Immunogenicity and Contraceptive Potential of a Classical Swine Fever Viral Vector Live Vaccine Strain Containing Pig Gonadotropin-Releasing Hormone
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Flc-Lom-Gnrh Using a Classical Swine Virus Viral Vector
2.2. Rescue of Flc-LOM-Gnrhx3 Virus
2.3. Growth of the Flc-Lom-Gnrh Virus in Cells
2.4. Inoculation of Pigs with Flc-Lom-Gnrhx3
2.5. Anti-Csfv E2 Antibody Elisa and Serum Neutralization Assay
2.6. In-House Elisa to Measure Anti-Gnrh Levels
2.7. Concentrations of Testosterone
2.8. Statistical Analysis
3. Results
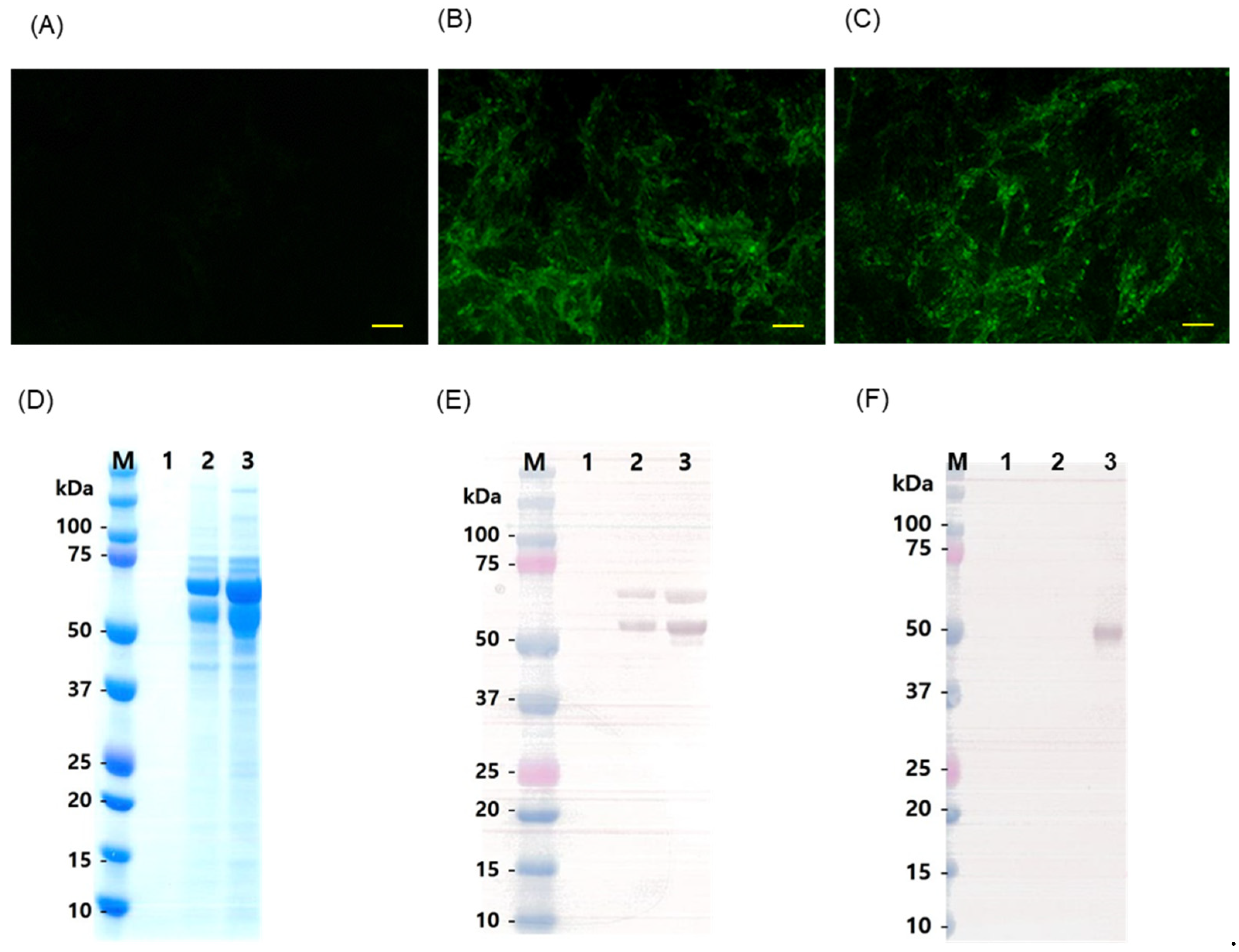
3.1. Cloning of Flc-LOM-GnRHx3
3.2. Rescue of Flc-LOM-GnRHx3 Virus
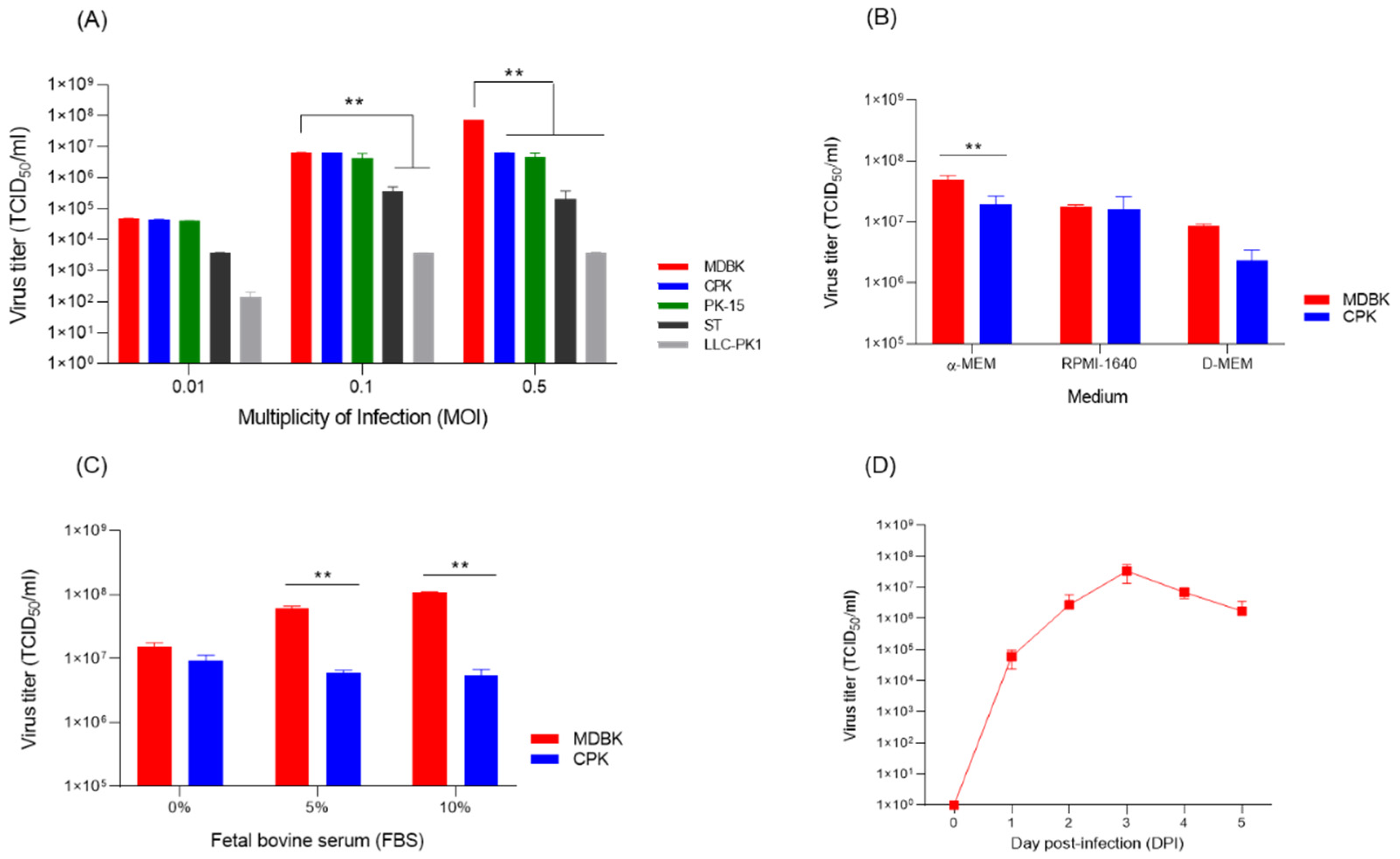
3.3. Optimal Culture Conditions for the Flc-Lom-Gnrhx3 Virus
3.4. Anti-CSFV Antibody Production in Pigs
3.5. Detection of Anti-Gnrh Antibody and Testosterone Concentrations in Pigs
3.6. Changes in the Size and Weight of the Testes
3.7. Changes in the Seminiferous Tubules of the Testis, and in the Epididymal Ducts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faruck, M.O.; Koirala, P.; Yang, J.; D’occhio, M.J.; Skwarczynski, M.; Toth, I. Polyacrylate-GnRH Peptide Conjugate as an Oral Contraceptive Vaccine Candidate. Pharmaceutics 2021, 13, 1081. [Google Scholar] [CrossRef]
- Lefebvre, J.; Gauthier, G.; Giroux, J.-F.; Reed, A.; Reed, E.T.; Bélanger, L. The greater snow goose Anser caerulescens atlanticus: Managing an overabundant population. Ambio 2017, 46, 262–274. [Google Scholar] [CrossRef]
- Payo-Payo, A.; Oro, D.; Igual, J.M.; Jover, L.; Sanpera, C.; Tavecchia, G. Population control of an overabundant species achieved through consecutive anthropogenic perturbations. Ecol. Appl. 2015, 25, 2228–2239. [Google Scholar] [CrossRef]
- Lueders, I.; Young, D.; Maree, L.; van der Horst, G.; Luther, I.; Botha, S.; Tindall, B.; Fosgate, G.; Ganswindt, A.; Bertschinger, H.J. Effects of GnRH vaccination in wild and captive African Elephant bulls (Loxodonta africana) on reproductive organs and semen quality. PLOS ONE 2017, 12, e0178270. [Google Scholar] [CrossRef] [PubMed]
- Somgird, C.; Homkong, P.; Sripiboon, S.; Brown, J.L.; Stout, T.A.; Colenbrander, B.; Mahasawangkul, S.; Thitaram, C. Potential of a gonadotropin-releasing hormone vaccine to suppress musth in captive male Asian elephants (Elephas maximus). Anim. Reprod. Sci. 2016, 164, 111–120. [Google Scholar] [CrossRef]
- Kustritz, M.V.R. Population Control in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Miesner, M.D.; Anderson, D.E. Surgical Management of Common Disorders of Feedlot Calves. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 407–424. [Google Scholar] [CrossRef]
- Massei, G.; Cowan, D. Fertility control to mitigate human–wildlife conflicts: A review. Wildl. Res. 2014, 41, 1–21. [Google Scholar] [CrossRef]
- Kirkpatrick, J.F.; Lyda, R.O.; Frank, K.M. Contraceptive Vaccines for Wildlife: A Review. Am. J. Reprod. Immunol. 2011, 66, 40–50. [Google Scholar] [CrossRef]
- Naz, R.K.; Gupta, S.K.; Gupta, J.C.; Vyas, H.K.; Talwar, G.P. Recent advances in contraceptive vaccine development: A mini-review. Hum. Reprod. 2005, 20, 3271–3283. [Google Scholar] [CrossRef]
- Millar, R.P. GnRHs and GnRH receptors. Anim. Reprod. Sci. 2005, 88, 5–28. [Google Scholar] [CrossRef]
- Schally, A.; Arimura, A.; Baba, Y.; Nair, R.; Matsuo, H.; Redding, T.; Debeljuk, L.; White, W. Isolation and properties of the FSH and LH-releasing hormone. Biochem. Biophys. Res. Commun. 1971, 43, 393–399. [Google Scholar] [CrossRef]
- Casper, R.F. Clinical uses of gonadotropin-releasing hormone analogues. CMAJ Can. Med. Assoc. J. 1991, 144, 153–158. [Google Scholar]
- Bartfai, G. Clinical applications of gonadotrophin-releasing hormone and its analogues. Hum. Reprod. 1988, 3, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Batorek, N.; Čandek-Potokar, M.; Bonneau, M.; Van Milgen, J. Meta-analysis of the effect of immunocastration on production performance, reproductive organs and boar taint compounds in pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef]
- Nautrup, B.P.; Van Vlaenderen, I.; Mah, C.K. The effect of immunization against gonadotropin-releasing factor in market gilts: Meta-analyses of parameters relevant for pig producers, pork packers and retailers/consumers. Res. Vet. Sci. 2020, 131, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Gionfriddo, J.P.; Denicola, A.J.; Miller, L.A.; Fagerstone, K.A. Efficacy of GnRH immunocontraception of wild white-tailed deer in New Jersey. Wildl. Soc. Bull. 2011, 35, 142–148. [Google Scholar] [CrossRef]
- Levy, J.K.; Friary, J.A.; Miller, L.A.; Tucker, S.J.; Fagerstone, K.A. Long-term fertility control in female cats with GonaCon™, a GnRH immunocontraceptive. Theriogenology 2011, 76, 1517–1525. [Google Scholar] [CrossRef]
- Massei, G.; Cowan, D.P.; Coats, J.; Bellamy, F.; Quy, R.; Pietravalle, S.; Brash, M.; Miller, L.A. Long-term effects of immuno-contraception on wild boar fertility, physiology and behaviour. Wildl. Res. 2012, 39, 378–385. [Google Scholar] [CrossRef]
- Quy, R.J.; Massei, G.; Lambert, M.S.; Coats, J.; Miller, L.A.; Cowan, D.P. Effects of a GnRH vaccine on the movement and activity of free-living wild boar (Sus scrofa). Wildl. Res. 2014, 41, 185–193. [Google Scholar] [CrossRef]
- Massei, G.; Koon, K.-K.; Law, S.-I.; Gomm, M.; Mora, D.S.; Callaby, R.; Palphramand, K.; Eckery, D.C. Fertility control for managing free-roaming feral cattle in Hong Kong. Vaccine 2018, 36, 7393–7398. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Baker, D.L.; Davis, T.L.; Conner, M.M.; Lothridge, A.H.; Nett, T.M. Effects of Gonadotropin-Releasing Hormone Immunization on Reproductive Function and Behavior in Captive Female Rocky Mountain Elk (Cervus elaphus nelsoni). Biol. Reprod. 2011, 85, 1152–1160. [Google Scholar] [CrossRef]
- Yadav, S.; Weng, H.-Y. Estimating the scale of adverse animal welfare consequences of movement restriction and mitigation strategies in a classical swine fever outbreak. BMC Vet. Res. 2017, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Ganges, L.; Crooke, H.R.; Bohórquez, J.A.; Postel, A.; Sakoda, Y.; Becher, P.; Ruggli, N. Classical swine fever virus: The past, present and future. Virus Res. 2020, 289, 198151. [Google Scholar] [CrossRef]
- Thiel, H.J.; Stark, R.; Weiland, E.; Rümenapf, T.; Meyers, G. Hog cholera virus: Molecular composition of virions from a pestivirus. J. Virol. 1991, 65, 4705–4712. [Google Scholar] [CrossRef]
- Elbers, K.; Tautz, N.; Becher, P.; Stoll, D.; Rümenapf, T.; Thiel, H.J. Processing in the pestivirus E2-NS2 region: Identification of proteins p7 and E2p7. J. Virol. 1996, 70, 4131–4135. [Google Scholar] [CrossRef]
- Park, G.-S.; Lim, S.-I.; Hong, S.-H.; Song, J.-Y. Establishment and characterization of an infectious cDNA clone of a classical swine fever virus LOM strain. J. Vet. Sci. 2012, 13, 81–91. [Google Scholar] [CrossRef]
- Choe, S.; Shin, J.; Kim, K.-S.; Song, S.; Cha, R.M.; Jung, B.-I.; Hyun, B.-H.; Park, B.-K.; An, D.-J. Protection of Piglets with Maternally Derived Antibodies from Sows Inoculated with an Attenuated Live Marker Classical Swine Fever Vaccine (Flc-LOM-BErns). Pathogens 2020, 9, 608. [Google Scholar] [CrossRef]
- Han, X.; Zhou, Y.; Zeng, Y.; Sui, F.; Liu, Y.; Tan, Y.; Cao, X.; Du, X.; Meng, F.; Zeng, X. Effects of active immunization against GnRH versus surgical castration on hypothalamic-pituitary function in boars. Theriogenology 2017, 97, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ebner, F.; Schwiertz, P.; Steinfelder, S.; Pieper, R.; Zentek, J.; Schütze, N.; Baums, C.G.; Alber, G.; Geldhof, P.; Hartmann, S. Pathogen-Reactive T Helper Cell Analysis in the Pig. Front. Immunol. 2017, 8, 565. [Google Scholar] [CrossRef]
- Goodwin, D.; Varamini, P.; Simerska, P.; D’oCchio, M.J.; Toth, I. Design, synthesis and evaluation of a gonadotropin releasing hormone-based subunit vaccine in rams (Ovis aries). Vaccine 2015, 33, 1453–1458. [Google Scholar] [CrossRef]
- Goodwin, D.; Simerska, P.; Chang, C.-H.; Mansfeld, F.M.; Varamini, P.; D’oCchio, M.J.; Toth, I. Active immunisation of mice with GnRH lipopeptide vaccine candidates: Importance of T helper or multi-dimer GnRH epitope. Bioorganic Med. Chem. 2014, 22, 4848–4854. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, L.; Tian, C.; Zhang, G.; Huo, G.; Tang, L.; Li, Y. Immunogenicity of Recombinant Classic Swine Fever Virus CD8+ T Lymphocyte Epitope and Porcine Parvovirus VP2 Antigen Coexpressed by Lactobacillus casei in Swine via Oral Vaccination. Clin. Vaccine Immunol. 2011, 18, 1979–1986. [Google Scholar] [CrossRef]
- Tarradas, J.; Monsó, M.; Muñoz, M.; Rosell, R.; Fraile, L.; Frías, M.T.; Domingo, M.; Andreu, D.; Sobrino, F.; Ganges, L. Partial protection against classical swine fever virus elicited by dendrimeric vaccine-candidate peptides in domestic pigs. Vaccine 2011, 29, 4422–4429. [Google Scholar] [CrossRef]
- Pauly, T.; Elbers, K.; Konig, M.; Lengsfeld, T.; Saalmuller, A.; Thiel, H.-J. Classical swine fever virus-specific cytotoxic T lymphocytes and identification of a T cell epitope. J. Gen. Virol. 1995, 76, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Armengol, E.; Wiesmüller, K.-H.; Wienhold, D.; Büttner, M.; Pfaff, E.; Jung, G.; Saalmüller, A. Identification of T-cell epitopes in the structural and non-structural proteins of classical swine fever virus. J. Gen. Virol. 2002, 83, 551–560. [Google Scholar] [CrossRef]
- Tarradas, J.; Monsó, M.; Fraile, L.; de la Torre, B.G.; Muñoz, M.; Rosell, R.; Riquelme, C.; Pérez, L.J.; Nofrarías, M.; Domingo, M.; et al. A T-cell epitope on NS3 non-structural protein enhances the B and T cell responses elicited by dendrimeric constructions against CSFV in domestic pigs. Vet. Immunol. Immunopathol. 2012, 150, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-C.; Lin, H.-H.; Lin, C.-H.; Chung, W.-B. Identification of Cytotoxic T Lymphocyte Epitopes on Swine Viruses: Multi-Epitope Design for Universal T Cell Vaccine. PLOS ONE 2013, 8, e84443. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, P.; Liu, L.; Qian, S.; Chen, H.; Li, X. Virus-Like Particles of Chimeric Recombinant Porcine Circovirus Type 2 as Antigen Vehicle Carrying Foreign Epitopes. Viruses 2014, 6, 4839–4855. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, J.; Bi, Y.; Yang, L.; Meng, S.; Zhou, Y.; Jia, X.; Meng, S.; Sun, L.; Liu, W. Synthetic B- and T-cell epitope peptides of porcine reproductive and respiratory syndrome virus with Gp96 as adjuvant induced humoral and cell-mediated immunity. Vaccine 2013, 31, 1838–1847. [Google Scholar] [CrossRef]
- Burmakina, G.; Malogolovkin, A.; Tulman, E.R.; Xu, W.; Delhon, G.; Kolbasov, D.; Rock, D.L. Identification of T-cell epitopes in African swine fever virus CD2v and C-type lectin proteins. J. Gen. Virol. 2019, 100, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, J.; Nahar, U.J.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; Skwarczynski, M.; Toth, I. A dual-adjuvanting strategy for peptide-based subunit vaccines against group A Streptococcus: Lipidation and polyelectrolyte complexes. Bioorganic Med. Chem. 2020, 28, 115823. [Google Scholar] [CrossRef] [PubMed]
- Azmi, F.; Ahmad Fuaad, A.A.H.; Skwarczynski, M.; Toth, I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum. Vaccines Immunother. 2014, 10, 778–796. [Google Scholar] [CrossRef]
- Choe, S.; Kim, J.-H.; Kim, K.-S.; Song, S.; Kang, W.-C.; Kim, H.-J.; Park, G.-N.; Cha, R.M.; Cho, I.-S.; Hyun, B.-H.; et al. Impact of a Live Attenuated Classical Swine Fever Virus Introduced to Jeju Island, a CSF-Free Area. Pathogens 2019, 8, 251. [Google Scholar] [CrossRef]
- Choe, S.; Kim, J.-H.; Kim, K.-S.; Song, S.; Cha, R.M.; Kang, W.-C.; Kim, H.-J.; Park, G.-N.; Shin, J.; Jo, H.-N.; et al. Adverse Effects of Classical Swine Fever Virus LOM Vaccine and Jeju LOM Strains in Pregnant Sows and Specific Pathogen-Free Pigs. Pathogens 2019, 9, 18. [Google Scholar] [CrossRef]
- Lim, S.-I.; Song, J.-Y.; Kim, J.; Hyun, B.-H.; Kim, H.-Y.; Cho, I.-S.; Kim, B.; Woo, G.-H.; Lee, J.-B.; An, D.-J. Safety of classical swine fever virus vaccine strain LOM in pregnant sows and their offspring. Vaccine 2016, 34, 2021–2026. [Google Scholar] [CrossRef]
- Choe, S.; Park, G.-N.; Kim, K.-S.; Shin, J.; An, B.-H.; An, D.-J. Immunogenicity of a classical swine fever bait vaccine (Flc-LOM- BErns) in hybrid-wild boars. Vaccine 2024, 43, 126517. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.A.; Fagerstone, K.A.; Eckery, D.C. TWENTY YEARS OF IMMUNOCONTRACEPTIVE RESEARCH: LESSONS LEARNED. J. Zoo Wildl. Med. 2013, 44. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.A.; Gionfriddo, J.P.; Fagerstone, K.A.; Rhyan, J.C.; Killian, G.J. The single shot GnRH immunocontraceptive vaccine (GonaConTM) in white-tailed deer: Comparison of several GnRH preparations. Am. J. Reprod. Immunol. 2008, 60, 214–223. [Google Scholar] [CrossRef]
- Killian, G.; Thain, D.; Diehl, N.K.; Rhyan, J.; Miller, L. Four-year contraception rates of mares treated with single-injection porcine zona pellucida and GnRH vaccines and intrauterine devices. Wildl. Res. 2008, 35, 531–539. [Google Scholar] [CrossRef]
- Killian, G.; Kreeger, T.J.; Rhyan, J.; Fagerstone, K.; Miller, L. Observations on the Use of GonaconTM in Captive Female elk (Cervus Elaphus). J. Wildl. Dis. 2009, 45, 184–188. [Google Scholar] [CrossRef]
- Yoder, C.A.; Miller, L.A. Effect of GonaCon™ vaccine on black-tailed prairie dogs: Immune response and health effects. Vaccine 2010, 29, 233–239. [Google Scholar] [CrossRef]
- Benka, V.A.; Levy, J.K. Vaccines for feline contraception: GonaCon GnRH-hemocyanin conjugate immunocontraceptive. J. Feline Med. Surg. 2015, 17, 758–765. [Google Scholar] [CrossRef]
- Dalmau, A.; Velarde, A.; Rodriguez, P.; Pedernera, C.; Lionch, P.; Fäbrega, E.; Casal, N.; Mainau, E.; Gispert, M.; King, V.; et al. Use of anti-GnRF vaccine to suppress estrus in cross-bred Iberian female pigs. Theriogenology 2015, 84, 342–347. [Google Scholar] [CrossRef]
- Karaconji, B.; Lloyd, B.; Campbell, N.; Meaney, D.; Ahern, T. Effect of an anti-gonadotropin-releasing factor vaccine on sexual and aggressive behaviour in male pigs during the finishing period under Australian field conditions. Aust. Vet. J. 2015, 93, 121–123. [Google Scholar] [CrossRef]
- Dunshea, F.R.; Colantoni, C.; Howard, K.; McCauley, I.; Jackson, P.; A Long, K.; Lopaticki, S.; A Nugent, E.; A Simons, J.; Walker, J.; et al. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Andersson, H.; Chen, G.; Andersson, K.; Madej, A.; Lundström, K. Effect of a Gonadotropin-releasing Hormone Vaccine (ImprovacTM) on Steroid Hormones, Boar Taint Compounds and Performance in Entire Male Pigs. Reprod. Domest. Anim. 2007, 43, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Janett, F.; Lanker, U.; Jörg, H.; Meijcrink, E.; Thun, R. Suppression of reproductive cyclicity by active immunization against GnRH in the adult ewe. Schweiz. Arch. Tierheilkd. 2009, 151, 53–59. [Google Scholar] [CrossRef]
- Lueders, I.; Hildebrandt, T.B.; Gray, C.; Botha, S.; Rich, P.; Niemuller, C. Supression of testicular function in a male Asian elephant (Elephas maximus) treated with gonadotropin-releasing hormone vaccines. J. Zoo Wildl. Med. 2014, 45, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Janett, F.; Stump, R.; Burger, D.; Thun, R. Suppression of testicular function and sexual behavior by vaccination against GnRH (Equity™) in the adult stallion. Anim. Reprod. Sci. 2009, 115, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Janett, F.; Gerig, T.; Tschuor, A.; Amatayakul-Chantler, S.; Walker, J.; Howard, R.; Bollwein, H.; Thun, R. Vaccination against gonadotropin-releasing factor (GnRF) with Bopriva significantly decreases testicular development, serum testosterone levels and physical activity in pubertal bulls. Theriogenology 2012, 78, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Theubet, G.; Thun, R.; Hilbe, M.; Janett, F. Effect of vaccination against GnRH (Bopriva®) in the male pubertal calf. Schweiz. Arch. Tierheilkd. 2010, 152, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Wicks, N.; Crouch, S.; Pearl, C.A. Effects of Improvac and Bopriva on the testicular function of boars ten weeks after immunization. Anim. Reprod. Sci. 2013, 142, 149–159. [Google Scholar] [CrossRef]
- Balet, L.; Janett, F.; Hüsler, J.; Piechotta, M.; Howard, R.; Amatayakul-Chantler, S.; Steiner, A.; Hirsbrunner, G. Immunization against gonadotropin-releasing hormone in dairy cattle: Antibody titers, ovarian function, hormonal levels, and reversibility. J. Dairy Sci. 2014, 97, 2193–2203. [Google Scholar] [CrossRef]
- Lugar, D.W.; Rhoads, M.L.; Clark-Deener, S.G.; Callahan, S.R.; Revercomb, A.K.; Prusa, K.J.; Estienne, M.J. Immunological castration temporarily reduces testis size and function without long-term effects on libido and sperm quality in boars. Animal 2017, 11, 643–649. [Google Scholar] [CrossRef]
- Bohrer, B.M.; Flowers, W.L.; Kyle, J.M.; Johnson, S.S.; King, V.L.; Spruill, J.L.; Thompson, D.P.; Schroeder, A.L.; Boler, D.D. Effect of gonadotropin releasing factor suppression with an immunological on growth performance, estrus activity, carcass characteristics, and meat quality of market gilts. J. Anim. Sci. 2014, 92, 4719–4724. [Google Scholar] [CrossRef]
- Boedeker, N.C.; Hayek, L.-A.C.; Murray, S.; de Avila, D.M.; Brown, J.L. Effects of a Gonadotropin-Releasing Hormone Vaccine On Ovarian Cyclicity And Uterine Morphology Of An Asian Elephant (Elephas Maximus). J. Zoo Wildl. Med. 2012, 43, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Brunius, C.; Zamaratskaia, G.; Andersson, K.; Chen, G.; Norrby, M.; Madej, A.; Lundström, K. Early immunocastration of male pigs with Improvac®–Effect on boar taint, hormones and reproductive organs. Vaccine 2011, 29, 9514–9520. [Google Scholar] [CrossRef]
- Oliviero, C.; Lindh, L.; Peltoniemi, O. Board Invited Review: Immunocontraception as a possible tool to reduce feral pig populations: Recent and future perspectives. J. Anim. Sci. 2019, 97, 2283–2290. [Google Scholar] [CrossRef]
- Albrecht, A.K. Review on the consequences of using Improvac TM in modern pig production. Vet. Sci. Dev. 2013, 3, e1. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Non-invasive mucosal vaccine delivery: Advantages, challenges and the future. Expert Opin. Drug Deliv. 2020, 17, 435–437. [Google Scholar] [CrossRef]
- Brauer, A.; Lange, E.; Kaden, V. Oral immunisation of wild boar against classical swine fever: Uptake studies of new baits and investigations on the stability of lyophilised C-strain vaccine. Eur. J. Wildl. Res. 2006, 52, 271–276. [Google Scholar] [CrossRef]
- Rossi, S.; Staubach, C.; Blome, S.; Guberti, V.; Thulke, H.-H.; Vos, A.; Koenen, F.; Le Potier, M.-F. Controlling of CSFV in Eu-ropean wild boar using oral vaccination: A review. Front. Microbiol. 2015, 6, 1141. [Google Scholar] [CrossRef]
- Calenge, C.; Rossi, S. Bayesian modelling of hunting data may improve the understanding of host–parasite systems: Wild boar diseases and vaccination as an example. J. Theor. Biol. 2014, 343, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Feliziani, F.; Blome, S.; Petrini, S.; Giammarioli, M.; Iscaro, C.; Severi, G.; Convito, L.; Pietschmann, J.; Beer, M.; De Mia, G.M. First assessment of classical swine fever marker vaccine candidate CP7_E2alf for oral immunization of wild boar under field conditions. Vaccine 2014, 32, 2050–2055. [Google Scholar] [CrossRef]
- Moennig, V. The control of classical swine fever in wild boar. Front. Microbiol. 2015, 6, 1211. [Google Scholar] [CrossRef]
- Rossi, S.; Pol, F.; Forot, B.; Masse-Provin, N.; Rigaux, S.; Bronner, A.; Le Potier, M.-F. Preventive vaccination contributes to control classical swine fever in wild boar (Sus scrofa sp.). Vet. Microbiol. 2009, 142, 99–107. [Google Scholar] [CrossRef]
- Barasona, J.A.; Gallardo, C.; Cadenas-Fernández, E.; Jurado, C.; Rivera, B.; Rodríguez-Bertos, A.; Arias, M.; Sánchez-Vizcaíno, J.M. First Oral Vaccination of Eurasian Wild Boar Against African Swine Fever Virus Genotype II. Front. Vet. Sci. 2019, 6, 137. [Google Scholar] [CrossRef]
- Bosch-Camós, L.; López, E.; Rodriguez, F. African swine fever vaccines: A promising work still in progress. Porc. Heal. Manag. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Yang, D.-K.; Kim, H.-H.; Choi, S.-S.; Lee, S.H.; Cho, I.-S. A recombinant rabies virus (ERAGS) for use in a bait vaccine for swine. Clin. Exp. Vaccine Res. 2016, 5, 169–174. [Google Scholar] [CrossRef] [PubMed]


| Group | No. of Pigs | Pig Age | Vaccine | Dose | Route | Inoculation Time/Interval | Autopsy (WPV *) |
|---|---|---|---|---|---|---|---|
| G1 | 3 | 20 weeks | LOM-GnRHx3 | 105.0 TCID50/mL | Oral | Three times/ 2 weeks | 7 |
| G2 | 3 | 20 weeks | LOM-GnRHx3 | 105.0 TCID50/mL | IM | Three times/ 2 weeks | 7 |
| G3 | 3 | 20 weeks | Mock | - | - | - | 7 |
| Group | Seminiferous Tubules of the Testis | Epididymal Ducts | ||
|---|---|---|---|---|
| Diameter (µm) | Degeneration (%) | Histopathologic Lesions | Sperm Density | |
| G1 | 219.9 ± 15.1 | 20.83 ± 0.16 | Focal degeneration Focal inflammatory cell infiltration into the interstitium | Mild decrease |
| G2 | 194.33 ± 35.7 | 52.50 ± 0.15 | Multifocal degeneration Focal inflammatory cell infiltration into the interstitium | Marked decrease |
| G3 | 239.43 ± 15.2 | 6.94 ± 0.05 | Nothing remarkable | Nothing remarkable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, D.-J.; Shin, J.-H.; Choe, S.; Lee, Y.-H.; Jang, M.-K.; An, B.-H.; Park, G.-N.; Cho, Y.-S.; Chang, K.-S. Immunogenicity and Contraceptive Potential of a Classical Swine Fever Viral Vector Live Vaccine Strain Containing Pig Gonadotropin-Releasing Hormone. Vaccines 2025, 13, 1048. https://doi.org/10.3390/vaccines13101048
An D-J, Shin J-H, Choe S, Lee Y-H, Jang M-K, An B-H, Park G-N, Cho Y-S, Chang K-S. Immunogenicity and Contraceptive Potential of a Classical Swine Fever Viral Vector Live Vaccine Strain Containing Pig Gonadotropin-Releasing Hormone. Vaccines. 2025; 13(10):1048. https://doi.org/10.3390/vaccines13101048
Chicago/Turabian StyleAn, Dong-Jun, Ji-Hee Shin, SeEun Choe, Young-Hyeon Lee, Min-Kyung Jang, Byung-Hyun An, Gyu-Nam Park, Yun-Sang Cho, and Kyung-Soo Chang. 2025. "Immunogenicity and Contraceptive Potential of a Classical Swine Fever Viral Vector Live Vaccine Strain Containing Pig Gonadotropin-Releasing Hormone" Vaccines 13, no. 10: 1048. https://doi.org/10.3390/vaccines13101048
APA StyleAn, D.-J., Shin, J.-H., Choe, S., Lee, Y.-H., Jang, M.-K., An, B.-H., Park, G.-N., Cho, Y.-S., & Chang, K.-S. (2025). Immunogenicity and Contraceptive Potential of a Classical Swine Fever Viral Vector Live Vaccine Strain Containing Pig Gonadotropin-Releasing Hormone. Vaccines, 13(10), 1048. https://doi.org/10.3390/vaccines13101048







