Hepatitis B Serological Immunity and Exposure Among Blood Donors in Southern Croatia: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBV | Hepatitis B virus |
| HBsAg | Hepatitis B surface antigen |
| NAT | Nucleic acid testing |
| OBI | Occult hepatitis B infection |
| TTI | Transfusion-transmitted infection |
| anti-HBc | Antibody to hepatitis B core antigen |
| anti-HBs | Antibody to hepatitis B surface antigen |
| HCWs | Healthcare workers |
References
- Cargill, T.; Barnes, E. Therapeutic vaccination for treatment of chronic hepatitis B. Clin. Exp. Immunol. 2021, 205, 106–118. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021; WHO: Geneva, Switzerland, 2016; Available online: https://apps.who.int/iris/handle/10665/246177 (accessed on 15 June 2025).
- Candotti, D.; Laperche, S. Hepatitis B virus blood screening: Need for reappraisal of blood safety measures? Front. Med. 2018, 5, 29. [Google Scholar] [CrossRef]
- Dilberovic, A.; Arapovic, J.; Stanic, A.; Kola, L.; Martinovic, D. Detection of voluntary blood donor with previous hepatitis B infection—A case report. Med. Jadertina 2024, 54, 47–52. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.S. Current laboratory tests for diagnosis of hepatitis B virus infection. Int. J. Clin. Pract. 2021, 75, e14812. [Google Scholar] [CrossRef]
- Karimi, G.; Zadsar, M.; Vafaei, N.; Sharifi, Z.; Falah Tafti, M. Prevalence of antibody to hepatitis B core antigen and hepatitis B virus DNA in HBsAg-negative healthy blood donors. Virol. J. 2016, 13, 36. [Google Scholar] [CrossRef][Green Version]
- Manuela, M. Importance of Anti-HBc Antibody Detection in Identifying Voluntary Blood Donors with Occult Hepatitis B Virus Infection. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2019. [Google Scholar][Green Version]
- Safic Stanic, H.; Babic, I.; Maslovic, M.; Dogic, V.; Bingulac-Popovic, J.; Miletic, M.; Jurakovic-Loncar, N.; Vuk, T.; Strauss-Patko, M.; Jukic, I. Three-year experience in NAT screening of blood donors for transfusion-transmitted viruses in Croatia. Transfus. Med. Hemother. 2017, 44, 415–420. [Google Scholar] [CrossRef]
- Raimondo, G.; Pollicino, T.; Romano, L.; Zanetti, A.R. A 2010 update on occult hepatitis B infection. Pathol. Biol. 2010, 58, 254–257. [Google Scholar] [CrossRef]
- Rajcevic, S.; Medic, S.; Patic, A.; Dragnic, N.; Ristic, M.; Vukovic, V.; Petrovic, V. Seroprevalence study of anti-HBs antibodies in the general population of Vojvodina, Serbia. Medicina 2024, 60, 436. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 796–829. [Google Scholar] [CrossRef] [PubMed]
- Kaić, B.; Vilibic-Cavlek, T.; Kurecic Filipovic, S.; Nemeth-Blazic, T.; Pem-Novosel, I.; Visekruna Vucina, V.; Simunovic, A.; Zajec, M.; Radic, I.; Pavlic, J.; et al. Epidemiology of viral hepatitis. Acta Med. Croatica 2013, 67, 273–279. [Google Scholar]
- Zhao, H.; Zhou, X.; Zhou, Y.H. Hepatitis B vaccine development and implementation. Hum. Vaccines Immunother. 2020, 16, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Zanetti, A.R. Hepatitis B vaccination: A historical overview with a focus on the Italian achievements. Viruses 2022, 14, 1515. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection; WHO: Geneva, Switzerland, 2015; Available online: https://iris.who.int/bitstream/handle/10665/154590/9789241549059_eng.pdf (accessed on 10 July 2025).
- Stasi, C.; Silvestri, C.; Voller, F. Hepatitis B vaccination and immunotherapies: An update. Clin. Exp. Vaccine Res. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Kucinar, J.; Ljubin-Sternak, S.; Kaic, B.; Lazaric-Stefanovic, L.; Kolaric, B. Prevalence of viral hepatitis in Croatian adult population undergoing routine check-up, 2010–2011. Cent. Eur. J. Public Health 2014, 22, 29–33. [Google Scholar] [CrossRef]
- Seekircher, L.; Mühlbacher, A.; Tschiderer, L.; Wachter, G.A.; Astl, M.; Schennach, H.; Siller, A.; Willeit, P. Anti-HBs seroprevalence in blood donors from Tyrol, Austria. Vaccines 2024, 12, 1156. [Google Scholar] [CrossRef] [PubMed]
- Miletic, M.; Bingulac-Popovic, J.; Stojic Vidovic, M.; Hecimovic, A.; Berendika, M.; Babic, I.; Dogic, V.; Samardzija, M.; Barisic, K.; Jukic, I.; et al. Anti-HBc prevalence among Croatian blood donors in a 14-year period (2004–2017): Assessment of trends, risks and need for implementing routine testing. Transfus. Clin. Biol. 2019, 26, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Samardzija, M.; Drenjancevic, D.; Miletic, M.; Slavulj, B.; Jukic, I.; Zibar, L.; Mihaljevic, S.; Ferenac Kis, M.; Samardzija, M. The impact of positive anti-HBc marker on permanent deferral of voluntary blood donors in Eastern Croatia and estimation of occult hepatitis B virus infection rate. Acta Clin. Croat. 2020, 59, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Abbott Diagnostics. ARCHITECT Anti-HBc II Reagent Kit, Package Insert G47715R09. Available online: https://medilinkltd.com/wp-content/uploads/2023/07/Anti-HBc-II-2.pdf (accessed on 20 July 2025).
- Abbott Diagnostics. ARCHITECT Anti-HBs Reagent Kit, Package Insert G80543R03. Available online: https://medilinkltd.com/wp-content/uploads/2023/07/Anti-HBs.pdf (accessed on 20 July 2025).
- Zbinden, A.; Ries, J.; Redli, P.M.; Shah, C.; Glauser, A.; Goslings, D.; Huzly, D.; Boni, J.; Gottschalk, J.; Frey, B.M. Prevalence of occult hepatitis B virus infection in blood donors with negative ID-NAT in Switzerland. Transfus. Med. Hemother. 2022, 49, 338–345. [Google Scholar] [CrossRef]
- O’Brien, S.F.; Reedman, C.N.; Osiowy, C.; Bolotin, S.; Yi, Q.-L.; Lourenco, L.; Lewin, A.; Binka, M.; Caffrey, N.; Drews, S.J. Hepatitis B blood donor screening data: An under-recognized resource for Canadian public health surveillance. Viruses 2023, 15, 409. [Google Scholar] [CrossRef]
- Aijaz, R.; Siddiqui, H.; Soomro, A.; Kanwal, M.; Hussain, W.; Khalique, S. Prevalence of antibodies to hepatitis B core antigen in hepatitis B surface antigen negative healthy blood donors. Pak. J. Health Sci. 2022, 3, 175–179. [Google Scholar] [CrossRef]
- Ismail, F.; Shambesh, M.; Franka, E.; Agila, A. Frequency of hepatitis B core antibody and hepatitis B virus DNA among apparently healthy male blood donors in Eastern Libya. Libyan J. Med. Sci. 2018, 2, 34–39. [Google Scholar] [CrossRef]
- Ismail, F.; Shambesh, M.K.; Aboutwerat, A.; Elbackush, M. Serological and molecular characterization of total hepatitis B core antibodies in blood donors in Tripoli, Libya. Libyan J. Infect. Dis. 2010, 4, 31–37. [Google Scholar]
- Khalaf, A.A.; BenDarif, E.T.; Gibreel, T.M.; Alhadi, A.J.; Abugalia, M.O.; Mohamed, A.E.; Gebril, N.M.; Jomaa, F.; Daeki, A.O. Seroprevalence and associated risk factors of HBV and HCV infections in the population of Ghudduwah Village, South Libya. J. Infect. Dev. Ctries 2025, 19, 117–123. [Google Scholar] [CrossRef]
- Shambesh, M.; Franka, E.; Ismail, F.; Gebril, N.; Azabi, K.; Amar, F. Anti-HBc and HBV-DNA among blood donors in North Africa; Western Libya. Int. Blood Res. Rev. 2015, 3, 152–159. [Google Scholar] [CrossRef]
- Abdelaziz, H.M.; Fahmy, S.; Soliman, A.; Yousef, E.M. Anti-HB core screening significance among healthy blood donors in Fayoum, Egypt. J. Anc. Dis. Prev. Rem. 2016, 4, 2. [Google Scholar] [CrossRef]
- Shastry, S.; Bhat, S.S. Prevention of post-transfusion hepatitis by screening of antibody to hepatitis B core antigen in healthy blood donors. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011062. [Google Scholar] [CrossRef]
- Kolayli, C.C.; Topbas, M.; Ozkaya, E.; Koksal, I.; Beyhun, N.E.; Kaklikkaya, N.; Can, G.; Yilmaz, M.; Hamzaoglu, K.; Sayin, E.; et al. Seroprevalence and risk factors of hepatitis B, C and D in adults in Trabzon, Türkiye. Mikrobiyol. Bul. 2024, 58, 293–308. [Google Scholar] [CrossRef] [PubMed]
- El-Ghitany, E.M.; Farghaly, A.G. Serological pattern of hepatitis B virus among HBsAg-negative blood donors in Alexandria, Egypt. East Mediterr. Health J. 2013, 19, 600–607. [Google Scholar] [PubMed]
- Fabris, P.; Baldo, V.; Baldovin, T.; Bellotto, E.; Rassu, M.; Trivello, R.; Tramarin, A.; Tositti, G.; Floreani, A. Changing epidemiology of HCV and HBV infections in Northern Italy: A survey in the general population. J. Clin. Gastroenterol. 2008, 42, 527–532. [Google Scholar] [CrossRef]
- Ogunfemi, M.K.; Olawumi, H.O.; Olokoba, A.B.; Kagu, M.B.; Biliaminu, S.A.; Durowade, K.A.; Durotoye, I.A.; Shittu, A.O. Prevalence of antibody to hepatitis B core antigen among hepatitis B surface antigen-negative blood donors in Ilorin, Nigeria: A cross-sectional study. Malawi Med. J. 2017, 29, 32–36. [Google Scholar] [CrossRef]
- Salawu, L.; Adegoke, A.O.; Aboderin, A.O.; Huraina, H.A. Hepatitis B viral markers in surface antigen negative blood donors: The need to look beyond antibody negativity. West Afr. J. Med. 2011, 30, 292–295. [Google Scholar] [PubMed]
- Croatian Institute of Transfusion Medicine. Homepage [Internet]. Croatian Institute of Transfusion Medicine. 2025. Available online: https://hztm.hr/ (accessed on 10 September 2025).
- Drlje, I.T.; Arapović, J. Prevalence of hepatitis B surface antigen (HBsAg) in blood donor population in Bosnia and Herzegovina: Impact of the pre-donation questionnaire implementation and mandatory hepatitis B virus (HBV) vaccination schedule—20 years’ experience of the University Clinical Hospital Mostar. Transfus. Clin. Biol. 2022, 29, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Joukar, F.; Mansour-Ghanaei, F.; Naghipour, M.R.; Asgharnezhad, M. Immune response to hepatitis B vaccine among north Iranian healthcare workers and its related factors. J. Infect. Dev. Ctries 2017, 11, 501–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahana, H.V.; Sarala, N.; Prasad, S.R. Decrease in anti-HBs antibodies over time in medical students and healthcare workers after hepatitis B vaccination. Biomed. Res. Int. 2017, 2017, 1327492. [Google Scholar] [CrossRef] [PubMed]
- Ippoliti, L.; Pizzo, A.; Paolino, A.; Coppeta, L.; Bizzarro, G.; Ferrari, C.; Mazza, A.; Salvi, C.; Buonomo, E.; Cenko, F.; et al. Evaluation of anti-HBs levels in a multi-ethnic cohort of health profession students. Vaccines 2025, 13, 771. [Google Scholar] [CrossRef]
- Bello, N.; Hudu, S.A.; Alshrari, A.S.; Imam, M.U.; Jimoh, A.O. Overview of Hepatitis B Vaccine Non-Response and Associated B Cell Amnesia: A Scoping Review. Pathogens 2024, 13, 554. [Google Scholar] [CrossRef]
- Leuridan, E.; Van Damme, P. Hepatitis B and the need for a booster dose. Clin. Infect. Dis. 2011, 53, 68–75. [Google Scholar] [CrossRef]
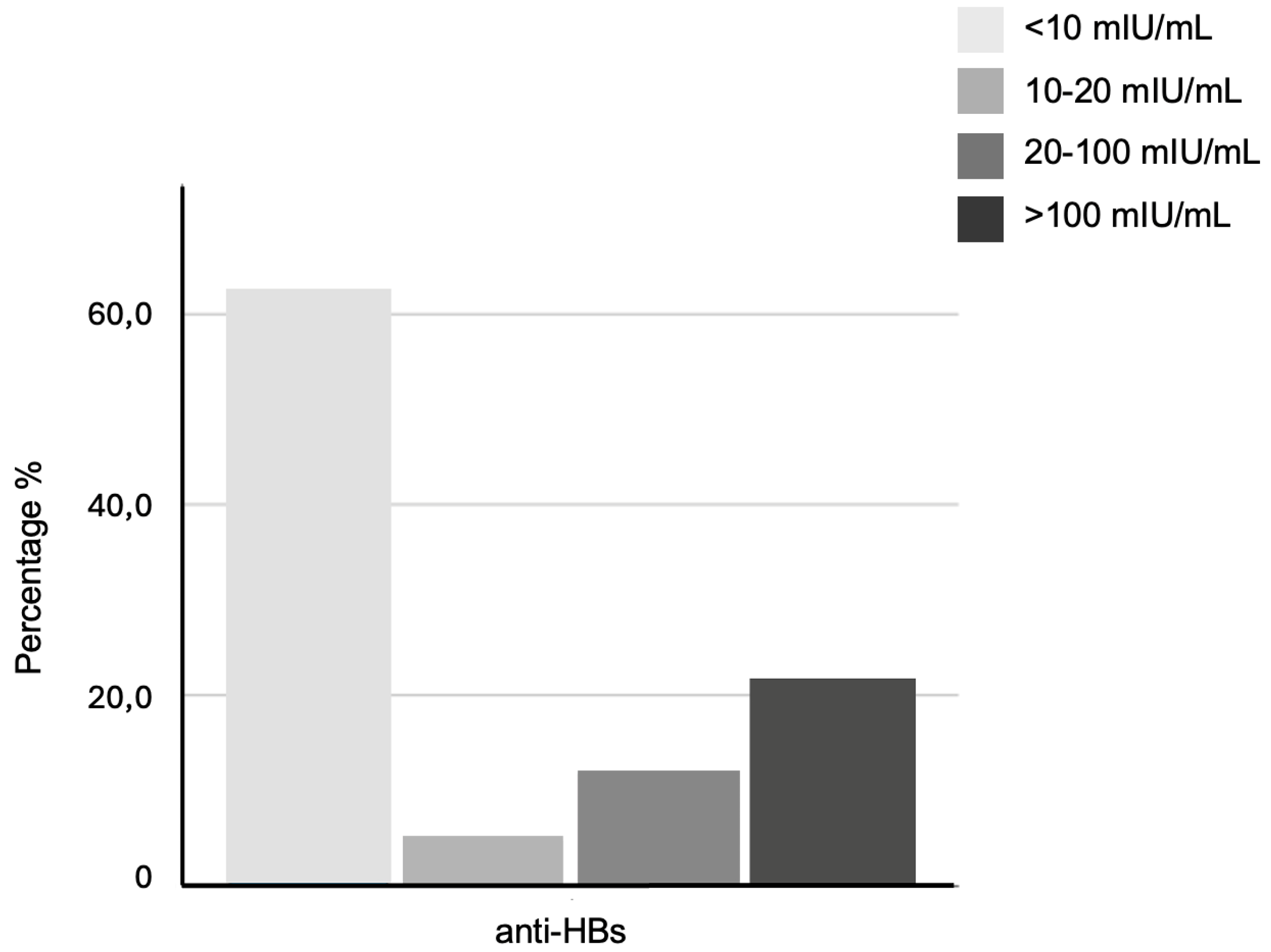

| Vaccination Status | Anti-HBc Negative, n/N (%) | Anti-HBc Positive n/N (%) | Pearson Chi-Square (p-Value) | Fisher’s Exact Test (p-Value) |
|---|---|---|---|---|
| Yes | 537/537 | 0/537 | 0.017 | 0.022 |
| (100.0%) | (0.0%) | |||
| No | 466/471 | 5/471 | ||
| (98.9%) | (1.1%) | |||
| Total | 1003/1008 (99.5%) | 5/1008 (0.5%) |
| Vaccination Status | Number of Donors | Donors with >10 mIU/mL (n, %) | Interpretation |
|---|---|---|---|
| Vaccinated | 537 | 379 (70.6%) | Vaccine-induced immunity |
| Unvaccinated | 471 | 5 (1.1%) | Resolved infection |
| Total | 1008 | 384 (38.1%) | Overall protective anti-HBs levels |
| Variable | Age Group | Gender | Vaccination Status | Time Since Vaccination (Years) | Healthcare Worker | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 18–37 (n = 490) | 38–65 (n = 518) | Male (n = 801) | Female (n = 207) | Yes (n = 537) | No (n = 471) | <15 (n = 197) | >15 (n = 340) | Yes (n = 88) | No (n = 920) | |
| Anti-HBs < 10 (n, %) | 135 (27.6%) | 489 (94.4%) | 503 (62.8%) | 121 (58.5%) | 158 (29.4%) | 466 (98.9%) | 45 (22.8%) | 113 (33.2%) | 29 (33.0%) | 595 (64.7%) |
| Anti-HBs 10–20 (n, %) | 44 (9.0%) | 4 (0.8%) | 41 (5.1%) | 7 (3.4%) | 48 (8.9%) | 0 (0.0%) | 22 (11.2%) | 26 (7.6%) | 7 (8.0%) | 41 (4.5%) |
| Anti-HBs 20–100 (n, %) | 108 (22.0%) | 11 (2.1%) | 87 (10.9%) | 32 (15.5%) | 117 (21.8%) | 2 (0.4%) | 40 (20.3%) | 77 (22.6%) | 17 (19.3%) | 102 (11.1%) |
| Anti-HBs > 100 (n, %) | 203 (41.4%) | 14 (2.7%) | 170 (21.2%) | 47 (22.7%) | 214 (39.9%) | 3 (0.6%) | 90 (45.7%) | 124 (36.5%) | 35 (39.8%) | 182 (19.8%) |
| Total | 490 (100%) | 518 (100%) | 801 (100%) | 207 (100%) | 537 (100%) | 471 (100%) | 197 (100%) | 340 (100%) | 88 (100%) | 920 (100%) |
| p-value (Pearson Chi-Square) | <0.001 * | 0.186 | <0.001 * | 0.024 * | <0.001 * | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilberovic, A.; Radman-Livaja, M.; Talic-Drlje, I.; Stanic, A.; Njire-Braticevic, M.; Tomicic, N.; Arapovic, J. Hepatitis B Serological Immunity and Exposure Among Blood Donors in Southern Croatia: A Cross-Sectional Study. Vaccines 2025, 13, 1027. https://doi.org/10.3390/vaccines13101027
Dilberovic A, Radman-Livaja M, Talic-Drlje I, Stanic A, Njire-Braticevic M, Tomicic N, Arapovic J. Hepatitis B Serological Immunity and Exposure Among Blood Donors in Southern Croatia: A Cross-Sectional Study. Vaccines. 2025; 13(10):1027. https://doi.org/10.3390/vaccines13101027
Chicago/Turabian StyleDilberovic, Admir, Mirela Radman-Livaja, Ivana Talic-Drlje, Ana Stanic, Marina Njire-Braticevic, Nikolina Tomicic, and Jurica Arapovic. 2025. "Hepatitis B Serological Immunity and Exposure Among Blood Donors in Southern Croatia: A Cross-Sectional Study" Vaccines 13, no. 10: 1027. https://doi.org/10.3390/vaccines13101027
APA StyleDilberovic, A., Radman-Livaja, M., Talic-Drlje, I., Stanic, A., Njire-Braticevic, M., Tomicic, N., & Arapovic, J. (2025). Hepatitis B Serological Immunity and Exposure Among Blood Donors in Southern Croatia: A Cross-Sectional Study. Vaccines, 13(10), 1027. https://doi.org/10.3390/vaccines13101027






