NIAID Workshop Report: Systematic Approaches for ESKAPE Bacteria Antigen Discovery
Abstract
1. Current Landscape—ESKAPE Bacterial Pathogens and Vaccines
2. Challenges and Opportunities
2.1. Large Variability in Clinical Presentation
2.2. Complex Pathogen Biology
2.3. Healthcare-Associated Infections
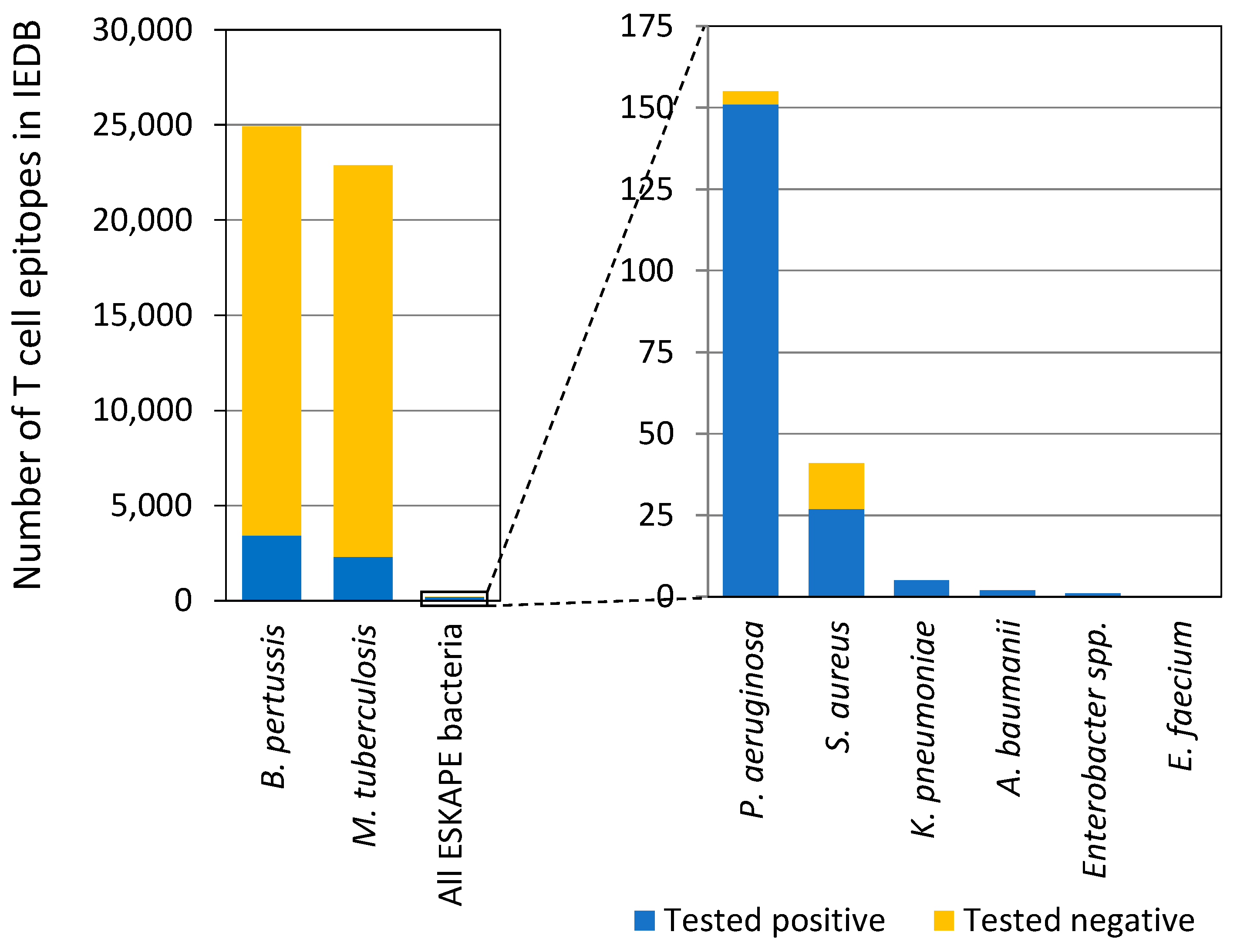
2.4. Limited Information in Human Immune Responses
2.5. Non-Clinically Relevant Vaccine Candidates
2.6. Limited Access to Biospecimens
2.7. Available Cutting-Edge Technologies
3. Emerging Technologies for Antigen Discovery and Vaccine Development
3.1. Host-Bacterium Multi-Dimensional Profiling for Antigen Discovery
3.2. Reverse Vaccinology
3.3. Systems Immunology and Serology
3.4. Mass Spectrometry-Based Immunopeptidomics for Antigen Discovery
3.5. Genome-Wide Peptide-Guided T Cell Epitope Discovery
3.6. Computational Protein Structure Prediction and Modeling
3.7. Computational Tools for T Cell Epitope-Based Vaccines
3.8. Vaccine Platforms and Technologies
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf (accessed on 13 November 2019).
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 27 February 2017).
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Creech, C.B.; Sheldon, E.A.; Seiden, D.J.; Kankam, M.K.; Baber, J.; Zito, E.; Hubler, R.; Eiden, J.; Severs, J.M.; et al. Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): Results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine 2017, 35, 375–384. [Google Scholar] [CrossRef]
- Wong Fok Lung, T.; Chan, L.C.; Prince, A.; Yeaman, M.R.; Archer, N.K.; Aman, M.J.; Proctor, R.A. Staphylococcus aureus adaptive evolution: Recent insights on how immune evasion, immunometabolic subversion and host genetics impact vaccine development. Front. Cell. Infect. Microbiol. 2022, 12, 1060810. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Bacterial Vaccines in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2022.
- Frost, I.; Sati, H.; Garcia-Vello, P.; Hasso-Agopsowicz, M.; Lienhardt, C.; Gigante, V.; Beyer, P. The role of bacterial vaccines in the fight against antimicrobial resistance: An analysis of the preclinical and clinical development pipeline. Lancet Microbe 2023, 4, e113–e125. [Google Scholar] [CrossRef] [PubMed]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report. 2022. Available online: https://stacks.cdc.gov/view/cdc/119025 (accessed on 30 June 2022).
- Rhee, C.; Kadri, S.S.; Dekker, J.P.; Danner, R.L.; Chen, H.C.; Fram, D.; Zhang, F.; Wang, R.; Klompas, M.; CDC Prevention Epicenters Program. Prevalence of Antibiotic-Resistant Pathogens in Culture-Proven Sepsis and Outcomes Associated with Inadequate and Broad-Spectrum Empiric Antibiotic Use. JAMA Netw. Open 2020, 3, e202899. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.E.; Hatfield, K.M.; Wolford, H.; Samore, M.H.; Scott, R.D.; Reddy, S.C.; Olubajo, B.; Paul, P.; Jernigan, J.A.; Baggs, J. National Estimates of Healthcare Costs Associated with Multidrug-Resistant Bacterial Infections Among Hospitalized Patients in the United States. Clin. Infect. Dis. 2021, 72 (Suppl. 1), S17–S26. [Google Scholar] [CrossRef]
- Leuzzi, R.; Bodini, M.; Thomsen, I.P.; Soldaini, E.; Bartolini, E.; Muzzi, A.; Clemente, B.; Galletti, B.; Manetti, A.G.O.; Giovani, C.; et al. Dissecting the Human Response to Staphylococcus aureus Systemic Infections. Front. Immunol. 2021, 12, 749432. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.M.; Horswill, A.R. Staphylococcus aureus bloodstream infections: Pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol. 2020, 53, 51–60. [Google Scholar] [CrossRef]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Fowler, V.G.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153. [Google Scholar] [CrossRef]
- Muthukrishnan, G.; Masters, E.A.; Daiss, J.L.; Schwarz, E.M. Mechanisms of Immune Evasion and Bone Tissue Colonization That Make Staphylococcus aureus the Primary Pathogen in Osteomyelitis. Curr. Osteoporos. Rep. 2019, 17, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Vale de Macedo, G.H.R.; Costa, G.D.E.; Oliveira, E.R.; Damasceno, G.V.; Mendonca, J.S.P.; Silva, L.D.S.; Chagas, V.L.; Bazan, J.M.N.; Alianca, A.; Miranda, R.C.M.; et al. Interplay between ESKAPE Pathogens and Immunity in Skin Infections: An Overview of the Major Determinants of Virulence and Antibiotic Resistance. Pathogens 2021, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, P.; Vasudevan, S.; David, H.; Shaktivel, A.; Shanmugam, K.; Neelakantan, P.; Solomon, A.P. Revisiting ESKAPE Pathogens: Virulence, resistance, and combating strategies focusing on quorum sensing. Front. Cell. Infect. Microbiol. 2023, 13, 1159798. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian-Ding, I. Challenges for Clinical Development of Vaccines for Prevention of Hospital-Acquired Bacterial Infections. Front. Immunol. 2020, 11, 1755. [Google Scholar] [CrossRef]
- Proctor, R. Respiration and Small Colony Variants of Staphylococcus aureus. Microbiol. Spectr. 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cain, A.K.; Barquist, L.; Goodman, A.L.; Paulsen, I.T.; Parkhill, J.; van Opijnen, T. A decade of advances in transposon-insertion sequencing. Nat. Rev. Genet. 2020, 21, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Leshchiner, D.; Rosconi, F.; Sundaresh, B.; Rudmann, E.; Ramirez, L.M.N.; Nishimoto, A.T.; Wood, S.J.; Jana, B.; Bujan, N.; Li, K.; et al. A genome-wide atlas of antibiotic susceptibility targets and pathways to tolerance. Nat. Commun. 2022, 13, 3165. [Google Scholar] [CrossRef] [PubMed]
- Rosconi, F.; Rudmann, E.; Li, J.; Surujon, D.; Anthony, J.; Frank, M.; Jones, D.S.; Rock, C.; Rosch, J.W.; Johnston, C.D.; et al. A bacterial pan-genome makes gene essentiality strain-dependent and evolvable. Nat. Microbiol. 2022, 7, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Rosini, R.; Nicchi, S.; Pizza, M.; Rappuoli, R. Vaccines Against Antimicrobial Resistance. Front. Immunol. 2020, 11, 1048. [Google Scholar] [CrossRef]
- Donnelly, J.; Medini, D.; Boccadifuoco, G.; Biolchi, A.; Ward, J.; Frasch, C.; Moxon, E.R.; Stella, M.; Comanducci, M.; Bambini, S.; et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 19490–19495. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.M.; Adu-Bobie, J.; Comanducci, M.; Arico, B.; Savino, S.; Santini, L.; Brunelli, B.; Bambini, S.; Biolchi, A.; Capecchi, B.; et al. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 2006, 103, 10834–10839. [Google Scholar] [CrossRef]
- Masignani, V.; Pizza, M.; Moxon, E.R. The Development of a Vaccine Against Meningococcus B Using Reverse Vaccinology. Front. Immunol. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Moriel, D.G.; Bertoldi, I.; Spagnuolo, A.; Marchi, S.; Rosini, R.; Nesta, B.; Pastorello, I.; Corea, V.A.; Torricelli, G.; Cartocci, E.; et al. Identification of protective and broadly conserved vaccine antigens from the genome of extraintestinal pathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 2010, 107, 9072–9077. [Google Scholar] [CrossRef] [PubMed]
- Pizza, M.; Scarlato, V.; Masignani, V.; Giuliani, M.M.; Arico, B.; Comanducci, M.; Jennings, G.T.; Baldi, L.; Bartolini, E.; Capecchi, B.; et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 2000, 287, 1816–1820. [Google Scholar] [CrossRef]
- Rojas-Lopez, M.; Martinelli, M.; Brandi, V.; Jubelin, G.; Polticelli, F.; Soriani, M.; Pizza, M.; Desvaux, M.; Rosini, R. Identification of lipid A deacylase as a novel, highly conserved and protective antigen against enterohemorrhagic Escherichia coli. Sci. Rep. 2019, 9, 17014. [Google Scholar] [CrossRef]
- Bassani-Sternberg, M.; Pletscher-Frankild, S.; Jensen, L.J.; Mann, M. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol. Cell. Proteom. 2015, 14, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.L.; Verbeke, R.; Asselman, C.; Aernout, I.; Gul, A.; Eggermont, D.; Boucher, K.; Thery, F.; Maia, T.M.; Demol, H.; et al. Immunopeptidomics-based design of mRNA vaccine formulations against Listeria monocytogenes. Nat. Commun. 2022, 13, 6075. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.; Cooke, M.F.; Huffman, A.; Xiang, Z.; Wong, M.U.; Wang, H.; Seetharaman, M.; Valdez, N.; He, Y. Vaxign2: The second generation of the first Web-based vaccine design program using reverse vaccinology and machine learning. Nucleic Acids Res. 2021, 49, W671–W678. [Google Scholar] [CrossRef] [PubMed]
- da Silva Antunes, R.; Garrigan, E.; Quiambao, L.G.; Dhanda, S.K.; Marrama, D.; Westernberg, L.; Wang, E.; Sutherland, A.; Armstrong, S.K.; Brickman, T.J.; et al. Genome-wide characterization of T cell responses to Bordetella pertussis reveals broad reactivity and similar polarization irrespective of childhood vaccination profiles. bioRxiv 2023. [CrossRef]
- Callaway, E. What’s next for AlphaFold and the AI protein-folding revolution. Nature 2022, 604, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.C.; Afonine, P.V.; Liebschner, D.; Croll, T.I.; McCoy, A.J.; Oeffner, R.D.; Williams, C.J.; Poon, B.K.; Richardson, J.S.; Read, R.J.; et al. Accelerating crystal structure determination with iterative AlphaFold prediction. Acta Crystallogr. Sect. D Struct. Biol. 2023, 79 Pt 3, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Anishchenko, I.; Pellock, S.J.; Chidyausiku, T.M.; Ramelot, T.A.; Ovchinnikov, S.; Hao, J.; Bafna, K.; Norn, C.; Kang, A.; Bera, A.K.; et al. De novo protein design by deep network hallucination. Nature 2021, 600, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Dauparas, J.; Anishchenko, I.; Bennett, N.; Bai, H.; Ragotte, R.J.; Milles, L.F.; Wicky, B.I.M.; Courbet, A.; de Haas, R.J.; Bethel, N.; et al. Robust deep learning-based protein sequence design using ProteinMPNN. Science 2022, 378, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Schoeder, C.T.; Gilchuk, P.; Sangha, A.K.; Ledwitch, K.V.; Malherbe, D.C.; Zhang, X.; Binshtein, E.; Williamson, L.E.; Martina, C.E.; Dong, J.; et al. Epitope-focused immunogen design based on the ebolavirus glycoprotein HR2-MPER region. PLoS Pathog. 2022, 18, e1010518. [Google Scholar] [CrossRef]
- Schoeder, C.T.; Schmitz, S.; Adolf-Bryfogle, J.; Sevy, A.M.; Finn, J.A.; Sauer, M.F.; Bozhanova, N.G.; Mueller, B.K.; Sangha, A.K.; Bonet, J.; et al. Modeling Immunity with Rosetta: Methods for Antibody and Antigen Design. Biochemistry 2021, 60, 825–846. [Google Scholar] [CrossRef]
- Correia, B.E.; Bates, J.T.; Loomis, R.J.; Baneyx, G.; Carrico, C.; Jardine, J.G.; Rupert, P.; Correnti, C.; Kalyuzhniy, O.; Vittal, V.; et al. Proof of principle for epitope-focused vaccine design. Nature 2014, 507, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; Schmitz, E.A.; Crowe, J.E., Jr.; Meiler, J. The human antibody sequence space and structural design of the V, J regions, and CDRH3 with Rosetta. mAbs 2022, 14, 2068212. [Google Scholar] [CrossRef] [PubMed]
- Sevy, A.M.; Panda, S.; Crowe, J.E., Jr.; Meiler, J.; Vorobeychik, Y. Integrating linear optimization with structural modeling to increase HIV neutralization breadth. PLoS Comput. Biol. 2018, 14, e1005999. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Finn, J.A.; Briney, B.; Sapparapu, G.; Singh, V.; King, H.; LaBranche, C.C.; Montefiori, D.C.; Meiler, J.; Crowe, J.E., Jr. Long antibody HCDR3s from HIV-naive donors presented on a PG9 neutralizing antibody background mediate HIV neutralization. Proc. Natl. Acad. Sci. USA 2016, 113, 4446–4451. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Haltaufderhyde, K.; Srikiatkhachorn, A.; Green, S.; Macareo, L.; Park, S.; Kalayanarooj, S.; Rothman, A.L.; Mathew, A. Activation of Peripheral T Follicular Helper Cells During Acute Dengue Virus Infection. J. Infect. Dis. 2018, 218, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Vardhana, S.; Baldo, L.; Morice, W.G., 2nd; Wherry, E.J. Understanding T cell responses to COVID-19 is essential for informing public health strategies. Sci. Immunol. 2022, 7, eabo1303. [Google Scholar] [CrossRef]
- De Groot, A.S.; Moise, L.; Terry, F.; Gutierrez, A.H.; Hindocha, P.; Richard, G.; Hoft, D.F.; Ross, T.M.; Noe, A.R.; Takahashi, Y.; et al. Better Epitope Discovery, Precision Immune Engineering, and Accelerated Vaccine Design Using Immunoinformatics Tools. Front. Immunol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Moise, L.; Gutierrez, A.; Kibria, F.; Martin, R.; Tassone, R.; Liu, R.; Terry, F.; Martin, B.; De Groot, A.S. iVAX: An integrated toolkit for the selection and optimization of antigens and the design of epitope-driven vaccines. Hum. Vaccin. Immunother. 2015, 11, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Rappuoli, R. From empiricism to rational design: A personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014, 14, 505–514. [Google Scholar] [CrossRef]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V.; et al. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. Publisher Correction: A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 129. [Google Scholar] [CrossRef]
- Kapulu, M.C.; Nakakana, U.; Scire, A.S.; Sarakinou, E.; Conti, V.; Rossi, O.; Acquaviva, A.; Necchi, F.; Obiero, C.W.; Martin, L.B.; et al. Complement-mediated serum bactericidal activity of antibodies elicited by the Shigella sonnei GMMA vaccine in adults from a shigellosis-endemic country: Exploratory analysis of a Phase 2a randomized study. Front. Immunol. 2022, 13, 971866. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Nakakana, U.N.; Berlanda Scorza, F. Towards a Four-Component GMMA-Based Vaccine against Shigella. Vaccines 2022, 10, 328. [Google Scholar] [CrossRef]
- Boerth, E.M.; Gong, J.; Roffler, B.; Thompson, C.M.; Song, B.; Malley, S.F.; Hirsch, A.; MacLennan, C.A.; Zhang, F.; Malley, R.; et al. Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A. Vaccines 2023, 11, 1671. [Google Scholar] [CrossRef]
- Chichili, G.R.; Smulders, R.; Santos, V.; Cywin, B.; Kovanda, L.; Van Sant, C.; Malinoski, F.; Sebastian, S.; Siber, G.; Malley, R. Phase 1/2 study of a novel 24-valent pneumococcal vaccine in healthy adults aged 18 to 64 years and in older adults aged 65 to 85 years. Vaccine 2022, 40, 4190–4198. [Google Scholar] [CrossRef]
- Martin, P.; Alaimo, C. The Ongoing Journey of a Shigella Bioconjugate Vaccine. Vaccines 2022, 10, 212. [Google Scholar] [CrossRef] [PubMed]
| Vaccine Candidates | Enterococcus faecium “E” | Staphylococcus aureus “S” | Klebsiella pneumoniae “K” | Acinetobacter baumannii “A” | Pseudomonas aeruginosa “P” | Enterobacter ssp. “E” |
|---|---|---|---|---|---|---|
| Pre-clinical | 0 | 14 | 5 | 5 | 4 | 0 |
| Clinical | 0 | 2 | 1 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sastalla, I.; Kwon, K.; Huntley, C.; Taylor, K.; Brown, L.; Samuel, T.; Zou, L. NIAID Workshop Report: Systematic Approaches for ESKAPE Bacteria Antigen Discovery. Vaccines 2025, 13, 87. https://doi.org/10.3390/vaccines13010087
Sastalla I, Kwon K, Huntley C, Taylor K, Brown L, Samuel T, Zou L. NIAID Workshop Report: Systematic Approaches for ESKAPE Bacteria Antigen Discovery. Vaccines. 2025; 13(1):87. https://doi.org/10.3390/vaccines13010087
Chicago/Turabian StyleSastalla, Inka, Keehwan Kwon, Clayton Huntley, Kimberly Taylor, Liliana Brown, Tamika Samuel, and Lanling Zou. 2025. "NIAID Workshop Report: Systematic Approaches for ESKAPE Bacteria Antigen Discovery" Vaccines 13, no. 1: 87. https://doi.org/10.3390/vaccines13010087
APA StyleSastalla, I., Kwon, K., Huntley, C., Taylor, K., Brown, L., Samuel, T., & Zou, L. (2025). NIAID Workshop Report: Systematic Approaches for ESKAPE Bacteria Antigen Discovery. Vaccines, 13(1), 87. https://doi.org/10.3390/vaccines13010087







