Impediments to Progress Toward Polio Eradication During 2014–2024: Effectively Addressing the Current Challenges
Abstract
1. Introduction
2. Materials and Methods
3. Progress Toward Wild Poliovirus Eradication, 2014–2024
3.1. Global WPV1 Outbreaks
3.2. WPV1 Transmission in Endemic Countries
3.2.1. Nigeria
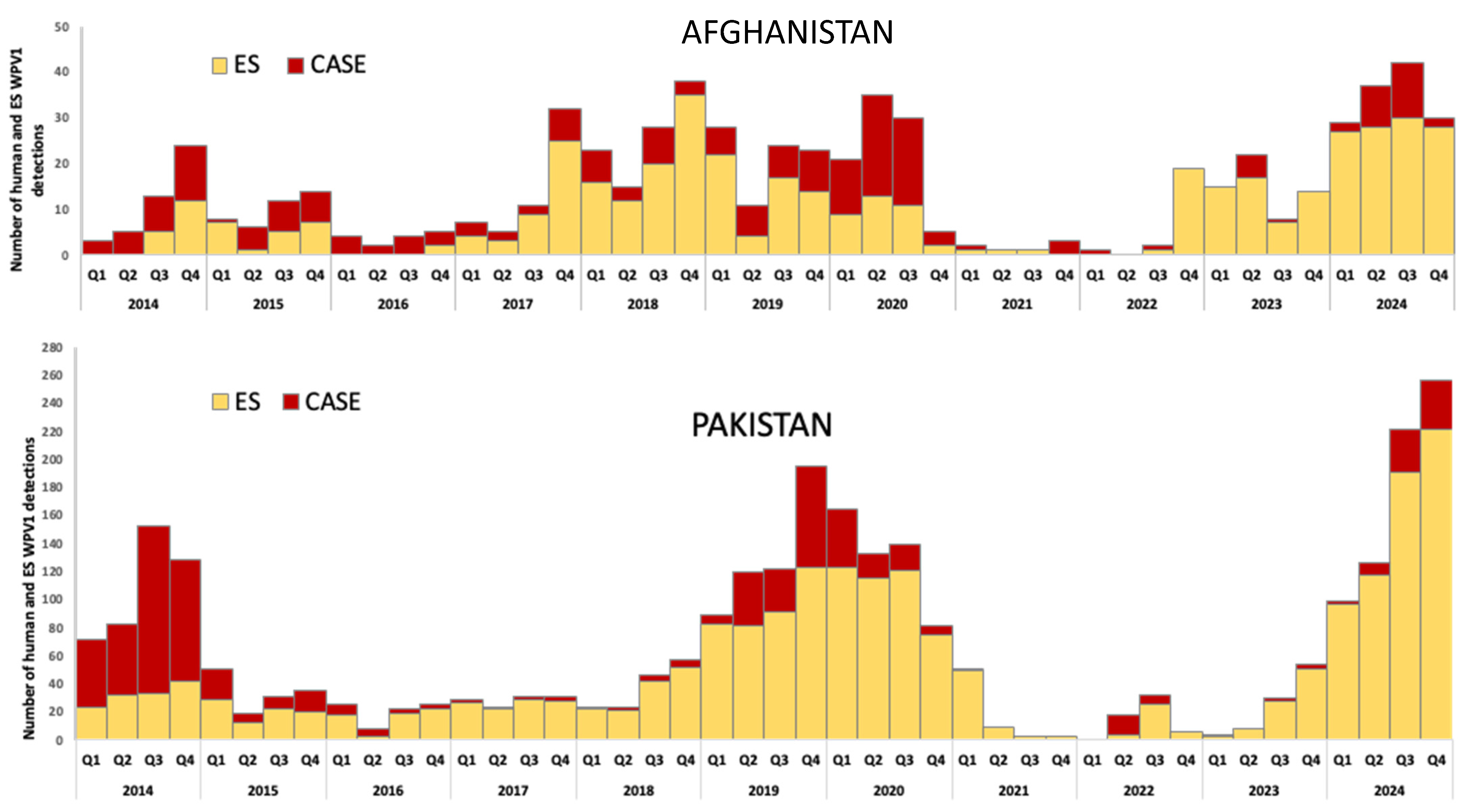
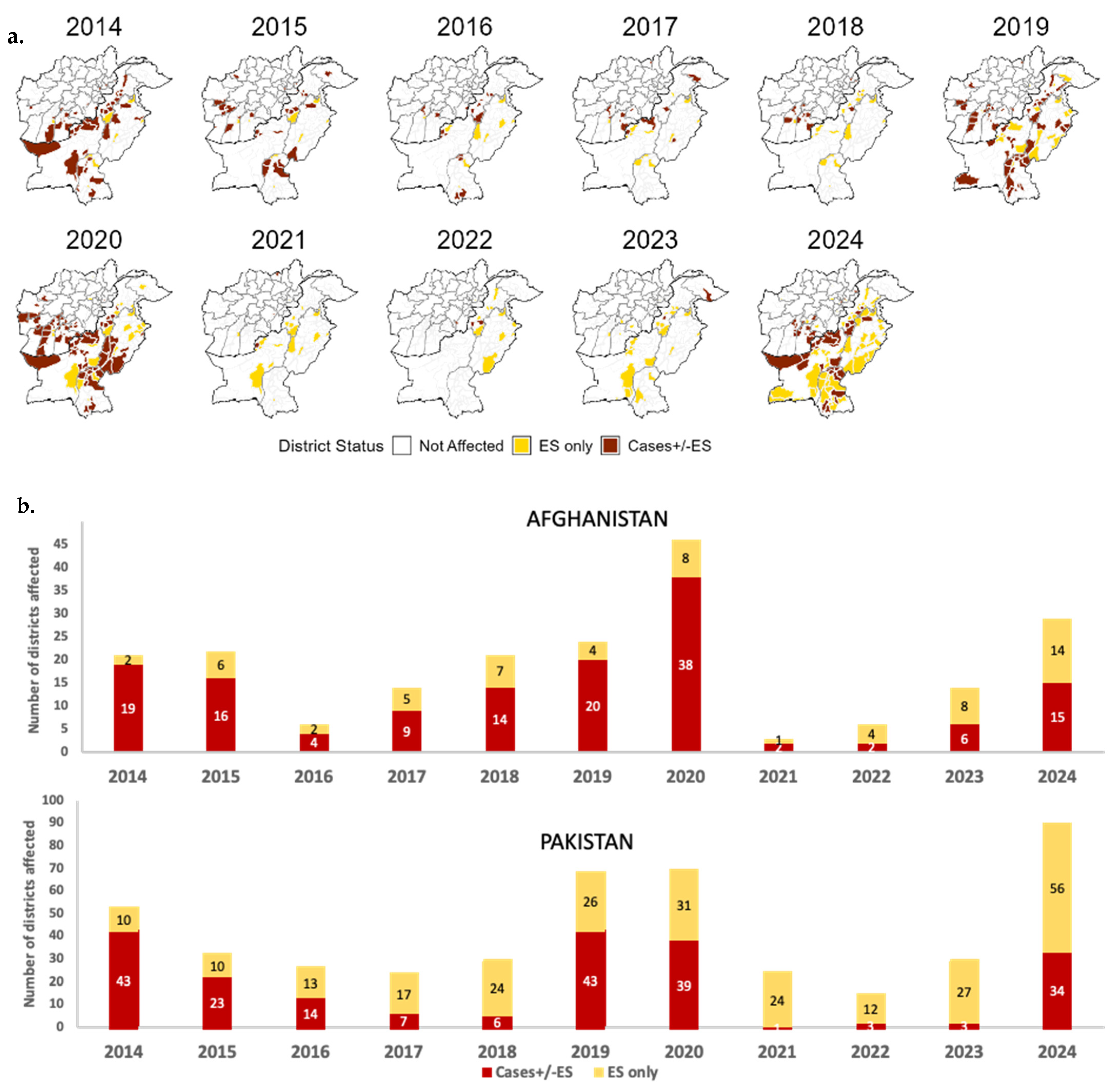
3.2.2. Afghanistan and Pakistan
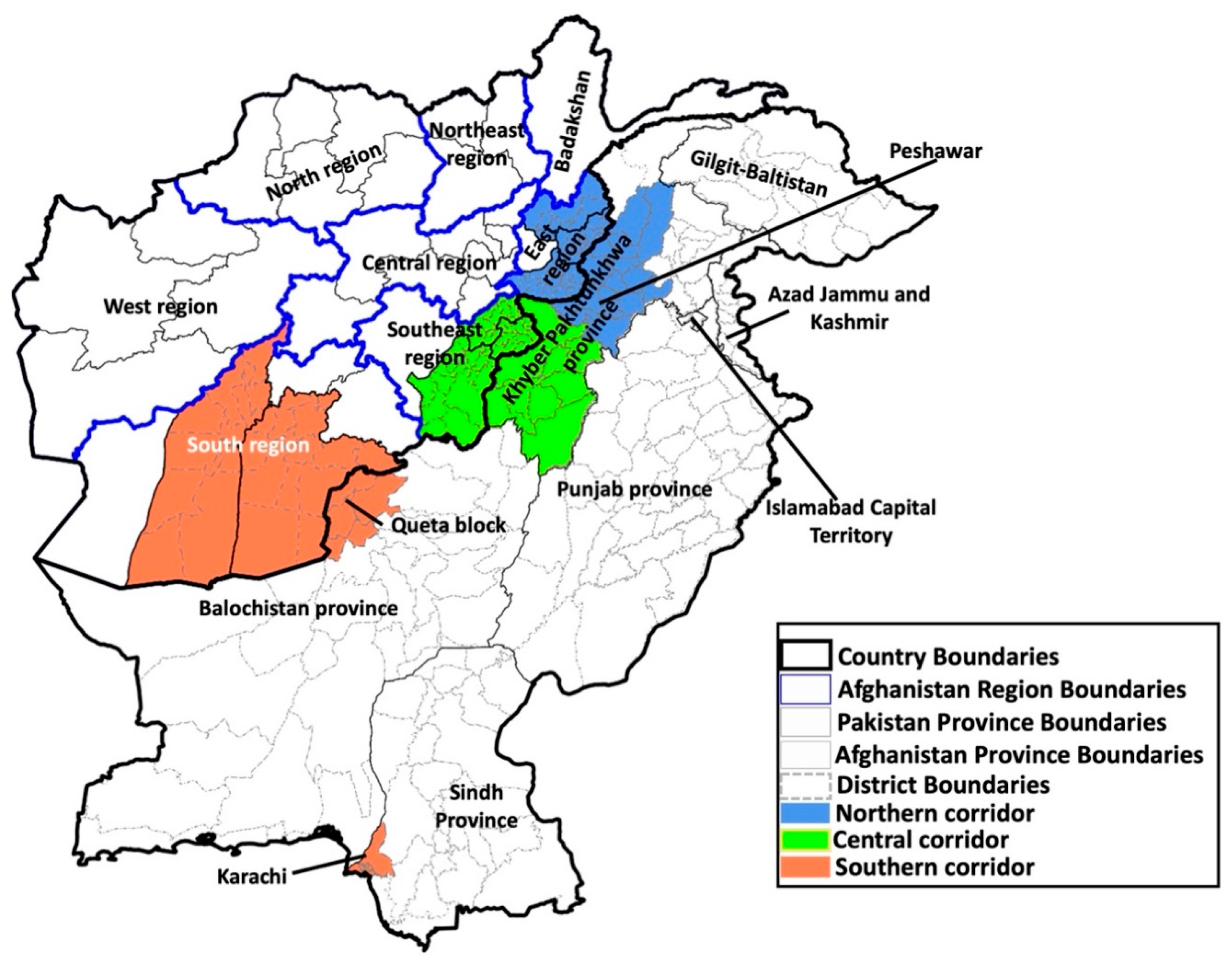
Afghanistan Country-Specific Issues
Pakistan Country-Specific Issues
4. Progress Toward Ending Transmission of cVDPVs
4.1. Background
4.1.1. History of Use of Different OPV Presentations
4.1.2. The 2016 tOPV-bOPV Switch
4.2. cVDPV Transmission, 2000–2024 (Figure 4a,b, 2014–2024)
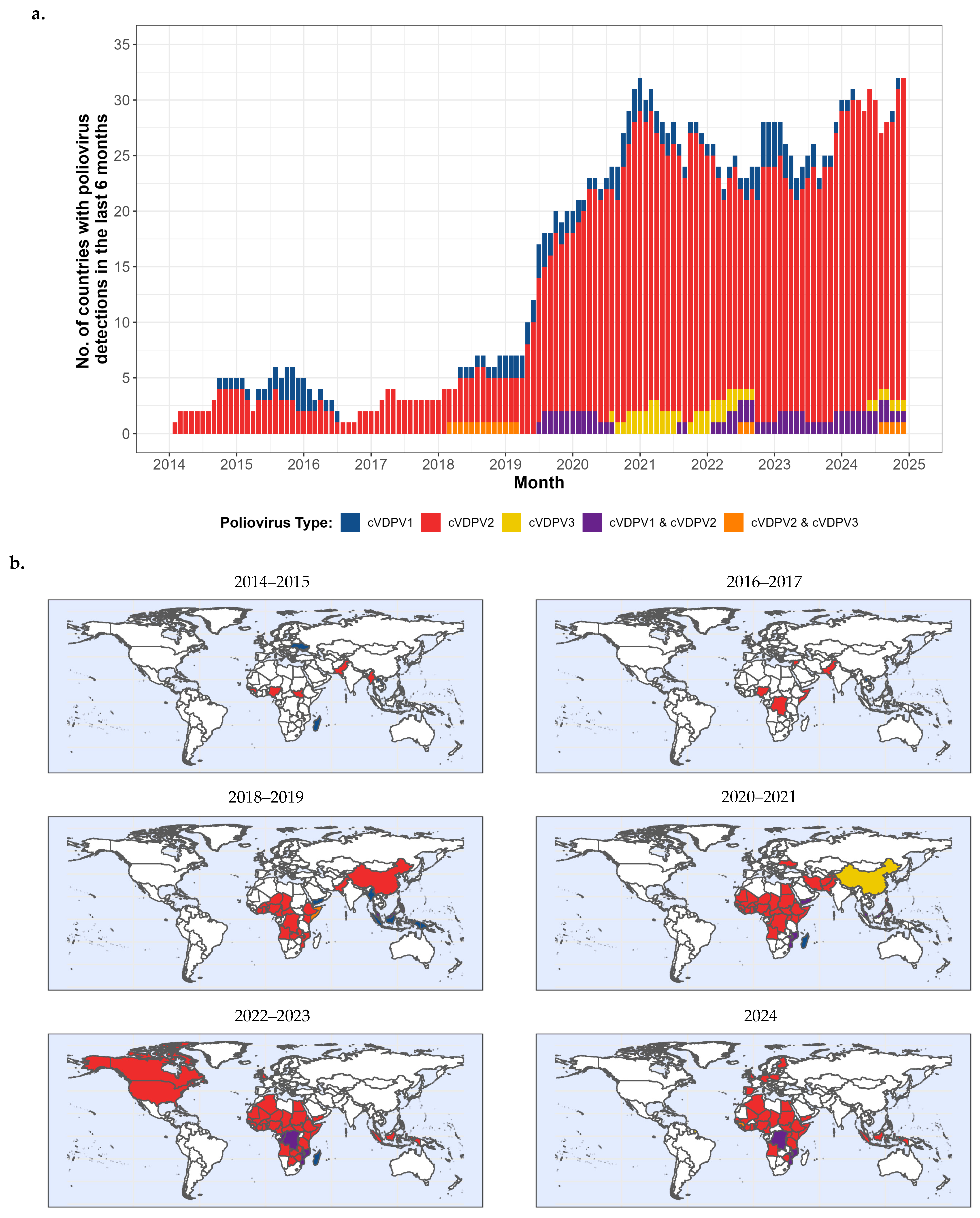
4.2.1. Reported cVDPV1 and cVDPV3 Outbreaks, 2000–2024
4.2.2. Reported cVDPV2 Outbreaks, 2000–2024 (Figure 4a,b, 2014–2024)
Outbreaks Prior to 2014
cVDPV2 Outbreaks, 2014–2024
5. Addressing Challenges to Polio Eradication
5.1. Overview
5.2. Recurring Challenges
5.2.1. National Engagement
Mitigations
5.2.2. Limited Security and Access
Mitigations
5.2.3. Other Access Impediments
Mitigations
5.3. Selected Groups of Other Major Challenges
5.3.1. Residual Effects of COVID-19 Pandemic
Mitigations
5.3.2. Outbreak Response Capacity
Mitigations
5.3.3. Strategic Planning and Decision-Making
Mitigations
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Dedication
Disclaimer
Abbreviations and Initialisms
| AFP | Acute Flaccid Paralysis |
| aVDPV | Ambiguous Vaccine-Derived Poliovirus |
| aVDPV2 | Ambiguous Vaccine-Derived Poliovirus type 2 |
| bOPV | Bivalent Oral Poliovirus Vaccine (Sabin strain types 1 and 3) |
| CBV | Community-Based Vaccination (Pakistan) |
| COVID-19 | Coronavirus Disease 2019 due to SARS-CoV-2 |
| cVDPV | Circulating Vaccine-Derived Poliovirus |
| cVDPV1 | Circulating Vaccine-Derived Poliovirus type 1 |
| cVDPV2 | Circulating Vaccine-Derived Poliovirus type 2 |
| cVDPV3 | Circulating Vaccine-Derived Poliovirus type 3 |
| cVPDV2-n | Circulating Vaccine-Derived Poliovirus type 2 emergence from nOPV2 |
| DG | Director-General of the World Health Organization |
| DRC | Democratic Republic of the Congo |
| EOC | Emergency Operations Centre |
| ES | Environmental Surveillance |
| GPEI | Global Polio Eradication Initiative |
| IDP | Internally Displaced Person |
| IMB | Independent Monitoring Board (for GPEI) |
| IPV | Inactivated Poliovirus Vaccine (type 1, 2 and 3 antigens) |
| IQR | Interquartile Range |
| ISIS | Islamic State of Iraq and ash-Sham |
| KP | Khyber Pakhtunkhwa (Pakistan Province) |
| LQAS | Lot Quality Assurance Sampling (Survey) |
| mOPV1 | Monovalent Sabin Strain Poliovirus type 1 |
| mOPV2 | Monovalent Sabin Strain Poliovirus type 2 |
| mOPV3 | Monovalent Sabin Strain Poliovirus type 3 |
| NEOC | National Emergency Operations Centre (Pakistan) |
| nOPV2 | Novel Oral Poliovirus type 2 |
| OPV | Oral Poliovirus Vaccine |
| OPV2 | Oral Poliovirus Vaccine type 2 |
| PHEIC | Public Health Emergency of International Concern (International Health Regulations, 2005) |
| PV | Poliovirus |
| PV1 | Poliovirus type 1 |
| PV2 | Poliovirus type 2 |
| PV3 | Poliovirus type 3 |
| RI | Routine Immunization (Essential Immunization Services) |
| SAGE | Strategic Advisory Group of Experts on immunization (advising WHO on immunization policy) |
| SIAs | Supplementary Immunization Activities |
| tOPV | Trivalent Oral Poliovirus Vaccine (Sabin Strain types 1, 2 and 3) |
| UNICEF | United Nations Children’s Fund (formerly; now simply UNICEF) |
| UC | Union Council (Pakistan) |
| VDPV | Vaccine-Derived Poliovirus |
| VP1 | Viral Protein 1 (Poliovirus Capsid Surface Protein) |
| WHO | World Health Organization |
| WPV | Wild Poliovirus |
| WPV1 | Wild Poliovirus type 1 |
| WPV3 | Wild Poliovirus type 3 |
References
- World Health Organization. Forty-First World Health Assembly Resolutions and Decisions; World Health Organization: Geneva, Switerland, 1988; pp. 26–28. Available online: https://iris.who.int/bitstream/handle/10665/164197/WHA41_1988-REC-1_eng.pdf?sequence=1&isAllowed=y (accessed on 4 September 2025).
- Global Polio Eradication Initiative. Polio Eradication & Endgame Strategic Plan 2013–2018. WHO/POLIO/13.02. World Health Organization: Geneva, Switzerland, 2013; Available online: https://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_US.pdf (accessed on 4 September 2025).
- Wassilak, S.G.; Oberste, M.S.; Tangermann, R.H.; Diop, O.M.; Jafari, H.S.; Armstrong, G.L. Progress toward global interruption of wild poliovirus transmission, 2010–2013, and tackling the challenges to complete eradication. J. Infect. Dis. 2014, 210 (Suppl. 1), S5–S15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Global Polio Eradication Initiative. Annual Report 2014: On the Threshold of a Polio-Free World; World Health Organization: Geneva, Switerland, 2014; Available online: https://polioeradication.org/wp-content/uploads/2024/05/GPEI_AR2014_EN.pdf (accessed on 4 September 2025).[Green Version]
- Centers for Disease Control and Prevention. Progress toward eradication of polio—Worldwide, January 2011–March 2013. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 335–338. [Google Scholar][Green Version]
- Global Polio Eradication Initiative. Global Eradication of Wild Poliovirus Type 2 Declared: Declaration Further Milestone for Globally-Coordinated Vaccine Switch in 2016. Press Release 20 September. 2015. Available online: https://polioeradication.org/news/global-eradication-of-wild-poliovirus-type-2-declared/ (accessed on 4 September 2025).[Green Version]
- Global Polio Eradication Initiative. Two out of Three Wild Poliovirus Strains Eradicated: Global Eradication of Wild Poliovirus Type 3 Declared on World Polio Day. Press Release 24 October. 2019. Available online: https://polioeradication.org/news/two-out-of-three-wild-poliovirus-strains-eradicated/ (accessed on 4 September 2025).[Green Version]
- Bahl, S.; Kumar, R.; Menabde, N.; Thapa, A.; McFarland, J.; Swezy, V.; Tangermann, R.H.; Jafari, H.S.; Elsner, L.; Wassilak, S.G.; et al. Polio-free certification and lessons learned—South-East Asia region, March 2014. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 941–946. [Google Scholar] [PubMed][Green Version]
- Cochi, S.L.; Jafari, H.S.; Armstrong, G.L.; Sutter, R.W.; Linkins, R.W.; Pallansch, M.A.; Kew, O.; Aylward, R.B. A world without polio. J. Infect. Dis. 2014, 210 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Etsano, A.; Gunnala, R.; Shuaib, F.; Damisa, E.; Mkanda, P.; Ticha, J.M.; Banda, R.; Korir, C.; Chevez, A.E.; Enemaku, O.; et al. Progress Toward Poliomyelitis Eradication—Nigeria, January 2014–July 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 878–882. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. GPEI Applauds WHO African Region for Wild Polio-Free Certification. Support from National Governments and Global Donors Critical to the Region’s Success Against Wild Polio and Must Continue to Achieve a Polio-Free World. Press Release 25 Augest. 2020. Available online: https://polioeradication.org/news/global-polio-eradication-initiative-applauds-who-african-region-for-wild-polio-free-certification/ (accessed on 4 September 2025).
- Hardy, C.M.; Rathee, M.; Chaudhury, S.; Wadood, M.Z.; Ather, F.; Henderson, E.; Martinez, M. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2023–September 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 1129–1134. [Google Scholar] [CrossRef]
- Mohamed, A.; Akbar, I.E.; Chaudhury, S.; Wadood, M.Z.; Ather, F.; Jorba, J.; Martinez, M. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2021–September 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1541–1546. [Google Scholar] [CrossRef]
- Bjork, A.; Akbar, I.E.; Chaudhury, S.; Wadood, M.Z.; Ather, F.; Jorba, J.; Martinez, M. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2022–June 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1020–1026. [Google Scholar] [CrossRef]
- Mbaeyi, C.; Baig, S.; Khan, Z.; Young, H.; Kader, M.; Jorba, J.; Safdar, M.R.; Jafari, H.; Franka, R. Progress Toward Poliomyelitis Eradication—Pakistan, January 2020–July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1359–1364. [Google Scholar] [CrossRef]
- Mbaeyi, C.; Baig, S.; Safdar, M.R.; Khan, Z.; Young, H.; Jorba, J.; Wadood, Z.M.; Jafari, H.; Alam, M.M.; Franka, R. Progress Toward Poliomyelitis Eradication—Pakistan, January 2021–July 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1313–1318. [Google Scholar] [CrossRef]
- Mbaeyi, C.; Baig, S.; Safdar, R.M.; Khan, Z.; Young, H.; Jorba, J.; Wadood, Z.M.; Jafari, H.; Alam, M.M.; Franka, R. Progress Toward Poliomyelitis Eradication—Pakistan, January 2022–June 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 880–885. [Google Scholar] [CrossRef]
- Mbaeyi, C.; Ul Haq, A.; Safdar, R.M.; Khan, Z.; Corkum, M.; Henderson, E.; Wadood, Z.M.; Alam, M.M.; Franka, R. Progress Toward Poliomyelitis Eradication—Pakistan, January 2023–June 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 788–792. [Google Scholar] [CrossRef]
- Hampton, L.M.; Farrell, M.; Ramirez-Gonzalez, A.; Menning, L.; Shendale, S.; Lewis, I.; Rubin, J.; Garon, J.; Harris, J.; Hyde, T.; et al. Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine-Worldwide, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Cochi, S. Addressing the Challenges and Opportunities of the Polio Endgame: Lessons for the Future. J. Infect. Dis. 2017, 216, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Zipursky, S.; Vandelaer, J.; Brooks, A.; Dietz, V.; Kachra, T.; Farrell, M.; Ottosen, A.; Sever, J.L.; Zaffran, M.J. Polio Endgame: Lessons Learned From the Immunization Systems Management Group. J. Infect. Dis. 2017, 216, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Gonzalez, A.; Farrell, M.; Menning, L.; Garon, J.; Everts, H.; Hampton, L.M.; Dolan, S.B.; Shendale, S.; Wanyoike, S.; Veira, C.L.; et al. Implementing the Synchronized Global Switch from Trivalent to Bivalent Oral Polio Vaccines-Lessons Learned From the Global Perspective. J. Infect. Dis. 2017, 216, S183–S192. [Google Scholar] [CrossRef]
- Macklin, G.R.; Goel, A.K.; Mach, O.; Tallis, G.; Ahmed, J.A.; O’Reilly, K.M.; Grassly, N.C.; Diop, O.M. Epidemiology of type 2 vaccine-derived poliovirus outbreaks between 2016 and 2020. Vaccine 2023, 41, A19–A24. [Google Scholar] [CrossRef]
- Macklin, G.R.; O’Reilly, K.M.; Grassly, N.C.; Edmunds, W.J.; Mach, O.; Santhana Gopala Krishnan, R.; Voorman, A.; Vertefeuille, J.F.; Abdelwahab, J.; Gumede, N.; et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science 2020, 368, 401–405. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Sutter, R.W. Evaluation of the 2016 Switch from tOPV to bOPV. 30 September 2024. Lessons Learned and Implications for an Anticipated bOPV Cessation. 2024. Available online: https://polioeradication.org/wp-content/uploads/2024/11/Switch-Report-20240930.pdf (accessed on 4 September 2025).
- Namageyo-Funa, A.; Greene, S.A.; Henderson, E.; Traoré, M.A.; Shaukat, S.; Bigouette, J.P.; Jorba, J.; Wiesen, E.; Bolu, O.; Diop, O.M.; et al. Update on Vaccine-Derived Poliovirus Outbreaks-Worldwide, January 2023–June 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 909–916. [Google Scholar] [CrossRef]
- Jorba, J.; Diop, O.M.; Iber, J.; Henderson, E.; Sutter, R.W.; Wassilak, S.G.F.; Burns, C.C. Update on Vaccine-Derived Polioviruses-Worldwide, January 2016–June 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1185–1191. [Google Scholar] [CrossRef]
- Van Damme, P.; De Coster, I.; Bandyopadhyay, A.S.; Revets, H.; Withanage, K.; De Smedt, P.; Suykens, L.; Oberste, M.S.; Weldon, W.C.; Costa-Clemens, S.A.; et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: A double-blind, single-centre phase 1 study. Lancet 2019, 394, 148–158. [Google Scholar] [CrossRef]
- Zaman, K.; Bandyopadhyay, A.S.; Hoque, M.; Gast, C.; Yunus, M.; Jamil, K.M.; Mainou, B.A.; Konopka-Anstadt, J.L.; Hendley, W.S.; Vincent, A.; et al. Evaluation of the safety, immunogenicity, and faecal shedding of novel oral polio vaccine type 2 in healthy newborn infants in Bangladesh: A randomised, controlled, phase 2 clinical trial. Lancet 2023, 401, 131–139. [Google Scholar] [CrossRef]
- Sáez-Llorens, X.; Bandyopadhyay, A.S.; Gast, C.; Leon, T.D.; DeAntonio, R.; Jimeno, J.; Caballero, M.I.; Aguirre, G.; Oberste, M.S.; Weldon, W.C.; et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: Two clinical trials. Lancet 2021, 397, 27–38. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.S.; Zipursky, S. A novel tool to eradicate an ancient scourge: The novel oral polio vaccine type 2 story. Lancet Infect. Dis. 2023, 23, e67–e71. [Google Scholar] [CrossRef]
- World Health Organization. Novel Oral Polio Vaccine Type 2 (nOPV2) Granted EUL Recommendation. WHO Has Issued an Emergency Use Listing Recommendation for the Type 2 Novel Oral Polio Vaccine (nOPV2). Press Release 13 November. 2020. Available online: https://polioeradication.org/news/novel-oral-polio-vaccine-type-2-nopv2-granted-interim-emergency-use-listing-recommendation/ (accessed on 4 September 2025).
- Asekun, A.; Nkwogu, L.; Bawa, S.; Usman, S.; Edukugho, A.; Ocheh, J.; Banda, R.; Nganda, G.W.; Nsubuga, P.; Archer, R.; et al. Deployment of novel oral polio vaccine type 2 under emergency use listing in Nigeria: The rollout experience. Pan Afr. Med. J. 2023, 45, 3. [Google Scholar] [CrossRef] [PubMed]
- Alleman, M.M.; Jorba, J.; Henderson, E.; Diop, O.M.; Shaukat, S.; Traoré, M.A.; Wiesen, E.; Wassilak, S.G.F.; Burns, C.C. Update on Vaccine-Derived Poliovirus Outbreaks-Worldwide, January 2020–June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Contributions of the Polio Network to the COVID-19 Response: Turning the Challenge into an Opportunity for Polio Transition; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240011533 (accessed on 4 September 2025).
- Burkholder, B.; Wadood, Z.; Kassem, A.M.; Ehrhardt, D.; Zomahoun, D. The immediate impact of the COVID-19 pandemic on polio immunization and surveillance activities. Vaccine 2021, 41, A2–A11. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Jafari, H.; Safdar, R.M.; Ahmed, J.A.; Mahamud, A.; Bandyopadhyay, A.S.; Shukla, H.; Quddus, A.; Zaffran, M.; Sutter, R.W.; et al. Modelling the spread of serotype-2 vaccine derived-poliovirus outbreak in Pakistan and Afghanistan to inform outbreak control strategies in the context of the COVID-19 pandemic. Vaccine 2023, 41 (Suppl. 1), A93–A104. [Google Scholar] [CrossRef]
- Zomahoun, D.J.; Burman, A.L.; Snider, C.J.; Chauvin, C.; Gardner, T.; Lickness, J.S.; Ahmed, J.A.; Diop, O.; Gerber, S.; Anand, A. Impact of COVID-19 Pandemic on Global Poliovirus Surveillance. MMWR Morb. Mortal. Wkly. Rep. 2021, 69, 1648–1652. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Voorman, A.; Pallansch, M.A.; Wassilak, S.G.F.; Cochi, S.L.; Badizadegan, K.; Thompson, K.M. The impact of disruptions caused by the COVID-19 pandemic on global polio eradication. Vaccine 2023, 41 (Suppl. 1), A12–A18. [Google Scholar] [CrossRef]
- Lopez Cavestany, R.; Eisenhawer, M.; Diop, O.M.; Verma, H.; Quddus, A.; Mach, O. The Last Mile in Polio Eradication: Program Challenges and Perseverance. Pathogens 2024, 13, 323. [Google Scholar] [CrossRef]
- Badizadegan, K.; Kalkowska, D.A.; Thompson, K.M. Polio by the Numbers—A Global Perspective. J. Infect. Dis. 2022, 226, 1309–1318. [Google Scholar] [CrossRef]
- Thompson, K.M.; Badizadegan, K. Evolution of global polio eradication strategies: Targets, vaccines, and supplemental immunization activities (SIAs). Expert Rev. Vaccines 2024, 23, 597–613. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.S.; Burke, R.M.; Hawes, K.M. Polio Eradication: Status, Struggles and Strategies. Pediatr. Infect. Dis. J. 2024, 43, e207–e211. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. Polio Eradication Strategy 2022–2026: Delivering on a Promise. 2021. Available online: https://iris.who.int/handle/10665/345967 (accessed on 4 September 2025).
- Independent Montoiring Board. 22nd Report. Closing in on Zero: Adapting to Complexity and Risk to the Path to End Polio; World Health Organization: Geneva, Switzerland, 2023; Available online: https://polioeradication.org/wp-content/uploads/2024/05/22nd-Report-of-The-Independent-Monitoring-Board-IMB.pdf (accessed on 4 September 2025).
- Independent Monitoring Board. 23rd Report. The Long Goodbye: Poliovirus Continues to Resist Extinction; World Health Organization: Geneva, Switzerland, 2024; Available online: https://polioeradication.org/wp-content/uploads/2024/09/23rd-IMB-Report-20240922.pdf (accessed on 4 September 2025).
- Estivariz, C.F.; Burns, C.C.; Macklin, G.R. Poliovirus Vaccine-Live. In Vaccines, 8th ed.; Orenstein, W.A., Offit, P.A., Edwards, K.M., Plotkin, S.A., Eds.; Elsevier: Philadelphia, PA, USA, 2023; pp. 914–968. [Google Scholar]
- Kishore, N.; Krow-Lucal, E.; Diop, O.M.; Jorba, J.; Avagnan, T.; Grabovac, V.; Kfutwah, A.K.W.; Johnson, T.; Joshi, S.; Sangal, L.; et al. Surveillance To Track Progress Toward Polio Eradication—Worldwide, 2022–2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 278–285. [Google Scholar] [CrossRef] [PubMed]
- VanderEnde, K.; Voorman, A.; Khan, S.; Anand, A.; Snider, C.J.; Goel, A.; Wassilak, S. New analytic approaches for analyzing and presenting polio surveillance data to supplement standard performance indicators. Vaccine X 2020, 4, 100059. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative. Guidelines on Environmental Surveillance for Detection of Polioviruses. Working Draft; March, 2015. 2015. Available online: http://polioeradication.org/wp-content/uploads/2016/07/GPLN_GuidelinesES_April2015.pdf (accessed on 4 September 2025).
- Hovi, T.; Blomqvist, S.; Nasr, E.; Burns, C.C.; Sarjakoski, T.; Ahmed, N.; Savolainen, C.; Roivainen, M.; Stenvik, M.; Laine, P.; et al. Environmental surveillance of wild poliovirus circulation in Egypt—Balancing between detection sensitivity and workload. J. Virol. Methods 2005, 126, 127–134. [Google Scholar] [CrossRef]
- Hovi, T.; Shulman, L.M.; van der Avoort, H.; Deshpande, J.; Roivainen, M.; De Gourville, E.M. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol. Infect. 2012, 140, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Asghar, H.; Diop, O.M.; Weldegebriel, G.; Malik, F.; Shetty, S.; El Bassioni, L.; Akande, A.O.; Al Maamoun, E.; Zaidi, S.; Adeniji, A.J.; et al. Environmental surveillance for polioviruses in the Global Polio Eradication Initiative. J. Infect. Dis. 2014, 210 (Suppl. 1), S294–S303. [Google Scholar] [CrossRef]
- Cowger, T.L.; Burns, C.C.; Sharif, S.; Gary, H.E., Jr.; Iber, J.; Henderson, E.; Malik, F.; Zahoor Zaidi, S.S.; Shaukat, S.; Rehman, L.; et al. The role of supplementary environmental surveillance to complement acute flaccid paralysis surveillance for wild poliovirus in Pakistan—2011–2013. PLoS ONE 2017, 12, e0180608. [Google Scholar] [CrossRef]
- O’Reilly, K.M.; Verity, R.; Durry, E.; Asghar, H.; Sharif, S.; Zaidi, S.Z.; Wadood, M.Z.M.; Diop, O.M.; Okayasu, H.; Safdar, R.M.; et al. Population sensitivity of acute flaccid paralysis and environmental surveillance for serotype 1 poliovirus in Pakistan: An observational study. BMC Infect. Dis. 2018, 18, 176. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. Standard Operating Procedures: Responding to a Poliovirus Event or Outbreak Version 4; World Health Organization: Geneva, Switzerland, 2022; Available online: https://polioeradication.org/wp-content/uploads/2024/05/9789240049154-eng.pdf (accessed on 4 September 2025).
- Jorba, J.; Campagnoli, R.; De, L.; Kew, O. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J. Virol. 2008, 82, 4429–4440. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Resurgence of wild poliovirus type 1 transmission and consequences of importation—21 countries, 2002–2005. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 145–150. [Google Scholar]
- O’Reilly, K.M.; Lamoureux, C.; Molodecky, N.A.; Lyons, H.; Grassly, N.C.; Tallis, G. An assessment of the geographical risks of wild and vaccine-derived poliomyelitis outbreaks in Africa and Asia. BMC Infect. Dis. 2017, 17, 367. [Google Scholar] [CrossRef]
- Mach, O.; Tangermann, R.H.; Wassilak, S.G.; Singh, S.; Sutter, R.W. Outbreaks of paralytic poliomyelitis during 1996–2012: The changing epidemiology of a disease in the final stages of eradication. J. Infect. Dis. 2014, 210 (Suppl. 1), S275–S282. [Google Scholar] [CrossRef]
- Mach, O.; Verma, H.; Khandait, D.W.; Sutter, R.W.; O’Connor, P.M.; Pallansch, M.A.; Cochi, S.L.; Linkins, R.W.; Chu, S.Y.; Wolff, C.; et al. Prevalence of asymptomatic poliovirus infection in older children and adults in northern India: Analysis of contact and enhanced community surveillance, 2009. J. Infect. Dis. 2014, 210 (Suppl. 1), S252–S258. [Google Scholar] [CrossRef]
- Kidd, S.; Goodson, J.L.; Aramburu, J.; Morais, A.; Gaye, A.; Wannemuehler, K.; Buffington, J.; Gerber, S.; Wassilak, S.; Uzicanin, A. Poliomyelitis outbreaks in Angola genetically linked to India: Risk factors and implications for prevention of outbreaks due to wild poliovirus importations. Vaccine 2011, 29, 3760–3766. [Google Scholar] [CrossRef]
- Chard, A.N.; Datta, S.D.; Tallis, G.; Burns, C.C.; Wassilak, S.G.F.; Vertefeuille, J.F.; Zaffran, M. Progress Toward Polio Eradication—Worldwide, January 2018–March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Davlantes, E. Notes from the Field: Initial Outbreak Response Activity Following Wild Poliovirus Type 1 Detection-Malawi, February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 776–777. [Google Scholar] [CrossRef]
- Davlantes, E.; Greene, S.A.; Tobolowsky, F.A.; Biya, O.; Wiesen, E.; Abebe, F.; Weldetsadik, M.B.; Eboh, V.A.; Chisema, M.N.; da Conceição Mário, B.; et al. Update on Wild Poliovirus Type 1 Outbreak-Southeastern Africa, 2021–2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, January 2010–June 2011. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1053–1057. [Google Scholar]
- Ado, J.M.; Etsano, A.; Shuaib, F.; Damisa, E.; Mkanda, P.; Gasasira, A.; Banda, R.; Korir, C.; Johnson, T.; Dieng, B.; et al. Progress toward poliomyelitis eradication in Nigeria. J. Infect. Dis. 2014, 210 (Suppl. 1), S40–S49. [Google Scholar] [CrossRef] [PubMed]
- Upfill-Brown, A.M.; Voorman, A.; Chabot-Couture, G.; Shuaib, F.; Lyons, H.M. Analysis of vaccination campaign effectiveness and population immunity to support and sustain polio elimination in Nigeria. BMC Med. 2016, 14, 60. [Google Scholar] [CrossRef]
- Nnadi, C.; Damisa, E.; Esapa, L.; Braka, F.; Waziri, N.; Siddique, A.; Jorba, J.; Nganda, G.W.; Ohuabunwo, C.; Bolu, O.; et al. Continued Endemic Wild Poliovirus Transmission in Security-Compromised Areas-Nigeria, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 190–193. [Google Scholar] [CrossRef]
- Forbi, J.C.; Musa, M.S.; Salawu, M.; Idris, J.M.; Ba’aba, A.I.; Higgins, J.; Musa, A.I.; Bashir, B.; Shettima, A.; Njeakor, N.; et al. Historical reconstruction of inaccessibility status in Local Government Areas (LGAs) of Borno and Yobe States, Nigeria, 2010–2020. Pan Afr Med J 2023, 45, 7. [Google Scholar] [CrossRef]
- Gammino, V.M.; Nuhu, A.; Chenoweth, P.; Manneh, F.; Young, R.R.; Sugerman, D.E.; Gerber, S.; Abanida, E.; Gasasira, A. Using geographic information systems to track polio vaccination team performance: Pilot project report. J. Infect. Dis. 2014, 210 (Suppl. 1), S98–S101. [Google Scholar] [CrossRef]
- Barau, I.; Zubairu, M.; Mwanza, M.N.; Seaman, V.Y. Improving polio vaccination coverage in Nigeria through the use of geographic information system technology. J. Infect. Dis. 2014, 210 (Suppl. 1), S102–S110. [Google Scholar] [CrossRef]
- Touray, K.; Mkanda, P.; Tegegn, S.G.; Nsubuga, P.; Erbeto, T.B.; Banda, R.; Etsano, A.; Shuaib, F.; Vaz, R.G. Tracking Vaccination Teams During Polio Campaigns in Northern Nigeria by Use of Geographic Information System Technology: 2013–2015. J. Infect. Dis. 2016, 213 (Suppl. 3), S67–S72. [Google Scholar] [CrossRef]
- Bolu, O.; Nnadi, C.; Damisa, E.; Braka, F.; Siddique, A.; Archer, W.R.; Bammeke, P.; Banda, R.; Higgins, J.; Edukugo, A.; et al. Progress Toward Poliomyelitis Eradication—Nigeria, January–December 2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 253–256. [Google Scholar] [CrossRef]
- Adamu, U.S.; Archer, W.R.; Braka, F.; Damisa, E.; Siddique, A.; Baig, S.; Higgins, J.; Sume, G.E.; Banda, R.; Korir, C.K.; et al. Progress Toward Poliomyelitis Eradication—Nigeria, January 2018–May 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Adamu, U.; Adewara, K.; Aladeshawe, A.; Aregay, A.; Barau, I.; Berens, A.; Bolu, O.; Dutton, N.; Iduma, N.; et al. Finding inhabited settlements and tracking vaccination progress: The application of satellite imagery analysis to guide the immunization response to confirmation of previously-undetected, ongoing endemic wild poliovirus transmission in Borno State, Nigeria. Int. J. Health Geogr. 2019, 18, 11. [Google Scholar] [CrossRef]
- Wiesen, E.; Dankoli, R.; Musa, M.; Higgins, J.; Forbi, J.; Idris, J.; Waziri, N.; Ogunbodede, O.; Mohammed, K.; Bolu, O.; et al. Conducting public health surveillance in areas of armed conflict and restricted population access: A qualitative case study of polio surveillance in conflict-affected areas of Borno State, Nigeria. Confl. Health 2022, 16, 20. [Google Scholar] [CrossRef]
- Nnadi, C.; Etsano, A.; Uba, B.; Ohuabunwo, C.; Melton, M.; Wa Nganda, G.; Esapa, L.; Bolu, O.; Mahoney, F.; Vertefeuille, J.; et al. Approaches to Vaccination Among Populations in Areas of Conflict. J. Infect. Dis. 2017, 216, S368–S372. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Franka, R.; Higgins, J.; Kovacs, S.D.; Forbi, J.C.; Wassilak, S.G.F.; Pallansch, M.A.; Thompson, K.M. Modeling Poliovirus Transmission in Borno and Yobe, Northeast Nigeria. Risk Anal. Off. Publ. Soc. Risk Anal. 2021, 41, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Kalkowska, D.A.; Thompson, K.M. Modeling Undetected Live Poliovirus Circulation After Apparent Interruption of Transmission: Borno and Yobe in Northeast Nigeria. Risk Anal. Off. Publ. Soc. Risk Anal. 2021, 41, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Hamisu, A.W.; Johnson, T.M.; Craig, K.; Mkanda, P.; Banda, R.; Tegegne, S.G.; Oyetunji, A.; Ningi, N.; Mohammed, S.M.; Adamu, M.I.; et al. Strategies for Improving Polio Surveillance Performance in the Security-Challenged Nigerian States of Adamawa, Borno, and Yobe During 2009–2014. J. Infect. Dis. 2016, 213 (Suppl. 3), S136–S139. [Google Scholar] [CrossRef]
- Simpson, D.M.; Sadr-Azodi, N.; Mashal, T.; Sabawoon, W.; Pardis, A.; Quddus, A.; Garrigos, C.; Guirguis, S.; Zahoor Zaidi, S.S.; Shaukat, S.; et al. Polio eradication initiative in Afghanistan, 1997–2013. J. Infect. Dis. 2014, 210 (Suppl. 1), S162–S172. [Google Scholar] [CrossRef]
- Alexander, J.P., Jr.; Zubair, M.; Khan, M.; Abid, N.; Durry, E. Progress and peril: Poliomyelitis eradication efforts in Pakistan, 1994–2013. J. Infect. Dis. 2014, 210 (Suppl. 1), S152–S161. [Google Scholar] [CrossRef]
- Farag, N.H.; Alexander, J.; Hadler, S.; Quddus, A.; Durry, E.; Wadood, M.Z.; Tangermann, R.H.; Ehrhardt, D. Progress toward poliomyelitis eradication—Afghanistan and Pakistan, January 2013–August 2014. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 973–977. [Google Scholar]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Cochi, S.L.; Ehrhardt, D.T.; Farag, N.H.; Hadler, S.C.; Hampton, L.M.; Martinez, M.; Wassilak, S.G.F.; Thompson, K.M. Modeling Poliovirus Transmission in Pakistan and Afghanistan to Inform Vaccination Strategies in Undervaccinated Subpopulations. Risk Anal. 2018, 38, 1701–1717. [Google Scholar] [CrossRef]
- Mbaeyi, C.; Saatcioglu, A.; Tangermann, R.H.; Hadler, S.; Ehrhardt, D. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2014–August 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Mbaeyi, C.; Shukla, H.; Smith, P.; Tangermann, R.H.; Martinez, M.; Jorba, J.C.; Hadler, S.; Ehrhardt, D. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2015–August 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1195–1199. [Google Scholar] [CrossRef][Green Version]
- Martinez, M.; Shukla, H.; Nikulin, J.; Wadood, M.Z.; Hadler, S.; Mbaeyi, C.; Tangermann, R.; Jorba, J.; Ehrhardt, D. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2016–June 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 854–858. [Google Scholar] [CrossRef]
- Martinez, M.; Shukla, H.; Ahmadzai, M.; Nikulin, J.; Wadood, M.Z.; Ahmed, J.; Mbaeyi, C.; Jorba, J.; Ehrhardt, D. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2017–May 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Shukla, H.; Nikulin, J.; Mbaeyi, C.; Jorba, J.; Ehrhardt, D. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2018–May 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 729–733. [Google Scholar] [CrossRef]
- Martinez, M.; Akbar, I.E.; Wadood, M.Z.; Shukla, H.; Jorba, J.; Ehrhardt, D. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2019–July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1464–1468. [Google Scholar] [CrossRef]
- Sadigh, K.S.; Akbar, I.E.; Wadood, M.Z.; Shukla, H.; Jorba, J.; Chaudhury, S.; Martinez, M. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2020–November 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 85–89. [Google Scholar] [CrossRef]
- Rana, M.S.; Asghar, R.J.; Usman, M.; Ikram, A.; Salman, M.; Umair, M.; Zaidi, S.S.Z.; Anas, M.; Ullah, N. The resurgence of wild poliovirus in Pakistan and Afghanistan: A new setback for polio eradication. J. Infect. 2022, 85, 334–363. [Google Scholar] [CrossRef]
- Bigouette, J.P.; Wilkinson, A.L.; Tallis, G.; Burns, C.C.; Wassilak, S.G.F.; Vertefeuille, J.F. Progress Toward Polio Eradication—Worldwide, January 2019–June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Nishtar, S. Pakistan, politics and polio. Bull. World Health Organ. 2010, 88, 159–160. [Google Scholar] [CrossRef]
- Hussain, S.F.; Boyle, P.; Patel, P.; Sullivan, R. Eradicating polio in Pakistan: An analysis of the challenges and solutions to this security and health issue. Glob. Health 2016, 12, 63. [Google Scholar] [CrossRef]
- Ahmed, Q.A.; Nishtar, S.; Memish, Z.A. Poliomyelitis in Pakistan: Time for the Muslim world to step in. Lancet 2013, 381, 1521–1523. [Google Scholar] [CrossRef]
- Habib, M.A.; Soofi, S.B.; Ali, N.; Hussain, I.; Tabassum, F.; Suhag, Z.; Anwar, S.; Ahmed, I.; Bhutta, Z.A. Knowledge and perceptions of polio and polio immunization in polio high-risk areas of Pakistan. J. Public Health Policy 2017, 38, 16–36. [Google Scholar] [CrossRef]
- Habib, M.A.; Tabassum, F.; Hussain, I.; Khan, T.J.; Syed, N.; Shaheen, F.; Soofi, S.B.; Bhutta, Z.A. Exploring Knowledge and Perceptions of Polio Disease and Its Immunization in Polio High-Risk Areas of Pakistan. Vaccines 2023, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Okayasu, H.; Nzioki, M.M.; Wadood, M.Z.; Chabot-Couture, G.; Quddus, A.; Walker, G.; Sutter, R.W. Lot quality assurance sampling to monitor supplemental immunization activity quality: An essential tool for improving performance in polio endemic countries. J. Infect. Dis. 2014, 210 (Suppl. 1), S333–S340. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Progress Toward Poliomyelitis Eradication—Afghanistan, January 2012–September 2013. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 928–933. [Google Scholar]
- Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Afghanistan and Pakistan, January 2004–February 2005. MMWR Morb. Mortal. Wkly. Rep. 2005, 54, 276–279. [Google Scholar]
- Safi, N.; Anwari, P.; Safi, H. Afghanistan’s health system under the Taliban: Key challenges. Lancet 2022, 400, 1179–1180. [Google Scholar] [CrossRef]
- Sabawoon, W.; Seino, S.; Pason, B.M.; Momin, N.W.S.; Kanamori, S.; Bender, C.; Takemura, K. Progress in Access and Oral Polio Vaccine Coverage Among Children Aged <5 Years in Polio Campaigns After the Political Change in Afghanistan. J. Infect. Dis. 2025, 231, e438–e445. [Google Scholar] [CrossRef] [PubMed]
- Farag, N.H.; Wadood, M.Z.; Safdar, R.M.; Ahmed, N.; Hamdi, S.; Tangermann, R.H.; Ehrhardt, D. Progress Toward Poliomyelitis Eradication—Pakistan, January 2014–September 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1271–1275. [Google Scholar] [CrossRef]
- Elhamidi, Y.; Mahamud, A.; Safdar, M.; Al Tamimi, W.; Jorba, J.; Mbaeyi, C.; Hsu, C.H.; Wadood, Z.; Sharif, S.; Ehrhardt, D. Progress Toward Poliomyelitis Eradication—Pakistan, January 2016–September 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1276–1280. [Google Scholar] [CrossRef]
- Hsu, C.; Mahamud, A.; Safdar, M.; Nikulin, J.; Jorba, J.; Bullard, K.; Agbor, J.; Kader, M.; Sharif, S.; Ahmed, J.; et al. Progress Toward Poliomyelitis Eradication—Pakistan, January 2017–September 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.M. Country ownership in global health. PLOS Glob. Public Health 2022, 2, e0000113. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E.; Carneiro, I.A. Transmissibility and persistence of oral polio vaccine viruses: Implications for the global poliomyelitis eradication initiative. Am. J. Epidemiol. 1999, 150, 1001–1021. [Google Scholar] [CrossRef]
- Chen, R.T.; Hausinger, S.; Dajani, A.S.; Hanfling, M.; Baughman, A.L.; Pallansch, M.A.; Patriarca, P.A. Seroprevalence of antibody against poliovirus in inner-city preschool children. Implications for vaccination policy in the United States. JAMA 1996, 275, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Kew, O.M.; Pallansch, M.A.; Omilianowski, D.R.; Rueckert, R.R. Changes in three of the four coat proteins of oral polio vaccine strain derived from type 1 poliovirus. J. Virol. 1980, 33, 256–263. [Google Scholar] [CrossRef]
- Kew, O.M.; Morris-Glasgow, V.; Landaverde, M.; Burns, C.; Shaw, J.; Garib, Z.; Andre, J.; Blackman, E.; Freeman, C.J.; Jorba, J.; et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 2002, 296, 356–359. [Google Scholar] [CrossRef]
- Kew, O.M.; Wright, P.F.; Agol, V.I.; Delpeyroux, F.; Shimizu, H.; Nathanson, N.; Pallansch, M.A. Circulating vaccine-derived polioviruses: Current state of knowledge. Bull. World Health Organ. 2004, 82, 16–23. [Google Scholar]
- Burns, C.C.; Diop, O.M.; Sutter, R.W.; Kew, O.M. Vaccine-derived polioviruses. J. Infect. Dis. 2014, 210 (Suppl. 1), S283–S293. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses—Worldwide, January 2006–August 2007. MMWR Morb. Mortal. Wkly. Rep. 2007, 56, 996–1001. [Google Scholar]
- Estivariz, C.F.; Krow-Lucal, E.R.; Mach, O. Immunodeficiency-Related Vaccine-Derived Poliovirus (iVDPV) Infections: A Review of Epidemiology and Progress in Detection and Management. Pathogens 2024, 13, 1128. [Google Scholar] [CrossRef]
- Adu, F.; Iber, J.; Bukbuk, D.; Gumede, N.; Yang, S.J.; Jorba, J.; Campagnoli, R.; Sule, W.F.; Yang, C.F.; Burns, C.; et al. Isolation of recombinant type 2 vaccine-derived poliovirus (VDPV) from a Nigerian child. Virus Res. 2007, 127, 17–25. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. Classification and Reporting of Vaccine-Derived Polioviruses (VDPVs). 2021. Available online: http://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf (accessed on 4 September 2025).
- Wassilak, S.; Pate, M.A.; Wannemuehler, K.; Jenks, J.; Burns, C.; Chenoweth, P.; Abanida, E.A.; Adu, F.; Baba, M.; Gasasira, A.; et al. Outbreak of type 2 vaccine-derived poliovirus in Nigeria: Emergence and widespread circulation in an underimmunized population. J. Infect. Dis. 2011, 203, 898–909. [Google Scholar] [CrossRef]
- Burns, C.C.; Shaw, J.; Jorba, J.; Bukbuk, D.; Adu, F.; Gumede, N.; Pate, M.A.; Abanida, E.A.; Gasasira, A.; Iber, J.; et al. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J. Virol. 2013, 87, 4907–4922. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Update on Vaccine-Derived Polioviruses—Worldwide, January 2008–June 2009. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 1002–1006. [Google Scholar]
- Centers for Disease Control and Prevention. Update on Vaccine-Derived Polioviruses—Worldwide, July 2009–March 2011. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 846–850. [Google Scholar]
- Centers for Disease Control and Prevention. Progress Toward Poliomyelitis Eradication—Nigeria, January 2008–July 2009. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 1150–1154. [Google Scholar]
- Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses—Worldwide, April 2011–June 2012. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 741–746. [Google Scholar]
- Alleman, M.M.; Jorba, J.; Riziki, Y.; Henderson, E.; Mwehu, A.; Seakamela, L.; Howard, W.; Kadiobo Mbule, A.; Nsamba, R.N.; Djawe, K.; et al. Vaccine-derived poliovirus serotype 2 outbreaks and response in the Democratic Republic of the Congo, 2017–2021. Vaccine 2023, 41 (Suppl. 1), A35–A47. [Google Scholar] [CrossRef]
- Jenkins, H.E.; Aylward, R.B.; Gasasira, A.; Donnelly, C.A.; Mwanza, M.; Corander, J.; Garnier, S.; Chauvin, C.; Abanida, E.; Pate, M.A.; et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N. Engl. J. Med. 2010, 362, 2360–2369. [Google Scholar] [CrossRef]
- Grassly, N.C.; Wenger, J.; Durrani, S.; Bahl, S.; Deshpande, J.M.; Sutter, R.W.; Heymann, D.L.; Aylward, R.B. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: A case-control study. Lancet 2007, 369, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Caceres, V.M.; Sutter, R.W. Sabin monovalent oral polio vaccines: Review of past experiences and their potential use after polio eradication. Clin. Infect. Dis. 2001, 33, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—India, January 2005–June 2006. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 772–776. [Google Scholar]
- Sutter, R.W.; John, T.J.; Jain, H.; Agarkhedkar, S.; Ramanan, P.V.; Verma, H.; Deshpande, J.; Singh, A.P.; Sreevatsava, M.; Malankar, P.; et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: A randomised, double-blind, controlled trial. Lancet 2010, 376, 1682–1688. [Google Scholar] [CrossRef]
- World Health Organization. Conclusions and recommendations of the Advisory Committee on Poliomyelitis Eradication, November 2009. Special consultation with polio-infected countries and global management team partners, Geneva, 18–19 November 2009. Wkly. Epidemiol. Rec. 2010, 85, 1–11. [Google Scholar]
- Kew, O.M.; Cochi, S.L.; Jafari, H.S.; Wassilak, S.G.; Mast, E.E.; Diop, O.M.; Tangermann, R.H.; Armstrong, G.L. Possible eradication of wild poliovirus type 3—Worldwide, 2012. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 1031–1033. [Google Scholar]
- Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—-India, January 2010–September 2011. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1482–1486. [Google Scholar]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, November 2012—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2013, 88, 1–16. [Google Scholar]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2012—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2012, 87, 201–216. [Google Scholar]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2013—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2013, 88, 201–216. [Google Scholar]
- World Health Organization. Meeting of the StrategicAdvisory Group of Experts on immunization, November 2013—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2014, 89, 1–19. [Google Scholar]
- World Health Organization. Meeting of the Strategic Advisory Group of Expert on immunization, April 2014—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2014, 89, 211–236. [Google Scholar]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2014—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2014, 89, 561–576. [Google Scholar]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2015: Conclusions and recommendations. Wkly. Epidemiol. Rec. 2015, 90, 261–276. [Google Scholar]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2015—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2015, 90, 681–699. [Google Scholar]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, April 2016—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2016, 91, 266–284. [Google Scholar]
- Thompson, K.M.; Duintjer Tebbens, R.J. Lessons From the Polio Endgame: Overcoming the Failure to Vaccinate and the Role of Subpopulations in Maintaining Transmission. J. Infect. Dis. 2017, 216, S176–S182. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.V.; Bandyopadhyay, A.S.; Gumede, N.; Mach, O.; Mkanda, P.; Ndoutabé, M.; Okiror, S.O.; Ramirez-Gonzalez, A.; Touray, K.; Wanyoike, S.; et al. Risk factors for the spread of vaccine-derived type 2 polioviruses after global withdrawal of trivalent oral poliovirus vaccine and the effects of outbreak responses with monovalent vaccine: A retrospective analysis of surveillance data for 51 countries in Africa. Lancet. Infect. Dis. 2022, 22, 284–294. [Google Scholar] [CrossRef]
- Macklin, G.; Peak, C.; Eisenhawer, M.; Kurji, F.; Mach, O.; Konz, J.; Gast, C.; Bachtiar, N.S.; Bandyopadhyay, A.S.; Zipursky, S. Enabling accelerated vaccine roll-out for Public Health Emergencies of International Concern (PHEICs): Novel Oral Polio Vaccine type 2 (nOPV2) experience. Vaccine 2023, 41, A122–A127. [Google Scholar] [CrossRef]
- Nathanson, N.; Kew, O.M. From emergence to eradication: The epidemiology of poliomyelitis deconstructed. Am. J. Epidemiol. 2010, 172, 1213–1229. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Public Health Dispatch: Update: Outbreak of Poliomyelitis—Dominican Republic and Haiti, 2000–2001. MMWR Morb. Mortal. Wkly. Rep. 2001, 50, 855–856. [Google Scholar]
- Centers for Disease Control and Prevention. Update on Vaccine-Derived Polioviruses. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 1093–1097. [Google Scholar]
- Diop, O.M.; Burns, C.C.; Wassilak, S.G.; Kew, O.M. Update on Vaccine-Derived Polioviruses-Worldwide, July 2012–December 2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 242–248. [Google Scholar] [PubMed]
- Diop, O.M.; Burns, C.C.; Sutter, R.W.; Wassilak, S.G.; Kew, O.M. Update on Vaccine-Derived Polioviruses-Worldwide, January 2014–March 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 640–646. [Google Scholar]
- Jorba, J.; Diop, O.M.; Iber, J.; Sutter, R.W.; Wassilak, S.G.; Burns, C.C. Update on Vaccine-Derived Polioviruses-Worldwide, January 2015–May 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Jorba, J.; Diop, O.M.; Iber, J.; Henderson, E.; Zhao, K.; Sutter, R.W.; Wassilak, S.G.F.; Burns, C.C. Update on Vaccine-Derived Polioviruses-Worldwide, January 2017–June 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1189–1194. [Google Scholar] [CrossRef]
- Jorba, J.; Diop, O.M.; Iber, J.; Henderson, E.; Zhao, K.; Quddus, A.; Sutter, R.; Vertefeuille, J.F.; Wenger, J.; Wassilak, S.G.F.; et al. Update on Vaccine-Derived Poliovirus Outbreaks-Worldwide, January 2018–June 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1024–1028. [Google Scholar] [CrossRef]
- Alleman, M.M.; Jorba, J.; Greene, S.A.; Diop, O.M.; Iber, J.; Tallis, G.; Goel, A.; Wiesen, E.; Wassilak, S.G.F.; Burns, C.C. Update on Vaccine-Derived Poliovirus Outbreaks-Worldwide, July 2019–February 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Bigouette, J.P.; Henderson, E.; Traoré, M.A.; Wassilak, S.G.F.; Jorba, J.; Mahoney, F.; Bolu, O.; Diop, O.M.; Burns, C.C. Update on Vaccine-Derived Poliovirus Outbreaks-Worldwide, January 2021–December 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Voorman, A.; Lyons, H.M. Measuring polio immunity to plan immunization activities. Vaccine 2016, 34, 5946–5952. [Google Scholar] [CrossRef]
- Voorman, A.; Hoff, N.A.; Doshi, R.H.; Alfonso, V.; Mukadi, P.; Muyembe-Tamfum, J.J.; Wemakoy, E.O.; Bwaka, A.; Weldon, W.; Gerber, S.; et al. Polio immunity and the impact of mass immunization campaigns in the Democratic Republic of the Congo. Vaccine 2017, 35, 5693–5699. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Circulation of a type 2 vaccine-derived poliovirus—Egypt, 1982–1993. Morb. Mortal. Wkly. Rep. 2001, 50, 41–42, 51. [Google Scholar]
- Yang, C.F.; Naguib, T.; Yang, S.J.; Nasr, E.; Jorba, J.; Ahmed, N.; Campagnoli, R.; van der Avoort, H.; Shimizu, H.; Yoneyama, T.; et al. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt from 1983 to 1993. J. Virol. 2003, 77, 8366–8377. [Google Scholar] [CrossRef] [PubMed]
- Rousset, D.; Rakoto-Andrianarivelo, M.; Razafindratsimandresy, R.; Randriamanalina, B.; Guillot, S.; Balanant, J.; Mauclere, P.; Delpeyroux, F. Recombinant vaccine-derived poliovirus in Madagascar. Emerg. Infect. Dis. 2003, 9, 885–887. [Google Scholar] [PubMed]
- Rakoto-Andrianarivelo, M.; Gumede, N.; Jegouic, S.; Balanant, J.; Andriamamonjy, S.N.; Rabemanantsoa, S.; Birmingham, M.; Randriamanalina, B.; Nkolomoni, L.; Venter, M.; et al. Reemergence of recombinant vaccine-derived poliovirus outbreak in Madagascar. J. Infect. Dis. 2008, 197, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Etsano, A.; Damisa, E.; Shuaib, F.; Nganda, G.W.; Enemaku, O.; Usman, S.; Adeniji, A.; Jorba, J.; Iber, J.; Ohuabunwo, C.; et al. Environmental Isolation of Circulating Vaccine-Derived Poliovirus After Interruption of Wild Poliovirus Transmission-Nigeria, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 770–773. [Google Scholar] [CrossRef][Green Version]
- Link-Gelles, R.; Lutterloh, E.; Schnabel Ruppert, P.; Backenson, P.B.; St George, K.; Rosenberg, E.S.; Anderson, B.J.; Fuschino, M.; Popowich, M.; Punjabi, C.; et al. Public Health Response to a Case of Paralytic Poliomyelitis in an Unvaccinated Person and Detection of Poliovirus in Wastewater-New York, June–August 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1065–1068. [Google Scholar] [CrossRef]
- Kasstan, B.; Chantler, T.; Marcus, B.; Mounier-Jack, S.; Saliba, V.; Edelstein, M. Linked poliovirus incidents in the UK, USA and Israel: Silent transmission or missed warnings of vaccine inequity? Vaccine 2023, 1, 2339–2342. [Google Scholar] [CrossRef]
- Klapsa, D.; Wilton, T.; Zealand, A.; Bujaki, E.; Saxentoff, E.; Troman, C.; Shaw, A.G.; Tedcastle, A.; Majumdar, M.; Mate, R.; et al. Sustained detection of type 2 poliovirus in London sewage between February and July, 2022, by enhanced environmental surveillance. Lancet 2022, 400, 1531–1538. [Google Scholar] [CrossRef]
- Seo, G.E.; Mandes, R.; Wright, N.D.; Hawkins, J.P.; Landgraff, A.; Lidder, R.; Mohammed, U.; Mangat, C.S.; Michel, A.S.; Fafard, J.; et al. Sporadic detection of vaccine-derived poliovirus type 2 using next-generation sequencing in Canadian wastewater in August of 2022. Sci. Rep. 2025, 15, 12913. [Google Scholar] [CrossRef]
- Grotto, I.; Agha, H.; Abu Al-Halaweh, A.; Davidovitch, N.; McKee, M.; Nitzan, D. Public health, war and cross-border challenges: The recent cVDPV2 polio outbreak in Gaza. EClinicalMedicine 2025, 81, 103136. [Google Scholar] [CrossRef] [PubMed]
- Huseynov, S.; Saxentoff, E.; Diedrich, S.; Martin, J.; Wieczorek, M.; Cabrerizo, M.; Blomqvist, S.; Jorba, J.; Hagan, J. Notes from the Field: Detection of Vaccine-Derived Poliovirus Type 2 in Wastewater-Five European Countries, September-December 2024. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 122–124. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. Kingdom of Saudi Arabia confirms US$500 Million Commitment to Global Polio Eradication Effort. 2025. Press Release 24 February. 2025. Available online: https://polioeradication.org/news/kingdom-of-saudi-arabia-confirms-us-500-million-commitment-to-global-polio-eradication-effort/ (accessed on 4 September 2025).
- Guarino, K.; Voorman, A.; Gasteen, M.; Stewart, D.; Wenger, J. Violence, insecurity, and the risk of polio: A systematic analysis. PLoS ONE 2017, 12, e0185577. [Google Scholar] [CrossRef] [PubMed]
- Ugwuoke, C.O.; Ajah, B.O.; Akor, L.; Ameh, S.O.; Lanshima, C.A.; Ngwu, E.C.; Eze, U.A.; Nwokedi, M. Violent crimes and insecurity on Nigerian highways: A tale of travelers’ trauma, nightmares and state slumber. Heliyon 2023, 9, e20489. [Google Scholar] [CrossRef]
- Mbaeyi, C. Polio vaccination activities in conflict-affected areas. Hum. Vaccines Immunother. 2023, 19, 2237390. [Google Scholar] [CrossRef]
- Mendes, A.; Mohamed, G.A.; Derow, M.; Stehling-Ariza, T.; Mohamed, A.; Mengistu, K.; Bullard, K.; Akbar, I.E.; Shukla, H.; Al Safadi, M.; et al. Persistent Transmission of Circulating Vaccine-Derived Poliovirus-Somalia, January 2017–March 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 575–580. [Google Scholar] [CrossRef]
- World Health Organization. Mass Polio Vaccination Campaign to Continue in the Gaza Strip. Press Release 19 February. 2025. Available online: https://www.who.int/news/item/19-02-2025-mass-polio-vaccination-campaign-to-continue-in-the-gaza-strip (accessed on 4 September 2025).
- British Broadcasting Corporation. Israel Agrees to Pauses in Fighting for Polio Vaccine Drive. 2024. Available online: https://www.bbc.com/news/articles/cn02z5kjn40o (accessed on 4 September 2025).
- Gammino, V.M.; Diaz, M.R.; Pallas, S.W.; Greenleaf, A.R.; Kurnit, M.R. Health services uptake among nomadic pastoralist populations in Africa: A systematic review of the literature. PLoS Negl. Trop. Dis. 2020, 14, e0008474. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Polio field census and vaccination of underserved populations—Northern Nigeria, 2012–2013. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 663–665. [Google Scholar]
- Waziri, N.E.; Ohuabunwo, C.J.; Nguku, P.M.; Ogbuanu, I.U.; Gidado, S.; Biya, O.; Wiesen, E.S.; Vertefeuille, J.; Townes, D.; Oyemakinde, A.; et al. Polio eradication in Nigeria and the role of the National Stop Transmission of Polio program, 2012–2013. J. Infect. Dis. 2014, 210 (Suppl. 1), S111–S117. [Google Scholar] [CrossRef] [PubMed]
- Gidado, S.O.; Ohuabunwo, C.; Nguku, P.M.; Ogbuanu, I.U.; Waziri, N.E.; Biya, O.; Wiesen, E.S.; Mba-Jonas, A.; Vertefeuille, J.; Oyemakinde, A.; et al. Outreach to underserved communities in northern Nigeria, 2012–2013. J. Infect. Dis. 2014, 210 (Suppl. 1), S118–S124. [Google Scholar] [CrossRef]
- Snider, C.J.; Boualam, L.; Tallis, G.; Takashima, Y.; Abeyasinghe, R.; Lo, Y.R.; Grabovac, V.; Avagyan, T.; Aslam, S.K.; Eltayeb, A.O.; et al. Concurrent outbreaks of circulating vaccine-derived poliovirus types 1 and 2 affecting the Republic of the Philippines and Malaysia, 2019–2021. Vaccine 2023, 41 (Suppl. 1), A58–A69. [Google Scholar] [CrossRef]
- Habib, M.A.; Soofi, S.; Cousens, S.; Anwar, S.; Haque, N.U.; Ahmed, I.; Ali, N.; Tahir, R.; Bhutta, Z.A. Community engagement and integrated health and polio immunisation campaigns in conflict-affected areas of Pakistan: A cluster randomised controlled trial. Lancet. Glob. Health 2017, 5, e593–e603. [Google Scholar] [CrossRef]
- Majidulla, A.; Sultan, M.A.; Zaman, A.; Shafique, M.; Ahmed, S.; Naz, F.; Nayyab, S.; Sohail, A. Engage less, provide more: Community health workers’ perspectives on how to overcome opposition to polio vaccination in Pakistan. Glob. Public Health 2025, 20, 2465645. [Google Scholar] [CrossRef] [PubMed]
- Warigon, C.; Mkanda, P.; Muhammed, A.; Etsano, A.; Korir, C.; Bawa, S.; Gali, E.; Nsubuga, P.; Erbeto, T.B.; Gerlong, G.; et al. Demand Creation for Polio Vaccine in Persistently Poor-Performing Communities of Northern Nigeria: 2013–2014. J. Infect. Dis. 2016, 213 (Suppl. 3), S79–S85. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.H.; Mehraj, J.; Khowaja, A.; Sodhar, I.A.; Chandio, S.A.; Rasool, S.; Zardari, A.A.; Hussain, I.; Bosan, A.; Stuckey, E.M.; et al. Community acceptance of services and effectiveness of health camps in high-risk areas of Karachi, Sindh, Pakistan, 2021. Front Public Health 2024, 12, 1498016. [Google Scholar] [CrossRef] [PubMed]
- Lassi, Z.S.; Naseem, R.; Salam, R.A.; Siddiqui, F.; Das, J.K. The Impact of the COVID-19 Pandemic on Immunization Campaigns and Programs: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 988. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2021: Conclusions and recommendations. Wkly. Epidemiol. Rec. 2021, 96, 613–632. [Google Scholar]
- Lush, L.; Attfield, W.; Baxendale, J.; Vuylsteke, Y.; McPherson, S.; Rutherford, S.; Page, P.; Weil, C.; Maynard, C.; Kastritis, P.; et al. Evaluation of Gavi’s Contribution to Reaching Zero-Dose and Missed Communities: Year 1 Annual Report; Gavi: Geneva, Switzerland, 2024; Available online: https://www.gavi.org/sites/default/files/evaluations/ZD-Evaluation_Year-1_Final%20Report.pdf (accessed on 4 September 2025).
- World Health Organization; UNICEF; Gavi; Bill & Melinda Gates Foundation. Global Partners Announce a New Effort—“The Big Catch-Up”—To Vaccinate Millions of Children and Restore Immunization Progress Lost During the Pandemic. Press Release 24 April. 2023. Available online: https://www.who.int/news/item/24-04-2023-global-partners-announce-a-new-effort-the-big-catch-up-to-vaccinate-millions-of-children-and-restore-immunization-progress-lost-during-the-pandemic (accessed on 4 September 2025).
- Gavi; The Vaccine Initiative. One Year of the Big Catch-Up: What Progress Has Been Made? 2024. Available online: https://www.gavi.org/vaccineswork/one-year-big-catch-what-progress (accessed on 4 September 2025).
- World Health Organization; UNICEF. Global Childhood Vaccination Coverage Holds Steady, yet over 14 Million Infants Remain Unvaccinated–WHO, UNICEF. Press Release 15 July. 2025. Available online: https://www.who.int/news/item/15-07-2025-global-childhood-vaccination-coverage-holds-steady-yet-over-14-million-infants-remain-unvaccinated-who-unicef (accessed on 4 September 2025).
- Darwar, R.; Biya, O.; Greene, S.A.; Jorba, J.; Al Safadi, M.; Franka, R.; Wiesen, E.; Durry, E.; Pallansch, M.A. Assessing country compliance with circulating vaccine-derived poliovirus type 2 outbreak response standard operating procedures: April 2016 to December 2020. Vaccine 2023, 41, A25–A34. [Google Scholar] [CrossRef]
- Geiger, K.; Heaghney, N.; Bigouette, J.P.; Bennett, S.D.; Kovacs, S.D.; Wassilak, S.G.F. Supplemental Immunization Activity Response Timeliness for Circulating Vaccine-Derived Poliovirus Outbreaks—Worldwide, 2016–2023. In Proceedings of the Epidemic Intelligence Service Conference 2024, Atlanta, GA, USA, 23–26 April 2024; p. 84. Available online: https://stacks.cdc.gov/view/cdc/154509/cdc_154509_DS1.pdf (accessed on 4 September 2025).
- Thompson, K.M.; Duintjer Tebbens, R.J.; Pallansch, M.A. Evaluation of response scenarios to potential polio outbreaks using mathematical models. Risk Anal. 2006, 26, 1541–1556. [Google Scholar] [CrossRef]
- Shaw, A.G.; Mampuela, T.K.; Lofiko, E.L.; Pratt, C.; Troman, C.; Bujaki, E.; O’Toole, Á.; Akello, J.O.; Aziza, A.A.; Lusamaki, E.K.; et al. Sensitive poliovirus detection using nested PCR and nanopore sequencing: A prospective validation study. Nat. Microbiol. 2023, 8, 1634–1640. [Google Scholar] [CrossRef]
- Ueno, M.K.; Kitamura, K.; Nishimura, Y.; Arita, M. Evaluation of Direct Detection Protocols for Poliovirus from Stool Samples of Acute Flaccid Paralysis Patients. Viruses 2023, 15, 2113. [Google Scholar] [CrossRef]
- Miles, S.J.; Harrington, C.; Sun, H.; Deas, A.; Oberste, M.S.; Nix, W.A.; Vega, E.; Gerloff, N. Validation of improved automated nucleic acid extraction methods for direct detection of polioviruses for global polio eradication. J. Virol. Methods 2024, 326, 114914. [Google Scholar] [CrossRef]
- Marcet, P.L.; Short, B.; Deas, A.; Sun, H.; Harrington, C.; Shaukat, S.; Alam, M.M.; Baba, M.; Faneye, A.; Namuwulya, P.; et al. Advancing poliovirus eradication: Lessons learned from piloting direct molecular detection of polioviruses in high-risk and priority geographies. Microbiol. Spectr. 2025, 13, e0227924. [Google Scholar] [CrossRef]
- Bahl, S.; Verma, H.; Bhatnagar, P.; Haldar, P.; Satapathy, A.; Kumar, K.N.; Horton, J.; Estivariz, C.F.; Anand, A.; Sutter, R. Fractional-Dose Inactivated Poliovirus Vaccine Immunization Campaign-Telangana State, India, June 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, A.; Mbaeyi, C.; Baig, M.A.; Burman, A.; Ahmed, J.A.; Akter, S.; Jatoi, F.A.; Mahamud, A.; Asghar, R.J.; Azam, N.; et al. Fractional-Dose Inactivated Poliovirus Vaccine Campaign-Sindh Province, Pakistan, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Grassly, N.C.; Wadood, M.Z.; Safdar, R.M.; Mahamud, A.S.; Sutter, R.W. Effect of Inactivated Poliovirus Vaccine Campaigns, Pakistan, 2014–2017. Emerg Infect Dis 2018, 24, 2113–2115. [Google Scholar] [CrossRef]
- Bashorun, A.O.; Badjie Hydara, M.; Adigweme, I.; Umesi, A.; Danso, B.; Johnson, N.; Sambou, N.A.; Fofana, S.; Kanu, F.J.; Jeyaseelan, V.; et al. Intradermal administration of fractional doses of the inactivated poliovirus vaccine in a campaign: A pragmatic, open-label, non-inferiority trial in The Gambia. Lancet Glob. Health 2022, 10, e257–e268. [Google Scholar] [CrossRef] [PubMed]
- Biya, O.; Manu, J.I.; Forbi, J.C.; Wa Nganda, G.; Ikwe, H.; Sule, A.; Edukugho, A.; Shehu, A.; Aliyu, N.; Barau, N.D.; et al. Notes from the Field: House-to-House Campaign Administration of Inactivated Poliovirus Vaccine-Sokoto State, Nigeria, November 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1290–1291. [Google Scholar] [CrossRef]
- Estivariz, C.F.; Kovacs, S.D.; Mach, O. Review of use of inactivated poliovirus vaccine in campaigns to control type 2 circulating vaccine derived poliovirus (cVDPV) outbreaks. Vaccine 2023, 41, A113–A121. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, September 2024—Conclusions and recommendations. Wkly. Epidemiol. Rec. 2024, 99, 719–740. [Google Scholar]
- Cochi, S.L.; Freeman, A.; Guirguis, S.; Jafari, H.; Aylward, B. Global polio eradication initiative: Lessons learned and legacy. J. Infect. Dis. 2014, 210 (Suppl. 1), S540–S546. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Assessing the Risks for Poliovirus Outbreaks in Polio-Free Countries—Africa, 2012–2013. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 768–772. [Google Scholar]
- Voorman, A.; Lyons, H.; Bennette, C.; Kovacs, S.; Makam, J.K.; Vertefeuille, J.F.; Tallis, G. Analysis of population immunity to poliovirus following cessation of trivalent oral polio vaccine. Vaccine 2023, 41 (Suppl. 1), A85–A92. [Google Scholar] [CrossRef]
- Kalkowska, D.A.; Wassilak, S.G.F.; Wiesen, E.; Burns, C.C.; Pallansch, M.A.; Badizadegan, K.; Thompson, K.M. Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences. Risk Anal. Off. Publ. Soc. Risk Anal. 2023, 44, 366–378. [Google Scholar] [CrossRef]
- Global Polio Eradication Initiative. Polio Eradication Strategy 2022–2026: Delivering on a Promise, Extension to 2029; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/382399/9789240109506-eng.pdf?sequence=1&isAllowed=y (accessed on 4 September 2025).
- Global Polio Eradication Initiative. WHO Executive Board Says Emergency Measures Needed to Stop Polio: Global Polio and Health Experts Urge Right Geopolitical Decisions to Stop ‘Perfect Storm’ for Poliovirus Transmission and Protect Eradication. Press Release 7 February 2025. Available online: https://polioeradication.org/news/who-executive-board-says-emergency-measures-needed-to-stop-polio/ (accessed on 4 September 2025).
- Kalkowska, D.A.; Duintjer Tebbens, R.J.; Thompson, K.M. Environmental Surveillance System Characteristics and Impacts on Confidence About No Undetected Serotype 1 Wild Poliovirus Circulation. Risk Anal. 2019, 39, 414–425. [Google Scholar] [CrossRef]
- Kroiss, S.J.; Ahmadzai, M.; Ahmed, J.; Alam, M.M.; Chabot-Couture, G.; Famulare, M.; Mahamud, A.; McCarthy, K.A.; Mercer, L.D.; Muhammad, S.; et al. Assessing the sensitivity of the polio environmental surveillance system. PLoS ONE 2018, 13, e0208336. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative. Global Polio Surveillance Action Plan, 2022–2024; World Health Organization: Geneva, Switzerland, 2022; Available online: https://polioeradication.org/wp-content/uploads/2022/05/GPSAP-2022-2024-EN.pdf (accessed on 4 September 2025).
- Pons-Salort, M.; Molodecky, N.A.; O’Reilly, K.M.; Wadood, M.Z.; Safdar, R.M.; Etsano, A.; Vaz, R.G.; Jafari, H.; Grassly, N.C.; Blake, I.M. Population Immunity against Serotype-2 Poliomyelitis Leading up to the Global Withdrawal of the Oral Poliovirus Vaccine: Spatio-temporal Modelling of Surveillance Data. PLoS Med. 2016, 13, e1002140. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Blake, I.M.; O’Reilly, K.M.; Wadood, M.Z.; Safdar, R.M.; Wesolowski, A.; Buckee, C.O.; Bandyopadhyay, A.S.; Okayasu, H.; Grassly, N.C. Risk factors and short-term projections for serotype-1 poliomyelitis incidence in Pakistan: A spatiotemporal analysis. PLoS Med. 2017, 14, e1002323. [Google Scholar] [CrossRef] [PubMed]
- Etsano, A.; Gunnala, R.; Shuaib, F.; Damisa, E.; Mkanda, P.; Banda, R.; Korir, C.; Enemaku, O.; Corkum, M.; Usman, S.; et al. Progress toward poliomyelitis eradication—Nigeria, January 2013–September 2014. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 1059–1063. [Google Scholar]
- Molodecky, N.A.; Sutter, R.W. Global withdrawal of Sabin oral poliovirus type 2 vaccine in 2016. Science 2025, 387, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Pons-Salort, M.; Burns, C.C.; Lyons, H.; Blake, I.M.; Jafari, H.; Oberste, M.S.; Kew, O.M.; Grassly, N.C. Preventing Vaccine-Derived Poliovirus Emergence during the Polio Endgame. PLoS Pathog. 2016, 12, e1005728. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.V.; Bandyopadhyay, A.S.; Grassly, N.C.; Gray, E.J.; Voorman, A.; Zipursky, S.; Blake, I.M. Global Impact of Mass Vaccination Campaigns on Circulating Type 2 Vaccine-Derived Poliovirus Outbreaks: An Interrupted Time-Series Analysis. J. Infect. Dis. 2025, 231, e446–e455. [Google Scholar] [CrossRef] [PubMed]
- Peak, C.M.; Lyons, H.; Voorman, A.; Gray, E.J.; Cooper, L.V.; Blake, I.M.; Hawes, K.M.; Bandyopadhyay, A.S. Monitoring the Risk of Type-2 Circulating Vaccine-Derived Poliovirus Emergence During Roll-Out of Type-2 Novel Oral Polio Vaccine. Vaccines 2024, 12, 1308. [Google Scholar] [CrossRef]
- Davlantes, E.; Jorba, J.; Henderson, E.; Bullard, K.; Deka, M.A.; Kfutwah, A.; Martin, J.; Bessaud, M.; Shulman, L.M.; Hawes, K.; et al. Notes from the Field: Circulating Vaccine-Derived Poliovirus Type 2 Emergences Linked to Novel Oral Poliovirus Vaccine Type 2 Use-Six African Countries, 2021–2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1041–1042. [Google Scholar] [CrossRef]
- Castro, C.J.; Oderinde, B.S.; Poston, K.D.; Mawashi, K.Y.; Bullard, K.; Akinola, M.; Meade, C.; Liu, H.; Hu, F.; Bullows, J.E.; et al. Complete genome sequences of nine double recombinant vaccine-derived novel oral poliovirus type 2 genomes from Nigeria 2023–2024. Microbiol. Resour. Announc. 2024, 13, e0088124. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A.; Badizadegan, K. Looking back at prospective modeling of outbreak response strategies for managing global type 2 oral poliovirus vaccine (OPV2) cessation. Front. Public Health 2023, 11, 1098419. [Google Scholar] [CrossRef]
- Thompson, K.M.; Tebbens, R.J. Eradication versus control for poliomyelitis: An economic analysis. Lancet 2007, 369, 1363–1371. [Google Scholar] [CrossRef]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Cochi, S.L.; Wassilak, S.G.; Linkins, J.; Sutter, R.W.; Aylward, R.B.; Thompson, K.M. Economic analysis of the global polio eradication initiative. Vaccine 2011, 29, 334–343. [Google Scholar] [CrossRef]
- Zimmermann, M.; Hagedorn, B.; Lyons, H. Projection of Costs of Polio Eradication Compared to Permanent Control. J. Infect. Dis. 2020, 221, 561–565. [Google Scholar] [CrossRef]
- Thompson, K.M.; Kalkowska, D.A. An Updated Economic Analysis of the Global Polio Eradication Initiative. Risk Anal. Off. Publ. Soc. Risk Anal. 2021, 41, 393–406. [Google Scholar] [CrossRef] [PubMed]
| Category | Specific Challenges | Potential Mitigation Measures |
|---|---|---|
| Funding Shortfall in 2025 | • Unexpected donor country funding decreases, with immediate impact on staffing | ⬪ Recruit more countries and high-net-worth individuals to participate as donors in global funding ⬪ Seek increased internal funding of operations by lower-middle-income countries with poliovirus transmission |
| Limited National Engagement | • Low operational accountability and limited logistic support • “False finger-marking” | ⬪ Coordinate advocacy by high-level representatives of GPEI partner agencies, other international organizations and other stakeholders ⬪ Place more international GPEI staff at national and subnational levels to enhance accountability |
| Limited Security and Access | • Civil war and insurgency | ⬪ Negotiate “days of tranquility” (temporary ceasefire) ⬪ Collaborate with humanitarian organizations to negotiate safe access ⬪ Implement variable, innovative efforts relevant to security level |
| • Targeted violence | ⬪ Have security personnel accompany immunization teams | |
| • Armed criminality, kidnapping | ⬪ Seek large-scale action by state governments | |
| Other Access Impediments | • Marginalized subpopulations | ⬪ Seek subpopulation engagement in campaign microplanning |
| • Hard-to-reach riverine and remote communities | ⬪ Seek necessary national logistical support: aircraft, off-road vehicles, motorcycles, watercraft and fuel | |
| • Low community acceptance of only oral poliovirus vaccine | ⬪ Provide multiantigen outreach vaccination ⬪ Provide “health camps” including health services for children of all ages ⬪ Provide other services, e.g., sanitation and clean water | |
| Residual Effects of COVID Pandemic | • Other health threats are higher priorities | ⬪ Coordinate high-level advocacy for promptness of outbreak responses |
| • Decreased routine immunization coverage | ⬪ Increase IPV delivery with periodic intensification of routine immunization and outreach services | |
| • Vaccine misinformation and “false finger-marking” | ⬪ Increase community engagement and recruit high-level and community opinion leaders equipped with persuasive messaging | |
| Outbreak Response Capacity | • Delayed and suboptimal quality outbreak response SIAs and “false finger-marking” | ⬪ GPEI partners and affected countries resume an emergency footing ⬪ Rapidly provide funds, human resources and vaccine ⬪ Coordinate high-level advocacy for promptness ⬪ Enhance supervision for SIA quality and accountability |
| • Delayed specimen and isolate handling and shipment | ⬪ Track shipping of specimens subnationally and internationally and intervene as needed | |
| Strategic Planning | • Premature focus on funding transition to health services * | ⬪ With appropriate integration of services in the interim, delay transitioning resources until there are reliable signs of reaching GPEI goals |
| • Prioritizing and monitoring rapid outbreak responses | ⬪ Ensure that resources are urgently directed to new outbreaks ⬪ Systematically evaluate risks and mitigate them before a risk becomes actualized |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wassilak, S.G.F.; Mohamed, A.; Bigouette, J.P. Impediments to Progress Toward Polio Eradication During 2014–2024: Effectively Addressing the Current Challenges. Vaccines 2025, 13, 1060. https://doi.org/10.3390/vaccines13101060
Wassilak SGF, Mohamed A, Bigouette JP. Impediments to Progress Toward Polio Eradication During 2014–2024: Effectively Addressing the Current Challenges. Vaccines. 2025; 13(10):1060. https://doi.org/10.3390/vaccines13101060
Chicago/Turabian StyleWassilak, Steven G. F., Abdinoor Mohamed, and John Paul Bigouette. 2025. "Impediments to Progress Toward Polio Eradication During 2014–2024: Effectively Addressing the Current Challenges" Vaccines 13, no. 10: 1060. https://doi.org/10.3390/vaccines13101060
APA StyleWassilak, S. G. F., Mohamed, A., & Bigouette, J. P. (2025). Impediments to Progress Toward Polio Eradication During 2014–2024: Effectively Addressing the Current Challenges. Vaccines, 13(10), 1060. https://doi.org/10.3390/vaccines13101060






