Accelerating Vaccine Adjuvant Screening: Early Follicular Dendritic Cell and Germinal Center B Cell Biomarkers Predict Protective Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Virus, and Infection
2.2. Immunization
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Viral Neutralizing Antibody Assay
2.5. Draining Lymph Node Isolation and Flow Cytometry
2.6. Fluorescent Labeling of Pre-F and Quantification of the Number of Pre-F-Specific GC B Cells
2.7. Statistical Analysis
3. Results
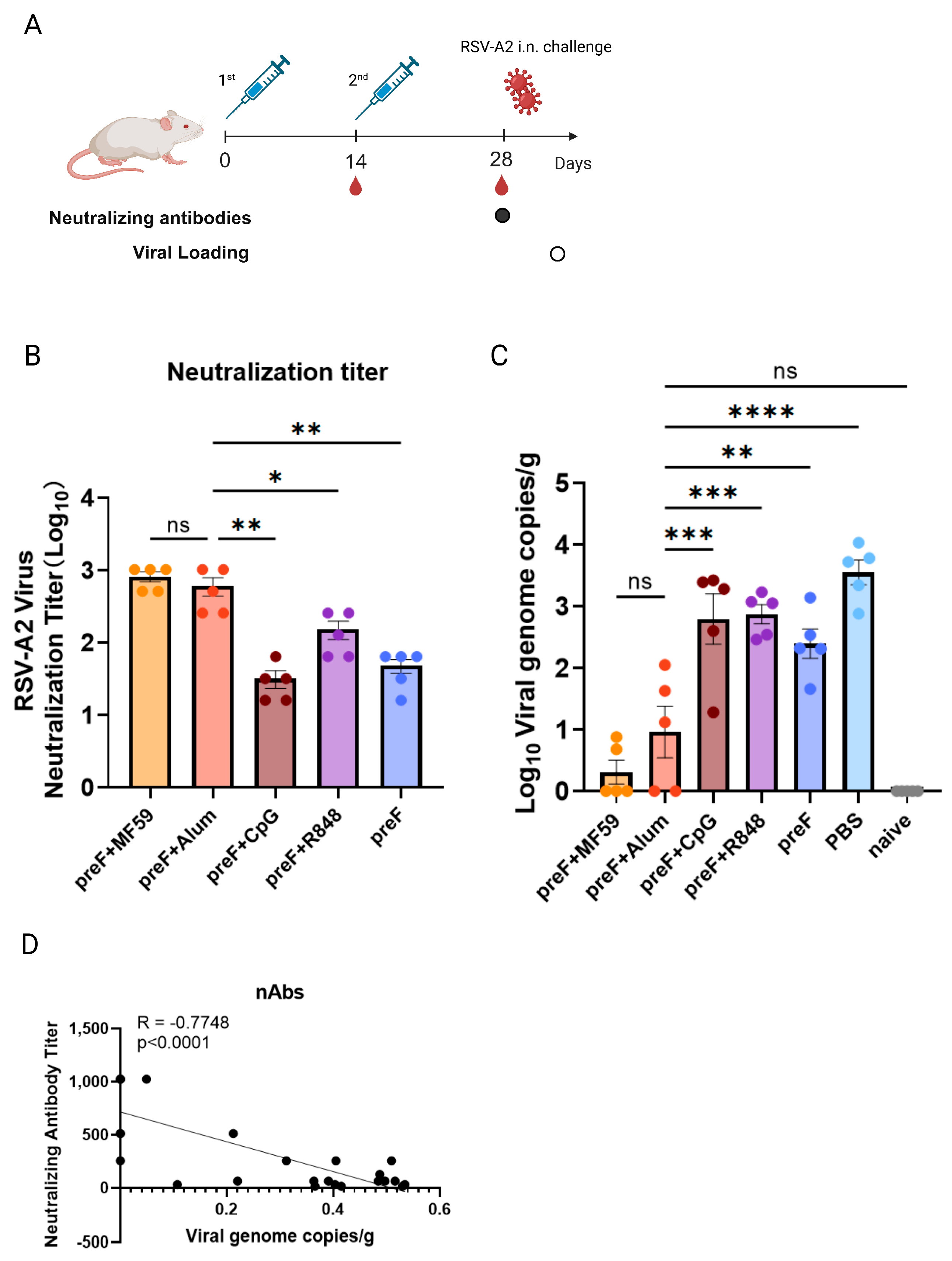
3.1. Variable Vaccine Protection Is Driven by Distinct Adjuvants
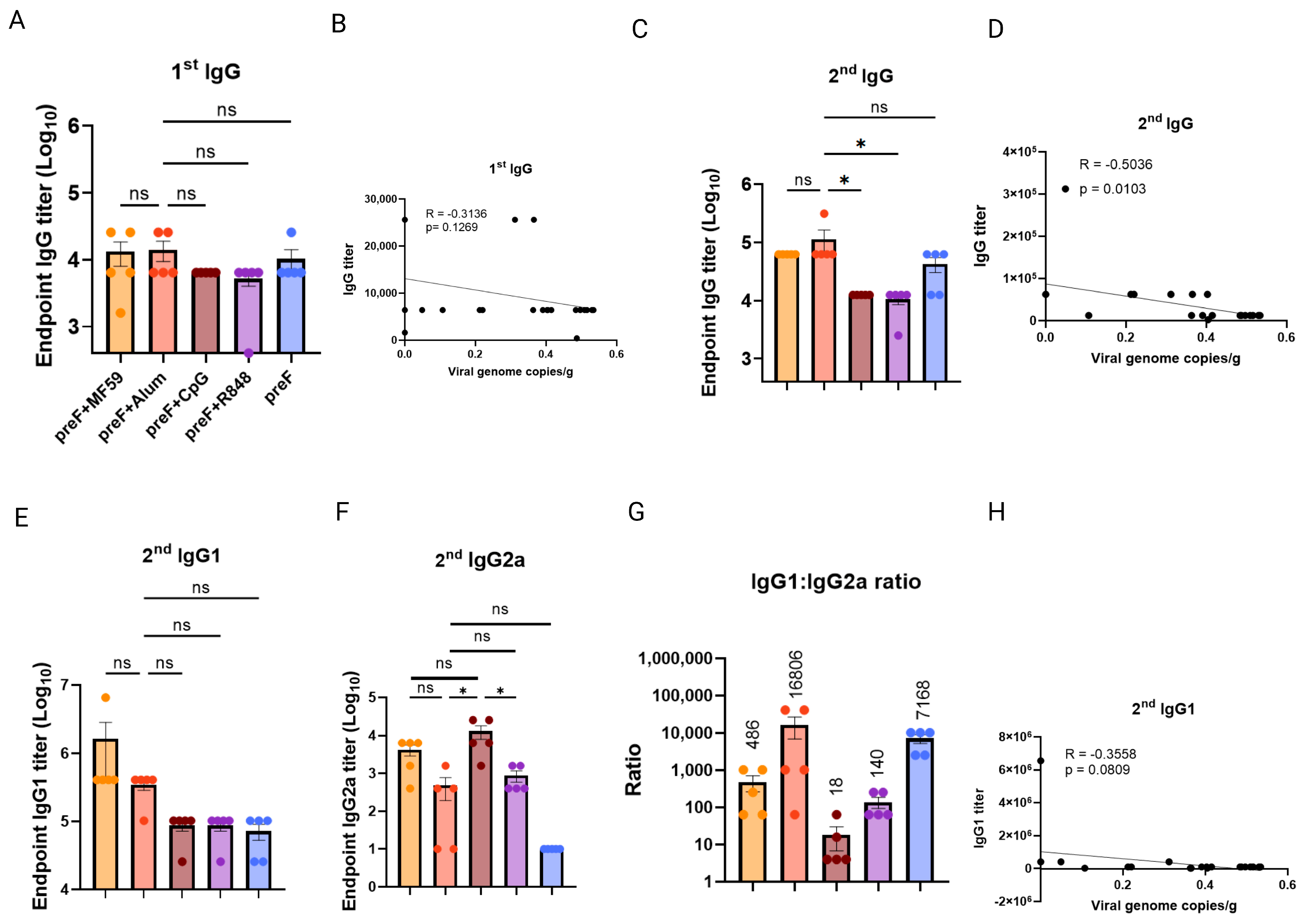
3.2. Binding Antibody Titers Lack Correlation with Protection Efficacy
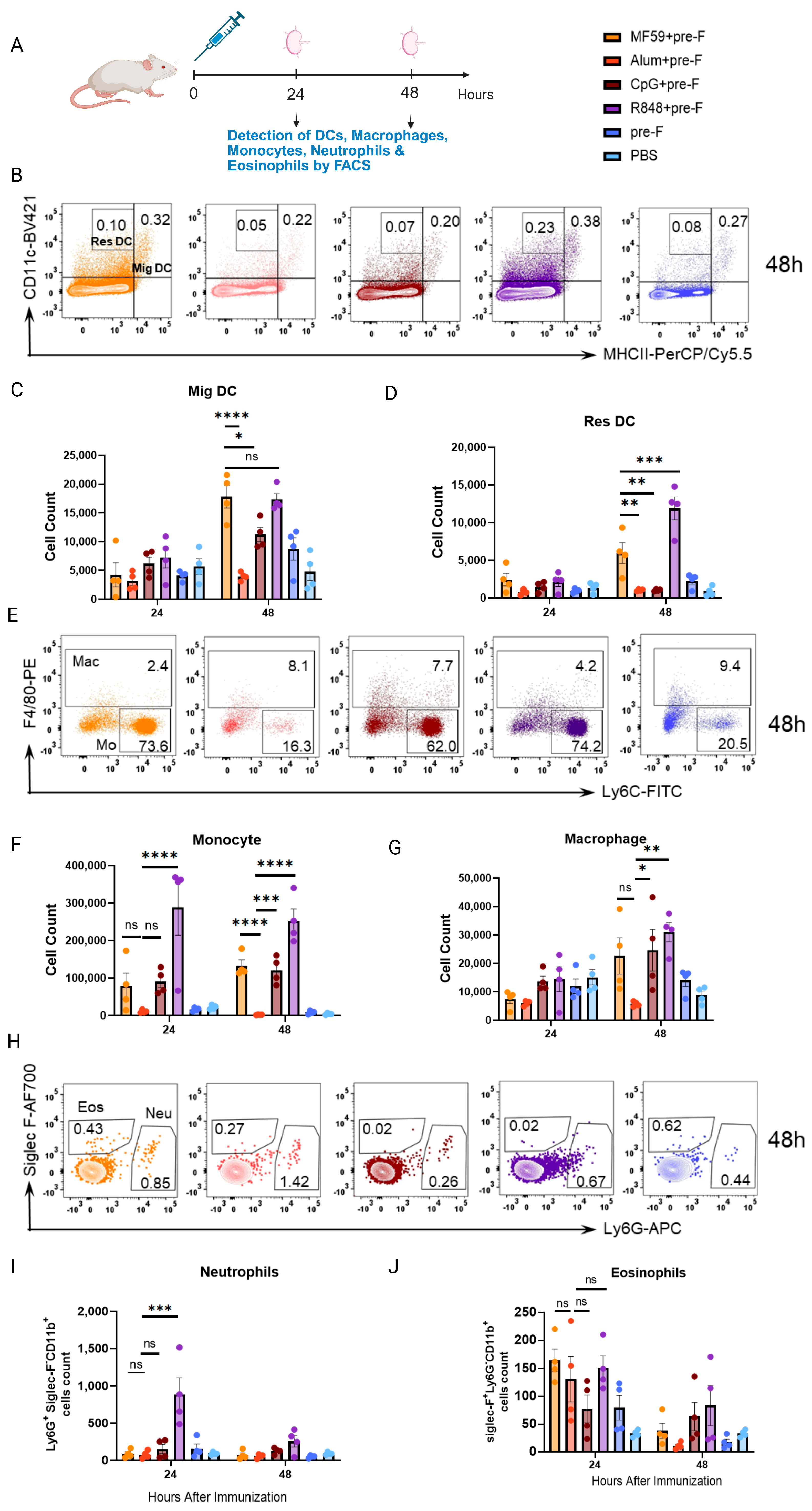
3.3. Innate Immune Cell Responses Do Not Correlate with Protection
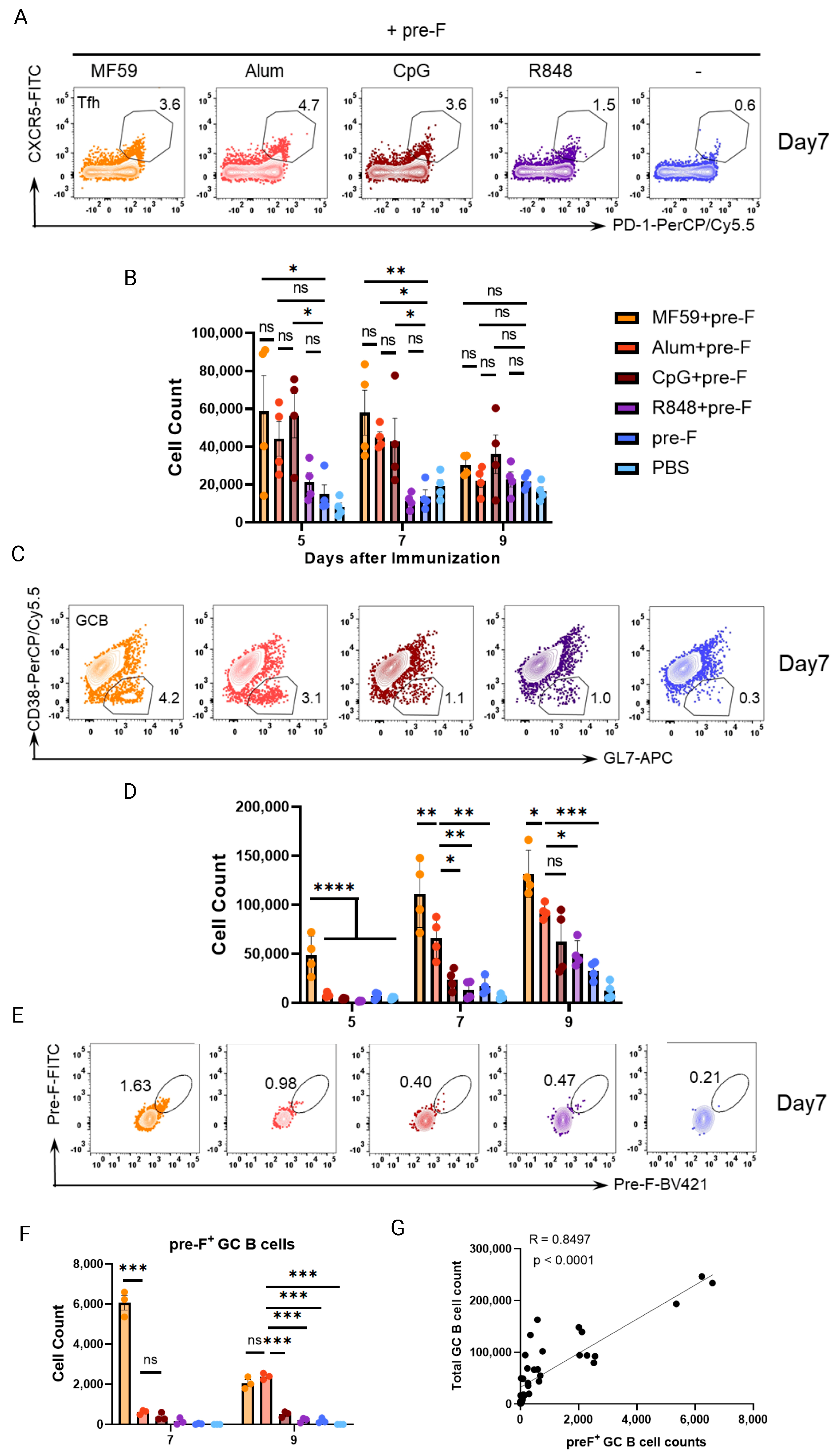
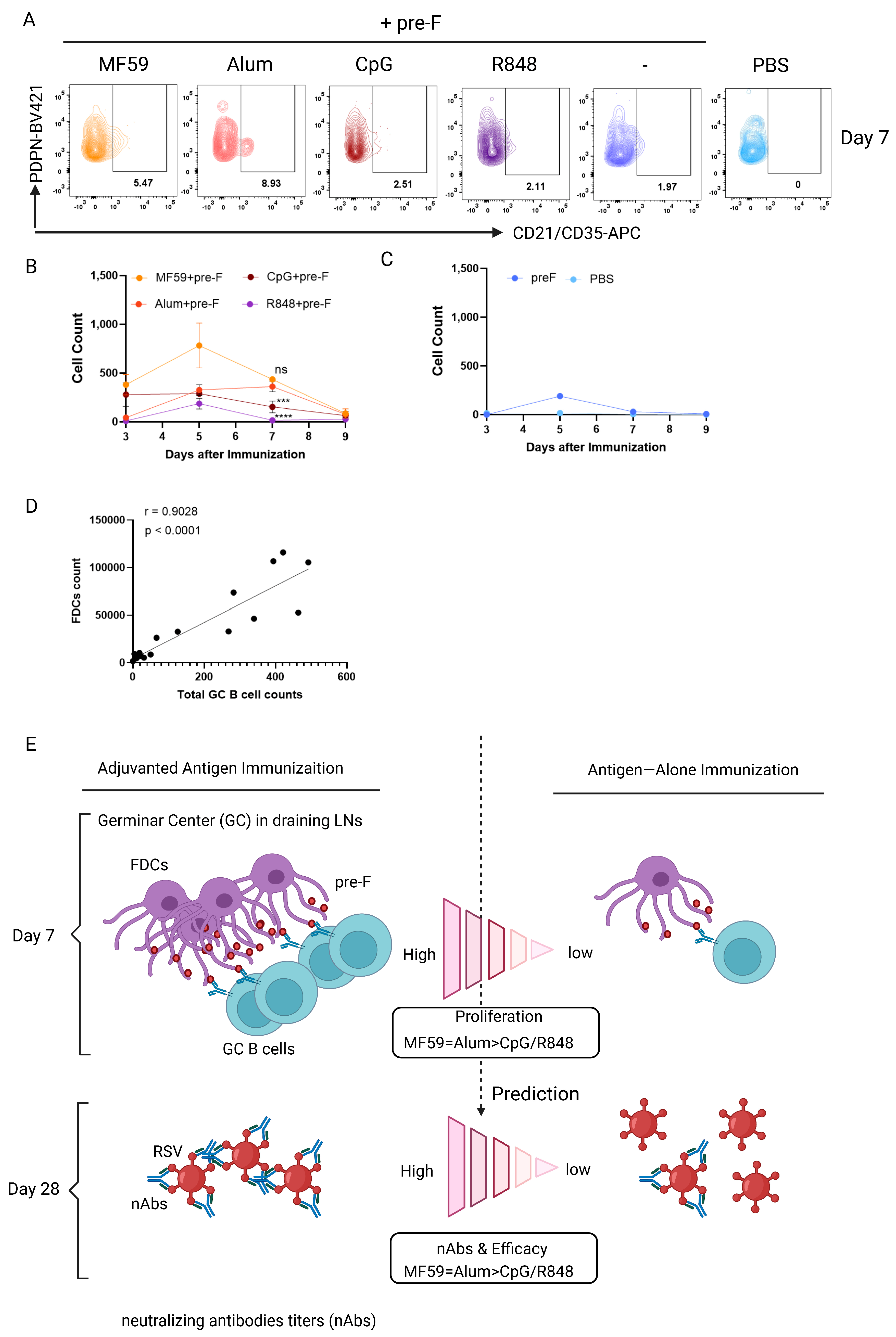
3.4. Early Germinal Center B Cell Responses Correlate with Vaccine−Mediated Protection
3.5. Follicular Dendritic Cell (FDC) Abundance Correlates with GC B Cell Response to Predict Protection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, K.A.; Rodriguez-Aponte, S.A.; Dalvie, N.C.; Lee, J.H.; Abraham, W.; Carnathan, D.G.; Jimenez, L.E.; Ngo, J.T.; Chang, J.Y.H.; Zhang, Z.; et al. Phosphate-mediated coanchoring of RBD immunogens and molecular adjuvants to alum potentiates humoral immunity against SARS-CoV-2. Sci. Adv. 2021, 7, eabj6538. [Google Scholar] [CrossRef]
- Hutchison, S.; Benson, R.A.; Gibson, V.B.; Pollock, A.H.; Garside, P.; Brewer, J.M. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012, 26, 1272–1279. [Google Scholar] [CrossRef]
- Khurana, S.; Verma, N.; Yewdell, J.W.; Hilbert, A.K.; Castellino, F.; Lattanzi, M.; Del Giudice, G.; Rappuoli, R.; Golding, H. MF59 Adjuvant Enhances Diversity and Affinity of Antibody-Mediated Immune Response to Pandemic Influenza Vaccines. Sci. Transl. Med. 2011, 3, 85ra48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Weinberger, B. Adjuvant strategies to improve vaccination of the elderly population. Curr. Opin. Pharmacol. 2018, 41, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kasturi Sudhir, P.; Kozlowski Pamela, A.; Nakaya Helder, I.; Burger Matheus, C.; Russo, P.; Pham, M.; Kovalenkov, Y.; Silveira Eduardo, L.V.; Havenar-Daughton, C.; Burton Samantha, L.; et al. Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques. J. Virol. 2017, 91, e01844-16. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Xu, W.; Deng, W.; Wang, Y.; Wang, M.; Wang, Q.; Hsieh, M.; Dong, J.; Wang, X.; et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs. Cell Res. 2022, 32, 269–287. [Google Scholar] [CrossRef]
- Giannini, S.L.; Hanon, E.; Moris, P.; Van Mechelen, M.; Morel, S.; Dessy, F.; Fourneau, M.A.; Colau, B.; Suzich, J.; Losonksy, G.; et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006, 24, 5937–5949. [Google Scholar] [CrossRef] [PubMed]
- Ols, S.; Yang, L.; Thompson, E.A.; Pushparaj, P.; Tran, K.; Liang, F.; Lin, A.; Eriksson, B.; Karlsson Hedestam, G.B.; Wyatt, R.T.; et al. Route of Vaccine Administration Alters Antigen Trafficking but Not Innate or Adaptive Immunity. Cell Rep. 2020, 30, 3964–3971.e7. [Google Scholar] [CrossRef]
- Swaminathan, G.; Thoryk, E.A.; Cox, K.S.; Meschino, S.; Dubey, S.A.; Vora, K.A.; Celano, R.; Gindy, M.; Casimiro, D.R.; Bett, A.J. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine 2016, 34, 110–119. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, K.M.; Carnathan, D.G.; Nogal, B.; Martin, J.T.; Rodriguez, O.L.; Upadhyay, A.A.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Viviano, F.; et al. Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 2019, 177, 1153–1171.e28. [Google Scholar] [CrossRef]
- Kim, H.; Niu, L.; Larson, P.; Kucaba, T.A.; Murphy, K.A.; James, B.R.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials 2018, 164, 38–53. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Tombácz In Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Sandgren, K.J.; Thompson, E.A.; Francica, J.R.; Seubert, A.; De Gregorio, E.; Barnett, S.; O’Hagan, D.T.; Sullivan, N.J.; et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 2017, 9, eaal2094. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, N.; Codrington, A.L.; El-Fenej, J.; Phondge, V.; Kumamoto, Y. Effective CD4 T cell priming requires repertoire scanning by CD301b+ migratory cDC2 cells upon lymph node entry. Sci. Immunol. 2021, 6, eabg0336. [Google Scholar] [CrossRef]
- Wang, X.; Cho, B.; Suzuki, K.; Xu, Y.; Green, J.A.; An, J.; Cyster, J.G. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J. Exp. Med. 2011, 208, 2497–2510. [Google Scholar] [CrossRef] [PubMed]
- van der Poel, C.E.; Bajic, G.; Macaulay, C.W.; van den Broek, T.; Ellson, C.D.; Bouma, G.; Victora, G.D.; Degn, S.R.E.; Carroll, M.C. Follicular Dendritic Cells Modulate Germinal Center B Cell Diversity through FcγRIIB. Cell Rep. 2019, 29, 2745–2755.e4. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.; Wolfreys, F.D.; Muppidi, J.R.; Xu, Y.; Cyster, J.G. S-Geranylgeranyl-l-glutathione is a ligand for human B cell-confinement receptor P2RY8. Nature 2019, 567, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Riaño, A.; Wang, S.; Boeing, S.; Minoughan, S.; Casal, A.; Spillane, K.M.; Ludewig, B.; Tolar, P. Long-term retention of antigens in germinal centers is controlled by the spatial organization of the follicular dendritic cell network. Nat. Immunol. 2023, 24, 1281–1294. [Google Scholar] [CrossRef]
- Dasoveanu, D.C.; Shipman, W.D.; Chia, J.J.; Chyou, S.; Lu, T.T. Regulation of Lymph Node Vascular-Stromal Compartment by Dendritic Cells. Trends Immunol. 2016, 37, 764–777. [Google Scholar] [CrossRef]
- Bian, L.; Zheng, Y.; Guo, X.; Li, D.; Zhou, J.; Jing, L.; Chen, Y.; Lu, J.; Zhang, K.; Jiang, C.; et al. Intramuscular Inoculation of AS02-Adjuvanted Respiratory Syncytial Virus (RSV) F Subunit Vaccine Shows Better Efficiency and Safety Than Subcutaneous Inoculation in BALB/c Mice. Front. Immunol. 2022, 13, 938598. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X.; Zhong, Y.; Li, C.; Dong, A.; He, Z.; Zhang, S.; Wang, B. A Recombinant G Protein Plus Cyclosporine A–Based Respiratory Syncytial Virus Vaccine Elicits Humoral and Regulatory T Cell Responses against Infection without Vaccine-Enhanced Disease. J. Immunol. 2016, 196, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Mboup, E.B.; Hamelin, M.-È.; Dubois, J.; Rosa-Calatrava, M.; Boivin, G. Vaccine Development for Human Pneumoviruses. Vaccines 2025, 13, 569. [Google Scholar] [CrossRef]
- Eto, D.; Lao, C.; DiToro, D.; Barnett, B.; Escobar, T.C.; Kageyama, R.; Yusuf, I.; Crotty, S. IL-21 and IL-6 Are Critical for Different Aspects of B Cell Immunity and Redundantly Induce Optimal Follicular Helper CD4 T Cell (Tfh) Differentiation. PLoS ONE 2011, 6, e17739. [Google Scholar] [CrossRef]
- Lindgren, G.; Ols, S.; Liang, F.; Thompson, E.A.; Lin, A.; Hellgren, F.; Bahl, K.; John, S.; Yuzhakov, O.; Hassett, K.J.; et al. Induction of Robust B cell responses after influenza mRNA vaccination is accompanied by circulating hemagglutinin-specific ICOS+ PD-1+ CXCR3+ T follicular helper cells. Front. Immunol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Foy, T.M.; Laman, J.D.; Ledbetter, J.A.; Aruffo, A.; Claassen, E.; Noelle, R.J. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J. Exp. Med. 1994, 180, 157–163. [Google Scholar] [CrossRef]
- Zabala-Peñafiel, A.; Gonzalez-Lombana, C.; Alameh, M.-G.; Sacramento, L.A.; Mou, Z.; Phan, A.T.; Aunins, E.A.; Tam, Y.K.; Uzonna, J.E.; Weissman, D.; et al. IL-12 mRNA-LNP promotes dermal resident memory CD4+ T cell development. Npj Vaccines 2025, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Watari, K.; Ohira, M.; Brito, J.S.; He, P.; Onyuru, J.; Zuniga, E.I.; Hoffman, H.M.; Karin, M. Mitochondrial DNA oxidation propagates autoimmunity by enabling plasmacytoid dendritic cells to induce TFH differentiation. Nat. Immunol. 2025, 26, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P.; Vong, A.M.; Castonguay, C.H.; Kugler-Umana, O.; Bautista, B.L.; Jones, M.C.; Kelly, K.A.; Xia, J.; Swain, S.L. Strong influenza-induced TFH generation requires CD4 effectors to recognize antigen locally and receive signals from continuing infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2111064119. [Google Scholar] [CrossRef]
- Anania, J.C.; Westin, A.; Adler, J.; Heyman, B. A Novel Image Analysis Approach Reveals a Role for Complement Receptors 1 and 2 in Follicular Dendritic Cell Organization in Germinal Centers. Front Immunol 2021, 12, 655753. [Google Scholar] [CrossRef]
- Bhagchandani, S.H.; Yang, L.; Lam, J.H.; Maiorino, L.; Ben-Akiva, E.; Rodrigues, K.A.; Romanov, A.; Suh, H.; Aung, A.; Wu, S.; et al. Two-dose priming immunization amplifies humoral immunity by synchronizing vaccine delivery with the germinal center response. Sci. Immunol. 2024, 9, eadl3755. [Google Scholar] [CrossRef]
- Heesters, B.A.; van Megesen, K.; Tomris, I.; de Vries, R.P.; Magri, G.; Spits, H. Characterization of human FDCs reveals regulation of T cells and antigen presentation to B cells. J. Exp. Med. 2021, 218, e20210790. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dasoveanu Dragos, C.; Chyou, S.; Tzeng, T.-C.; Rozo, C.; Liang, Y.; Stohl, W.; Fu, Y.-X.; Ruddle Nancy, H.; Lu Theresa, T. A Dendritic-Cell-Stromal Axis Maintains Immune Responses in Lymph Nodes. Immunity 2015, 42, 719–730. [Google Scholar] [CrossRef]
| Groups | pre-F/Mouse | Adjuvant/Mouse | Volume/Mouse |
|---|---|---|---|
| PBS | / | / | 100 μL |
| pre-F | 10 μg | / | 100 μL |
| pre-F+MF59 | 10 μg | MF59 50 μL | 100 μL |
| pre-F+Alum | 10 μg | Alum 100 μg | 100 μL |
| pre-F+CpG | 10 μg | CpG 20 μg | 100 μL |
| pre-F+R848 | 10 μg | R848 20 μg | 100 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Chen, M.; Lin, H.; Liu, Z.; Zhang, S.; He, Y.; Wang, B. Accelerating Vaccine Adjuvant Screening: Early Follicular Dendritic Cell and Germinal Center B Cell Biomarkers Predict Protective Efficacy. Vaccines 2025, 13, 1011. https://doi.org/10.3390/vaccines13101011
Zhong Y, Chen M, Lin H, Liu Z, Zhang S, He Y, Wang B. Accelerating Vaccine Adjuvant Screening: Early Follicular Dendritic Cell and Germinal Center B Cell Biomarkers Predict Protective Efficacy. Vaccines. 2025; 13(10):1011. https://doi.org/10.3390/vaccines13101011
Chicago/Turabian StyleZhong, Yiwei, Mingyue Chen, Hongzhe Lin, Zhenrui Liu, Shijie Zhang, Yue He, and Bin Wang. 2025. "Accelerating Vaccine Adjuvant Screening: Early Follicular Dendritic Cell and Germinal Center B Cell Biomarkers Predict Protective Efficacy" Vaccines 13, no. 10: 1011. https://doi.org/10.3390/vaccines13101011
APA StyleZhong, Y., Chen, M., Lin, H., Liu, Z., Zhang, S., He, Y., & Wang, B. (2025). Accelerating Vaccine Adjuvant Screening: Early Follicular Dendritic Cell and Germinal Center B Cell Biomarkers Predict Protective Efficacy. Vaccines, 13(10), 1011. https://doi.org/10.3390/vaccines13101011






