Robust and Long-Lasting Immunity and Protection in Mice Induced by Lipopolyplex-Delivered mRNA Vaccines Expressing the Prefusion Protein of Respiratory Syncytial Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Animals
2.2. Construction and Preparation of mRNA Vaccine Expressing RSV Pre-F Protein
2.3. Western Blotting
2.4. Immunofluorescence Assay
2.5. Immunizations
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. RSV Neutralisation Assays
2.8. Enzyme-Linked Immunospot Assay (ELISPOT)
2.9. Intracellular Cytokine Staining (ICS)
2.10. Challenge Experiment
2.11. Statistical Analysis
3. Results
3.1. Characterisation of RSV Pre-F LPP-mRNA Vaccines
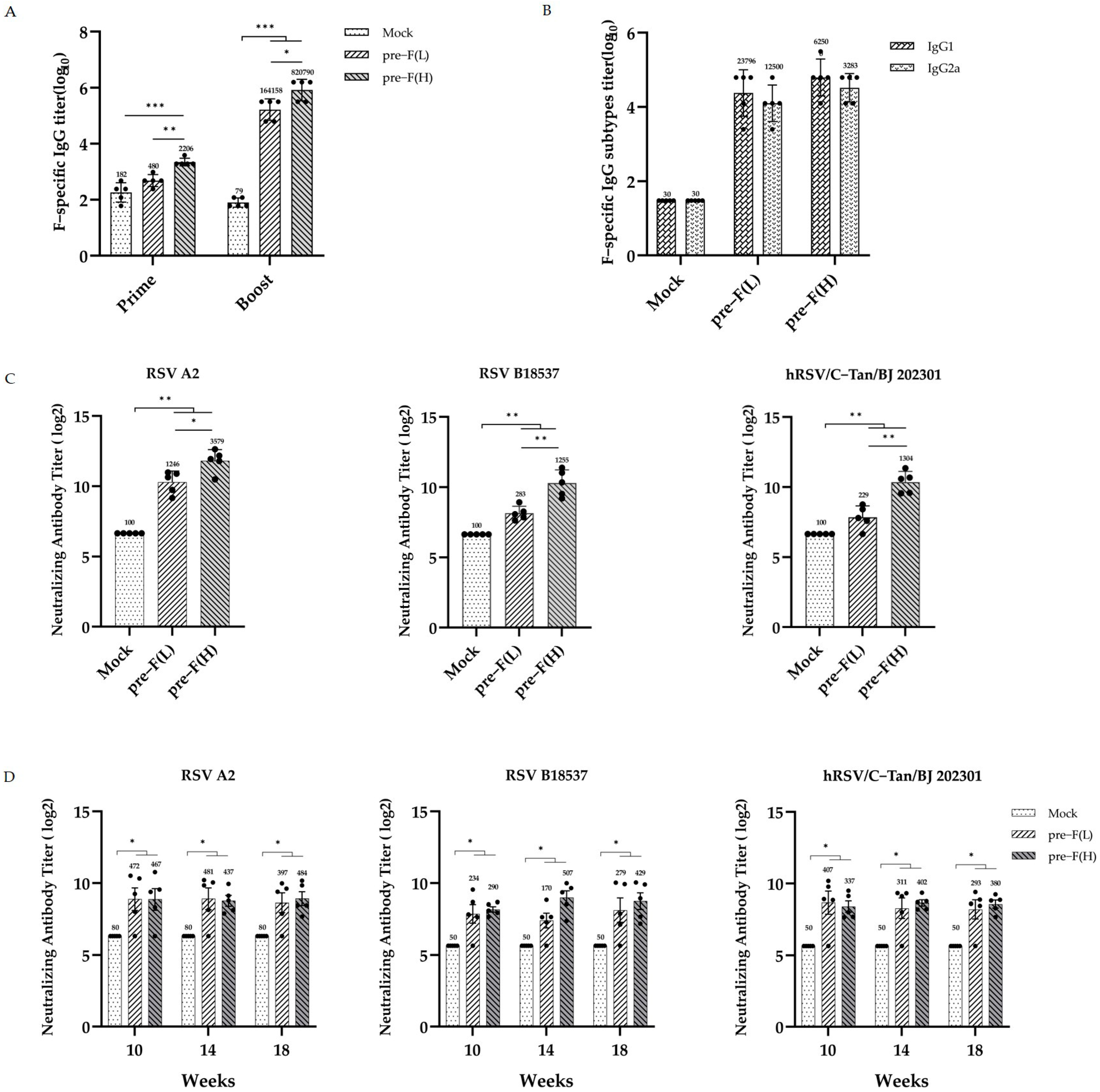
3.2. Significant and Sustained Humoral Immunity Responses Induced by RSV Pre-F LPP-mRNA
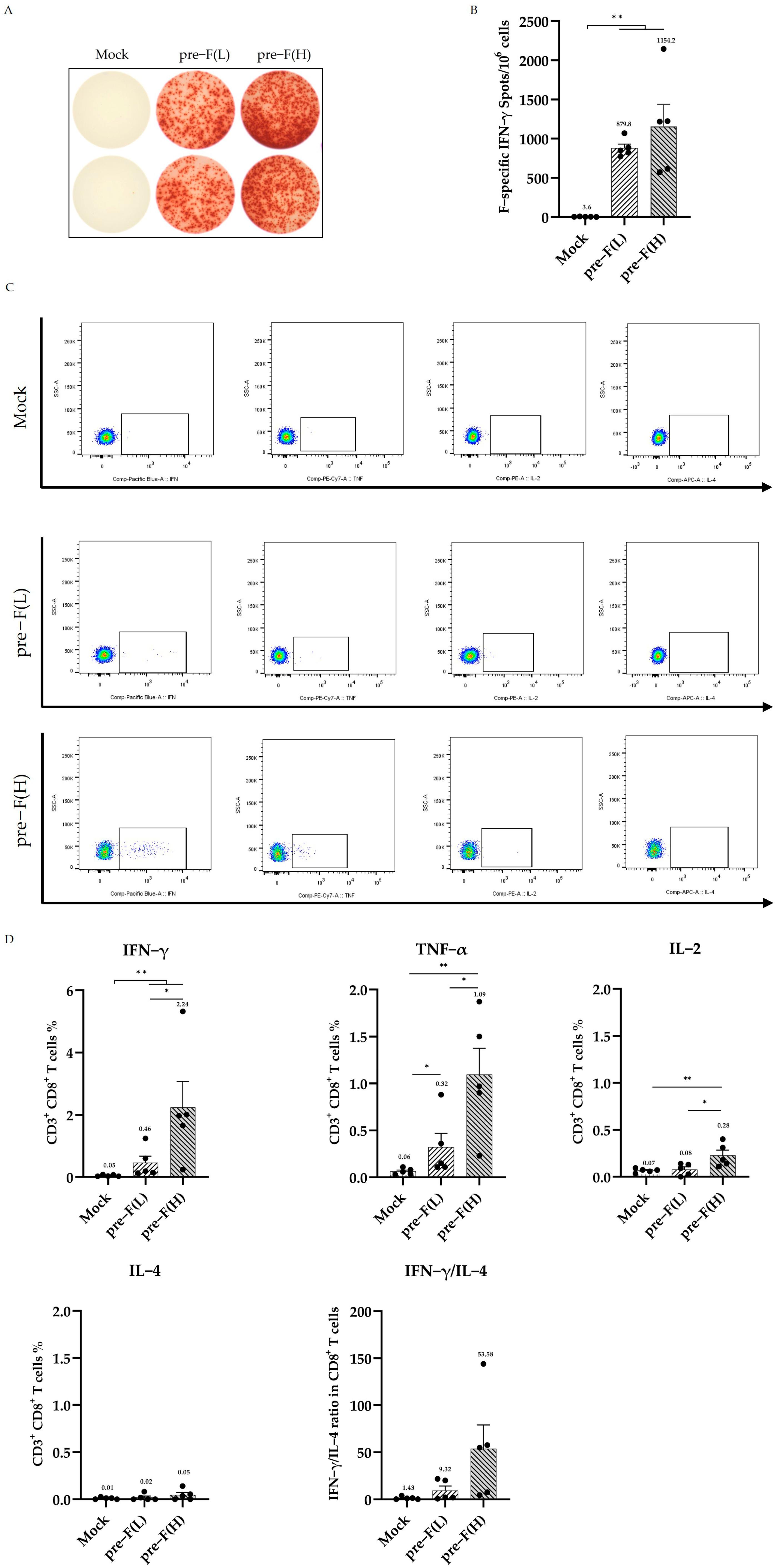
3.3. RSV Pre-F LPP-mRNA Elicits Significant F-Specific T Cell Responses
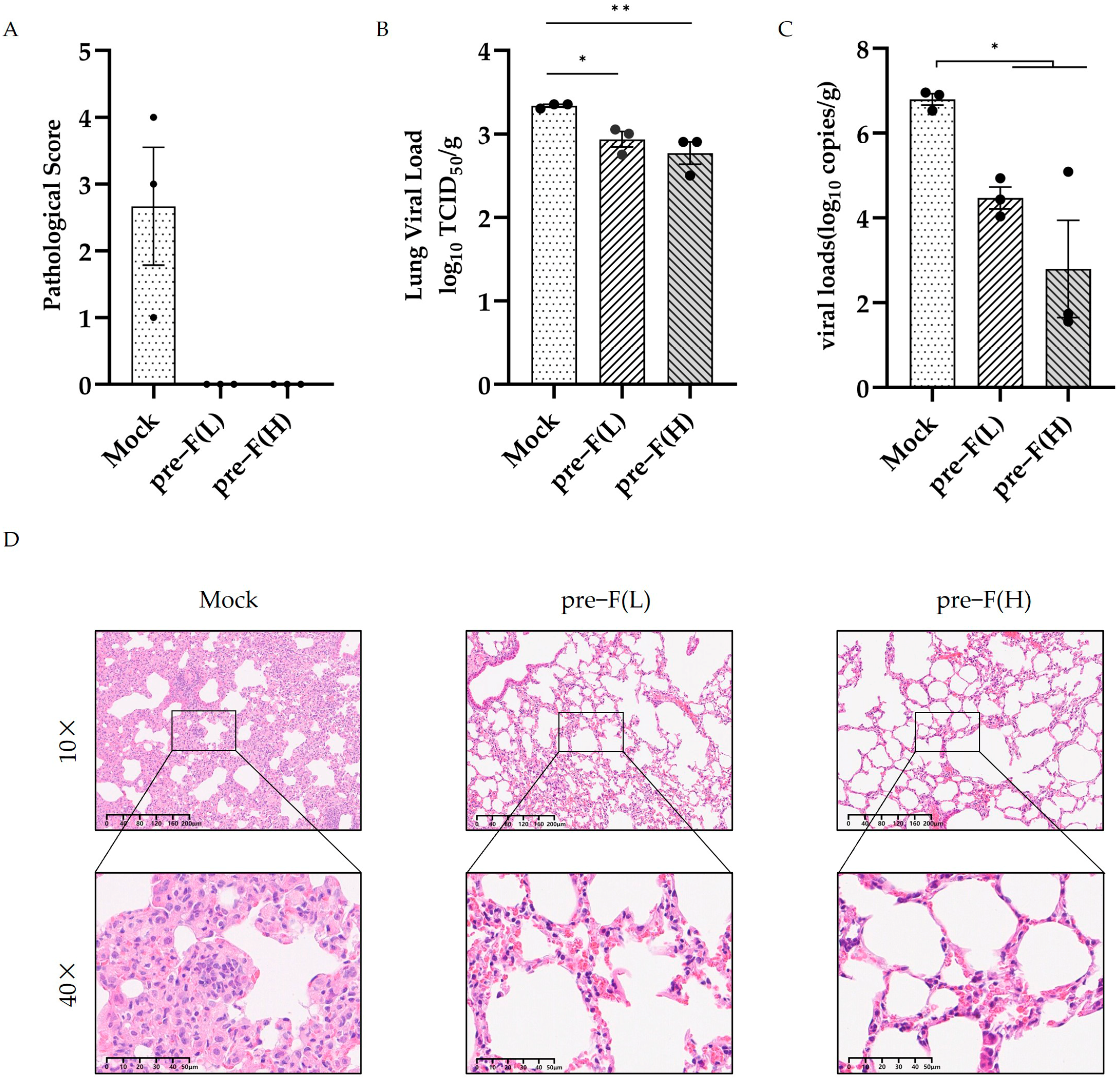
3.4. RSV Pre-F LPP-mRNA Induced Long-Lasting Protection Against RSV Infection in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Ruckwardt, T.J.; Morabito, K.M.; Graham, B.S. Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity 2019, 51, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Awosika, A.O.; Patel, P. Respiratory Syncytial Virus Prefusion F (RSVPreF3) Vaccine. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fleming-Dutra, K.E.; Jones, J.M.; Roper, L.E.; Prill, M.M.; Ortega-Sanchez, I.R.; Moulia, D.L.; Wallace, M.; Godfrey, M.; Broder, K.R.; Tepper, N.K.; et al. Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus-Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb. Mortal Wkly. Rep. 2023, 72, 1115–1122. [Google Scholar] [CrossRef]
- Magro, M.; Mas, V.; Chappell, K.; Vázquez, M.; Cano, O.; Luque, D.; Terrón, M.C.; Melero, J.A.; Palomo, C. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc. Natl. Acad. Sci. USA 2012, 109, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves mRNA-based RSV vaccine. Nat. Rev. Drug. Discov. 2024, 23, 487. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S. Vaccine development for respiratory syncytial virus. Curr. Opin. Virol. 2017, 23, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ngwuta, J.O.; Chen, M.; Modjarrad, K.; Joyce, M.G.; Kanekiyo, M.; Kumar, A.; Yassine, H.M.; Moin, S.M.; Killikelly, A.M.; Chuang, G.Y.; et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 2015, 7, 309ra162. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug. Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Trans. Target Ther. 2023, 8, 365. [Google Scholar] [CrossRef]
- Di, J.; Du, Z.; Wu, K.; Jin, S.; Wang, X.; Li, T.; Xu, Y. Biodistribution and Non-linear Gene Expression of mRNA LNPs Affected by Delivery Route and Particle Size. Pharm Res. 2022, 39, 105–114. [Google Scholar] [CrossRef]
- Yang, R.; Deng, Y.; Huang, B.; Huang, L.; Lin, A.; Li, Y.; Wang, W.; Liu, J.; Lu, S.; Zhan, Z.; et al. A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity. Signal Trans. Target Ther. 2021, 6, 213. [Google Scholar] [CrossRef]
- Stephens, L.M.; Varga, S.M. Function and Modulation of Type I Interferons during Respiratory Syncytial Virus Infection. Vaccines 2020, 8, 177. [Google Scholar] [CrossRef]
- Blais, N.; Gagné, M.; Hamuro, Y.; Rheault, P.; Boyer, M.; Steff, A.M.; Baudoux, G.; Dewar, V.; Demers, J.; Ruelle, J.L.; et al. Characterization of Pre-F-GCN4t, a Modified Human Respiratory Syncytial Virus Fusion Protein Stabilized in a Noncleaved Prefusion Conformation. J. Virol. 2017, 91, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Krarup, A.; Truan, D.; Furmanova-Hollenstein, P.; Bogaert, L.; Bouchier, P.; Bisschop, I.J.M.; Widjojoatmodjo, M.N.; Zahn, R.; Schuitemaker, H.; McLellan, J.S.; et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 2015, 6, 8143. [Google Scholar] [CrossRef]
- Persano, S.; Guevara, M.L.; Li, Z.; Mai, J.; Ferrari, M.; Pompa, P.P.; Shen, H. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials 2017, 125, 81–89. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, F.; He, M.; Fang, Y.; Ma, X.; Lu, S.; Li, E.; Xiao, H.; Zhu, H.; Wang, X.; et al. An inoculation site-retained mRNA vaccine induces robust immune responses against SARS-CoV-2 variants. J. Control. Release 2024, 366, 479–493. [Google Scholar] [CrossRef]
- Wan, J.; Yang, J.; Wang, Z.; Shen, R.; Zhang, C.; Wu, Y.; Zhou, M.; Chen, H.; Fu, Z.F.; Sun, H.; et al. A single immunization with core-shell structured lipopolyplex mRNA vaccine against rabies induces potent humoral immunity in mice and dogs. Emerg. Microbes Infect 2023, 12, 2270081. [Google Scholar] [CrossRef]
- Zhan, Y.; Deng, Y.; Huang, B.; Song, Q.; Wang, W.; Yang, Y.; Dai, L.; Wang, W.; Yan, J.; Gao, G.F.; et al. Humoral and cellular immunity against both ZIKV and poxvirus is elicited by a two-dose regimen using DNA and non-replicating vaccinia virus-based vaccine candidates. Vaccine 2019, 37, 2122–2130. [Google Scholar] [CrossRef]
- Zhao, Z.; Deng, Y.; Niu, P.; Song, J.; Wang, W.; Du, Y.; Huang, B.; Wang, W.; Zhang, L.; Zhao, P.; et al. Co-Immunization With CHIKV VLP and DNA Vaccines Induces a Promising Humoral Response in Mice. Front. Immunol. 2021, 12, 655743. [Google Scholar] [CrossRef]
- Huang, L.; Liu, M.Q.; Wan, C.Q.; Cheng, N.N.; Su, Y.B.; Zheng, Y.P.; Peng, X.L.; Yu, J.M.; Fu, Y.H.; He, J.S. The Protective Immunity Induced by Intranasally Inoculated Serotype 63 Chimpanzee Adenovirus Vector Expressing Human Respiratory Syncytial Virus Prefusion Fusion Glycoprotein in Balb/C Mice. Front. Microbiol. 2022, 13, 1041338. [Google Scholar] [CrossRef] [PubMed]
- Levely, M.E.; Bannow, C.A.; Smith, C.W.; Nicholas, J.A. Immunodominant T-Cell Epitope on the F Protein of Respiratory Syncytial Virus Recognized by Human Lymphocytes. J. Virol. 1991, 65, 3789–3796. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Wang, T.; Cheng, X.; Bing, J.; Li, J.; Deng, Y.; Shan, X.; Zhang, X.; Wang, D.; Sun, S.; et al. Immune Responses and Protection Profiles in Mice Induced by Subunit Vaccine Candidates Based on the Extracellular Domain Antigen of Respiratory Syncytial Virus G Protein Combined with Different Adjuvants. Vaccines 2024, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P.; Paredes, A.; Allison, J.E.; Taber, L.H.; Frank, A.L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 1981, 98, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Piedra, P.A.; Jewell, A.M.; Cron, S.G.; Atmar, R.L.; Glezen, W.P. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: Establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 2003, 21, 3479–3482. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P.; Taber, L.H.; Frank, A.L.; Kasel, J.A. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child 1986, 140, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.C.; Callahan, S.M.; Savchenko, K.G.; Stobart, C.C. A Contemporary View of Respiratory Syncytial Virus (RSV) Biology and Strain-Specific Differences. Pathogens 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Walsh, E.E.; Long, C.E.; Schnabel, K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect Dis. 1991, 163, 693–698. [Google Scholar] [CrossRef]
- Cannon, M.J.; Openshaw, P.J.; Askonas, B.A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 1988, 168, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Ostler, T.; Davidson, W.; Ehl, S. Virus clearance and immunopathology by CD8(+) T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur. J. Immunol. 2002, 32, 2117–2123. [Google Scholar] [CrossRef]
- Beňová, K.; Hancková, M.; Koči, K.; Kúdelová, M.; Betáková, T. T cells and their function in the immune response to viruses. Acta Virol. 2020, 64, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Agac, A.; Kolbe, S.M.; Ludlow, M.; Osterhaus, A.; Meineke, R.; Rimmelzwaan, G.F. Host Responses to Respiratory Syncytial Virus Infection. Viruses 2023, 15, 1999. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Simőes, E.A.; Anderson, L.J. Clinical and epidemiologic features of respiratory syncytial virus. Curr. Top. Microbiol. Immunol. 2013, 372, 39–57. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, X.; Han, R.; Cheng, X.; Bing, J.; Qi, Z.; Sun, S.; Wang, T.; Chu, Q.; Deng, Y.; Zhai, D.; et al. Robust and Long-Lasting Immunity and Protection in Mice Induced by Lipopolyplex-Delivered mRNA Vaccines Expressing the Prefusion Protein of Respiratory Syncytial Virus. Vaccines 2025, 13, 93. https://doi.org/10.3390/vaccines13010093
Shan X, Han R, Cheng X, Bing J, Qi Z, Sun S, Wang T, Chu Q, Deng Y, Zhai D, et al. Robust and Long-Lasting Immunity and Protection in Mice Induced by Lipopolyplex-Delivered mRNA Vaccines Expressing the Prefusion Protein of Respiratory Syncytial Virus. Vaccines. 2025; 13(1):93. https://doi.org/10.3390/vaccines13010093
Chicago/Turabian StyleShan, Xuchang, Ruiwen Han, Xueting Cheng, Jialuo Bing, Zhenyong Qi, Shucai Sun, Tangqi Wang, Qiaohong Chu, Yao Deng, Desheng Zhai, and et al. 2025. "Robust and Long-Lasting Immunity and Protection in Mice Induced by Lipopolyplex-Delivered mRNA Vaccines Expressing the Prefusion Protein of Respiratory Syncytial Virus" Vaccines 13, no. 1: 93. https://doi.org/10.3390/vaccines13010093
APA StyleShan, X., Han, R., Cheng, X., Bing, J., Qi, Z., Sun, S., Wang, T., Chu, Q., Deng, Y., Zhai, D., & Tan, W. (2025). Robust and Long-Lasting Immunity and Protection in Mice Induced by Lipopolyplex-Delivered mRNA Vaccines Expressing the Prefusion Protein of Respiratory Syncytial Virus. Vaccines, 13(1), 93. https://doi.org/10.3390/vaccines13010093





