 2020, 9, 658. [Google Scholar] [CrossRef]
- Ganges, L.; Crooke, H.R.; Bohorquez, J.A.; Postel, A.; Sakoda, Y.; Becher, P.; Ruggli, N. Classical swine fever virus: The past, present and future. Virus Res. 2020, 289, 198151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yu, M.; Weiland, E.; Morrissy, C.; Zhang, N.; Westbury, H.; Wang, L.F. Characterization of epitopes for neutralizing monoclonal antibodies to classical swine fever virus E2 and Erns using phage-displayed random peptide library. Arch. Virol. 2006, 151, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, B.K.; Rogers, K.; Fernandez-Sainz, I.J.; Holinka, L.G.; Borca, M.V.; Risatti, G.R. Effects of glycosylation on antigenicity and immunogenicity of classical swine fever virus envelope proteins. Virology 2011, 420, 135–145. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.; Kekungu, P.; Barman, N.N.; Kumar, S. Evaluation of surface glycoproteins of classical swine fever virus as immunogens and reagents for serological diagnosis of infections in pigs: A recombinant Newcastle disease virus approach. Arch. Virol. 2019, 164, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Kim, E.J.; Cho, S.C.; Moon, S.U.; Lee, M.H.; Ko, J.A.; Ko, S.B.; Lee, J.; Lee, C. Monitoring and evaluation of provincial classical swine fever immunization implementation with an E2 subunit vaccine in Jeju Island, South Korea. Clin. Exp. Vaccine Res. 2024, 13, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Isoda, N.; Baba, K.; Ito, S.; Ito, M.; Sakoda, Y.; Makita, K. Dynamics of Classical Swine Fever Spread in Wild Boar in 2018–2019, Japan. Pathogens 2020, 9, 119. [Google Scholar] [CrossRef]
- Choe, S.; Cha, R.M.; Yu, D.S.; Kim, K.S.; Song, S.; Choi, S.H.; Jung, B.I.; Lim, S.I.; Hyun, B.H.; Park, B.K.; et al. Rapid Spread of Classical Swine Fever Virus among South Korean Wild Boars in Areas near the Border with North Korea. Pathogens 2020, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Moß, C.; Reimann, I.; König, P.; Beer, M. Classical swine fever vaccines-State-of-the-art. Vet. Microbiol. 2017, 206, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.; Perez, L.J. Commentary: Genetic evolution of classical swine fever virus under immune environments conditioned by genotype 1-based modified live virus vaccine. Front. Vet. Sci. 2018, 5, 55. [Google Scholar] [CrossRef]
- Perez, L.J.; Diaz de Arce, H.; Perera, C.L.; Rosell, R.; Frias, M.T.; Percedo, M.I.; Tarradas, J.; Dominguez, P.; Nunez, J.I.; Ganges, L. Positive selection pressure on the B/C domains of the E2-gene of classical swine fever virus in endemic areas under C-strain vaccination. Infect. Genet. Evol. 2012, 12, 1405–1412. [Google Scholar] [CrossRef]
- Park, G.S.; Lim, S.I.; Hong, S.H.; Song, J.Y. Establishment and characterization of an infectious cDNA clone of a classical swine fever virus LOM strain. J. Vet. Sci. 2012, 13, 81–91. [Google Scholar] [CrossRef]
- Je, S.H.; Kwon, T.; Yoo, S.J.; Lee, D.U.; Lee, S.; Richt, J.A.; Lyoo, Y.S. Classical Swine Fever Outbreak after Modified Live LOM Strain Vaccination in Naive Pigs, South Korea. Emerg. Infect. Dis. 2018, 24, 798–800. [Google Scholar] [CrossRef]
- Coronado, L.; Rios, L.; Frias, M.T.; Amaran, L.; Naranjo, P.; Percedo, M.I.; Perera, C.L.; Prieto, F.; Fonseca-Rodriguez, O.; Perez, L.J. Positive selection pressure on E2 protein of classical swine fever virus drives variations in virulence, pathogenesis and antigenicity: Implication for epidemiological surveillance in endemic areas. Transbound. Emerg. Dis. 2019, 66, 2362–2382. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, K.; Kuninaga, N.; Kimura, Y.; Makita, K.; Isoda, N.; Shimizu, Y.; Sakoda, Y. Evaluation of Immune Status of Pigs against Classical Swine Fever for Three Years after the Initiation of Vaccination in Gifu Prefecture, Japan. Pathogens 2024, 13, 616. [Google Scholar] [CrossRef] [PubMed]


| Group * (n) | Vaccination Treatments | |
|---|---|---|
| CSFV E2 Protein (µg/Dose) | CpG Adjuvant (µg/Dose) | |
| Animal Trial I | ||
| E-2 (3) | 200 | 0 |
| E-1 (3) | 100 | 0 |
| E-1A (3) | 100 | 100 |
| E-0.5A (3) | 50 | 100 |
| C (3) | >64 | 0 |
| A (2) | 0 | 100 |
| Animal Trial II | ||
| E-1A (3) | 100 | 100 |
| E-0.5A (3) | 50 | 100 |
| N (3) | 0 | 0 |
| Group (n) * | CSFV Viral Loads in Serum, Nasal, and Saliva Samples Before and Post-Challenge (dpc) | |||||
|---|---|---|---|---|---|---|
| Ct | Log Copies/mL | |||||
| E-1A (3) | E-0.5A (3) | N (3) | E-1A (3) | E-0.5A (3) | N (3) | |
| Sera | ||||||
| −28 dpc | (−) | (−) | (−) | 0 a | 0 a | 0 a |
| 0 dpc | (−) | (−) | (−) | 0 a | 0 a | 0 a |
| 3 dpc | (−) | 32.2 ± 0.8 | 30.3 ± 0.8 | 0 a | 3.2 ± 1.2 ab | 5.4 ± 0.3 b |
| 7 dpc | (−) | 32.8 ⁘ | 18.0 ± 0.3 | 0 a | 1.5 ± 1.2 a | 9.1 ± 0.1 b |
| 10 dpc | (−) | (−) | 17.1 ± 0.8 | 0 a | 0 a | 9.4 ± 0.3 b |
| 14 dpc | (−) | (−) | N/A | 0 a | 0 a | N/A |
| Nasal | ||||||
| −28 dpc | (−) | (−) | (−) | 0 a | 0 a | 0 a |
| 0 dpc | (−) | (−) | (−) | 0 a | 0 a | 0 a |
| 3 dpc | (−) | (−) | 32.0 ⁘ | 0 a | 0 a | 1.7 ± 1.3 a |
| 7 dpc | (−) | (−) | 27.2 ± 1.1 | 0 a | 0 a | 6.5 ± 0.3 b |
| 10 dpc | (−) | (−) | 24.7 ± 0.0 | 0 a | 0 a | 7.2 ± 0.0 b |
| 14 dpc | (−) | (−) | N/A | 0 a | 0 a | N/A |
| Saliva | ||||||
| −28 dpc | (−) | (−) | (−) | 0 a | 0 a | 0 a |
| 0 dpc | (−) | (−) | (−) | 0 a | 0 a | 0 a |
| 3 dpc | (−) | (−) | (−) | 0 a | 0 a | 0 a |
| 7 dpc | 35.3 ± 0.7 | 37.6 ⁘ | 27.7 ± 1.0 | 3.4 ± 0.2 ab | 1.3 ± 1.3 a | 5.8 ± 0.3 b |
| 10 dpc | (−) | (−) | 26.2 ± 0.7 | 0 a | 0 a | 6.3 ± 0.2 b |
| 14 dpc | (−) | (−) | N/A | 0 a | 0 a | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Chen, C.-C.; Chung, W.-B.; Huang, Y.-L.; Ke, G.-M.; Chaung, H.-C. Efficacy Evaluation of an E2 Subunit Vaccine Against Highly Virulent Classical Swine Fever Virus Strain. Vaccines 2025, 13, 1072. https://doi.org/10.3390/vaccines13101072
Chen Y-C, Chen C-C, Chung W-B, Huang Y-L, Ke G-M, Chaung H-C. Efficacy Evaluation of an E2 Subunit Vaccine Against Highly Virulent Classical Swine Fever Virus Strain. Vaccines. 2025; 13(10):1072. https://doi.org/10.3390/vaccines13101072
Chicago/Turabian StyleChen, Yu-Chieh, Chi-Chih Chen, Wen-Bin Chung, Yen-Li Huang, Guan-Ming Ke, and Hso-Chi Chaung. 2025. "Efficacy Evaluation of an E2 Subunit Vaccine Against Highly Virulent Classical Swine Fever Virus Strain" Vaccines 13, no. 10: 1072. https://doi.org/10.3390/vaccines13101072
APA StyleChen, Y.-C., Chen, C.-C., Chung, W.-B., Huang, Y.-L., Ke, G.-M., & Chaung, H.-C. (2025). Efficacy Evaluation of an E2 Subunit Vaccine Against Highly Virulent Classical Swine Fever Virus Strain. Vaccines, 13(10), 1072. https://doi.org/10.3390/vaccines13101072






