Abstract
On 14–15 November 2023, the National Institute of Allergy and Infectious Diseases (NIAID) organized a workshop entitled “Systematic Approaches for ESKAPE Bacteria Antigen Discovery”. The goal of the workshop was to engage scientists from diverse relevant backgrounds to explore novel technologies that can be harnessed to identify and address current roadblocks impeding advances in antigen and vaccine discoveries for the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species). The workshop consisted of four sessions that addressed ESKAPE infections, antigen discovery and vaccine efforts, and new technologies including systems immunology and vaccinology approaches. Each session was followed by a panel discussion. In total, there were over 260 in-person and virtual attendees, with high levels of engagement. This report provides a summary of the event and highlights challenges and opportunities in the field of ESKAPE vaccine discovery.
1. Current Landscape—ESKAPE Bacterial Pathogens and Vaccines
The 2020–2025 U.S. National Action Plan to Combat Antibiotic Resistant Bacteria established a goal, among others, to accelerate research to develop vaccines that prevent infections with antimicrobial resistant (AMR) bacteria. In response, the U.S. National Institute of Allergy and Infectious Diseases (NIAID) hosted a workshop on 14–15 November 2023 to engage scientists from diverse relevant backgrounds to explore novel technologies that can be harnessed to identify and address current roadblocks impeding advances in antigen and vaccine discoveries for the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species) (Supplementary Material: Workshop Details S1).
The workshop began with presentations from Drs. Mayora Walters (CDC) and Timothy Cooke (Omniose), who presented information on the public health burden of ESKAPE pathogens, and the status of vaccines in development (Table 1). AMR bacteria pose a worldwide healthcare challenge, and the U.S. Centers for Disease Control and Prevention (CDC) have specifically identified ESKAPE pathogens as urgent threats based on their ability to cause high morbidity and mortality [1,2]. Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa are responsible for approximately 55% of global mortality caused by bacterial infections, with S. aureus reported as the leading bacterial cause of death [3]. Most deaths occur in young infants (<1 yr), older adults (>60 yrs), and in hospital settings. In their presentations, Drs. Walters and Cooke discussed how despite numerous efforts over several decades, attempts to combat ESKAPE bacterial infections with vaccines have faced multiple challenges. For example, while each ESKAPE pathogen has its own challenges and limitations, a common theme among all vaccine development efforts is the limited effort in the area of antigen discovery. Overall, ESKAPE vaccine efforts have focused on a limited number of bacterial proteins such as superantigens, adhesins, and iron-scavenging proteins [4,5,6], leading to limited strain coverage or relatively rapid immune escape [7]. Once antigens were down-selected, several development efforts were thwarted due to low vaccine efficacy and safety issues that caused early termination of clinical trials [8].

Table 1.
Number of ESKAPE vaccine candidates under global (pre-) clinical development in 2021 [8,9]. Two Staphylococcus aureus vaccine candidates are rTSST-1 variant vaccine (Phase 2) and GSK3878858A (Phase 1/2), and a Klebsiella pneumoniae vaccine candidate is KlebV4 (Phase 1/2).
Dr. Walters described how healthcare-associated infections (HAIs) in the USA are monitored by the National Healthcare Safety Network (NHSN), managed by the CDC. Reported HAIs include central line-associated bloodstream infections (CLABSIs), catheter-associated urinary tract infections (CAUTIs), surgical site infections (SSIs), and selected ventilator-associated events (VAEs). Data collected from this surveillance network are used to track emerging AMR pathogens in the US. For example, thirty eight percent of HAIs reported in the USA were associated with ESKAPE pathogens [10]. Importantly, an ESKAPE pathogen was isolated in more than 60% of cases of ventilator-associated pneumonia (VAP). S. aureus was responsible for the majority of VAP, CLABSI, and SSIs, while Klebsiella species caused the majority of catheter-associated UTIs. Between 2017 and 2019, decreasing numbers of infections were noted with MDR- P. aeruginosa and carbapenem-resistant Enterobacterales (CRE), while carbapenem-resistant Acinetobacter (CRA), vancomycin-resistant Enterococci (VRE) and methicillin-resistant S. aureus (MRSA) showed a stabilizing trend in the number of HAIs. Unfortunately, the decreasing rate of AMR ESKAPE infections was reversed during the COVID-19 pandemic [11]. In addition to the toll on human morbidity and mortality, HAIs were also associated with increased healthcare costs, with MRSA and CRA infections showing the highest attributable costs. As with hospital-acquired infections, ESKAPE pathogens also featured prominently in community-acquired infections, with E. coli and S. aureus being the leading causes that resulted in sepsis [12]. Altogether, these infections added USD 4.6 billion in US healthcare costs annually [13].
2. Challenges and Opportunities
The remainder of the workshop was dedicated to identifying challenges impeding antigen discovery and vaccine development and determining growing opportunities to address these challenges.
2.1. Large Variability in Clinical Presentation
The variability in clinical presentations of ESKAPE infections poses challenges in selecting a vaccine-target population critical for developing realistic vaccine approaches. ESKAPE organisms can infect multiple body sites resulting in different clinical symptoms (e.g., S. aureus causes soft-tissue infections, pneumonia, or blood-stream infections); can be asymptomatic, mild, severe, or fatal; or may be persistent or recurring [14,15,16,17]. In most cases, it is not known whether vaccines targeting antigens expressed during one clinical presentation can broadly protect against other presentations. For example, vaccines that are based on antigens expressed during bloodstream or tissue infections may not be effective against osteomyelitis caused by the same pathogen [18,19]. In practice, this may require multiple antigens to be identified and included in vaccine formulations to cover the variety of anatomical infection sites, or targeted vaccines may be required that are limited to distinct clinical presentations.
2.2. Complex Pathogen Biology
ESKAPE bacteria express a myriad of colonization, virulence, and immune evasion factors at different times and at different body sites during infection. In most cases, ESKAPE bacteria carry multiple genes with redundant functions that could come into play when immune pressure is experienced at one gene [4]. Additionally, horizontal gene transfer and often a high mutational rate of immune-exposed proteins allow these bacteria to acquire new or modify existing virulence factors, thus making vaccine approaches that focus on one or few antigens less likely to succeed [20].
2.3. Healthcare-Associated Infections
Most life-threatening infections occur during patient hospitalization, and to reduce the risk of HAIs, prophylactic vaccine interventions are desirable [8,10]. However, effective immunizations need to be administered weeks before the patient’s admission, thus limiting feasibility beyond elective procedures [21]. Short-term treatments such as prophylactic monoclonal antibody regimens might offer a viable alternative.
2.4. Limited Information in Human Immune Responses
Past vaccine research predominantly focused on antibodies and B cell responses that target a limited number of bacterial antigens. However, T cell responses to ESKAPE bacteria have been understudied. For example, the Immune-Epitope Database (IEDB) lacks abundant T cell epitope data for all ESKAPE bacteria (Figure 1); in fact, ESKAPE bacteria make up less than 0.3% of bacterial T cell epitope data in IEDB, and past epitope mapping studies have focused on very few selected protein targets, constraining T cell-focused vaccine strategies. Researchers are also exploring the possibility that early-life colonization by ESKAPE bacteria may create a humoral imprint, affecting human immune status and potentially rendering developed vaccines ineffective or even harmful.
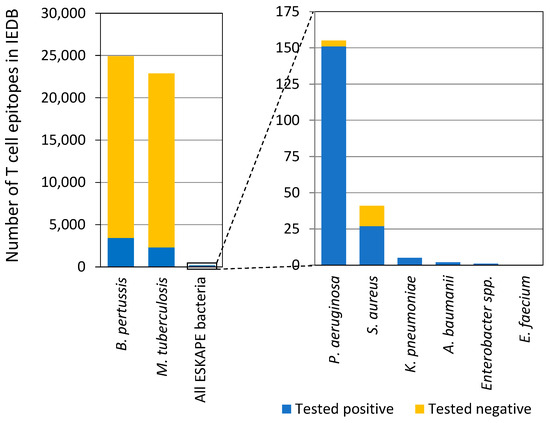
Figure 1.
T cell epitope data for ESKAPE bacteria in IEDB. Tested positive/negative indicates T cell assay outcome. This figure is adapted from Dr. Bjoern Peters’ presentation in the workshop.
2.5. Non-Clinically Relevant Vaccine Candidates
Many ESKAPE vaccine candidates show promise in laboratory and animal infection models, but their efficacies often fail to translate to human infection [22]. Better and alternative infection models, with a clearer understanding of correlates of protection, are needed to assist in vaccine development.
2.6. Limited Access to Biospecimens
There are existing panels of ESKAPE bacterial strains made available by NIAID, CDC, and FDA. However, there are limited publicly available well-maintained biobanks for human sera and tissue samples, as well as bacterial isolates from longitudinal ESKAPE clinical cases that include detailed metadata. Similarly, there are no agreed-upon criteria to regularly update panels to better represent circulating ESKAPE bacterial strains, and therefore even the existing strain panels might be outdated by the time they are used for vaccine testing. Such reagents and resources would enable the identification of immune correlates associated with infection and greatly benefit the research community.
2.7. Available Cutting-Edge Technologies
New and emerging technologies are mostly applied to viral research and are not always applied widely to advance antigen discovery and resolve obstacles that hinder ESKAPE vaccine development.
3. Emerging Technologies for Antigen Discovery and Vaccine Development
Emerging technologies can be harnessed to identify ESKAPE antigens. Similarly, new vaccine platforms and approaches can help overcome challenges and populate the ESKAPE bacteria vaccine pipeline. Some promising technologies that are not yet systematically employed for ESKAPE bacteria were discussed.
3.1. Host-Bacterium Multi-Dimensional Profiling for Antigen Discovery
Multi-dimensional host-bacterium profiles and bacterial gene interaction screens can support antigen discovery. This technology uses genome-wide bacterial gene deletion screens combined with comparative genomics and transcriptomics to investigate gene interactions and their impact on bacterial behavior and phenotypes under diverse conditions, such as exposure to drugs, different carbon sources, ions, host cells, and in vivo environments. Such approaches can reveal essential bacterial pathways and mechanisms, aid our understanding of bacterial biology contributing to antibiotic resistance, pathogenicity, and environmental adaptation, and may lead to the identification of new vaccine targets [23,24,25].
3.2. Reverse Vaccinology
Reverse vaccinology was first described in the early 2000s [26]. Instead of the traditional approach of isolating and growing pathogens in the lab, reverse vaccinology starts with the pathogen’s genetic sequence to predict which proteins might be suitable vaccine targets, thus allowing researchers to prioritize antigen characterization and eliminating lengthy growth and isolation methods for sub-optimal candidates. This approach has been successfully used for the discovery and development of GSK’s Neisseria meningitidis serogroup B (MenB) and can further be explored for ESKAPE pathogens [27,28,29,30,31,32].
3.3. Systems Immunology and Serology
Technologies such as IVIAT (in vivo induced antigen technology) identify bacterial immunogenic antigens expressed during human infection. Advanced “systems” approaches can combine immune genomics, transcriptomics, and metabolomics to comprehensively analyze both innate and adaptive human host responses during infections. Such systems immunology approaches can help identify correlates of infection and protection and ultimately new antigens for ESKAPE bacteria. Large-scale datasets from human and pathogen during infections allow the systematic and wide-ranging analysis of antibody responses to infection and/or vaccination. Systems serology combines traditional serology techniques with high-throughput technology to comprehensively study the characteristics and dynamics of antibodies. This approach, described at the workshop in the context of human Shigella challenge studies, can provide insights into immune system interactions, disease progression, and vaccine efficacy.
3.4. Mass Spectrometry-Based Immunopeptidomics for Antigen Discovery
Immunopeptidomics is a powerful approach to discover novel bacterial antigens. This technology aims to identify and characterize pathogen peptides that are presented by major histocompatibility complex (MHC) molecules. The method employs mass spectrometry and bioinformatics techniques to analyze the repertoire of peptides presented by MHC molecules, thus providing insights into the antigen presentation and immune surveillance mechanisms [33,34]. Additional computational tools for the design of epitope-driven vaccines such as the Vaxign 2 vaccine design platform [35], a machine learning-based vaccine candidate prediction and analysis system, presented at the workshop can evaluate the identified vaccine candidates for Listeria monocytogenes [34].
3.5. Genome-Wide Peptide-Guided T Cell Epitope Discovery
Peptide-guided T cell epitope discovery first predicts surface epitopes and then systematically screens peptide pools and peptides for their ability to bind to MHC molecules and to activate T cells [36]. This approach has been used to systematically identify T cell epitopes for viruses and—more limited—for bacteria with a focus on genome-wide M. tuberculosis and B. pertussis epitopes.
3.6. Computational Protein Structure Prediction and Modeling
Three-dimensional protein structures are critical to understand biological systems and functions of many uncharacterized ESKAPE proteins, and they can aid antigen discovery and vaccine development. The structural biology field has seen an exponential increase in resources and tools for computational 3D prediction and modeling [37]. In the artificial intelligence (AI) system, AlphaFold 2 revolutionized protein modeling with an accuracy of 94–98% in its predictions. Currently, more than 2 million predicted protein structure models are accessible to the public through the AlphaFold Database. The recent AlphaFold 3 has demonstrated much higher accuracy in predicting complex structures, including proteins with ligands, nucleic acids, antibodies, and other proteins [38,39,40]. Multiple high accuracy computational tools have been developed and evolved for structure-based antigen discovery, designing antigens and antibodies, and for modeling protein complexes with small and macromolecules; tools include “deep network hallucination”, AlphaFold3, RoseTTAFold and Protein MPNN [38,41,42,43,44]. Applications for these tools provide resources for antigen discovery, increasing the breadth of antibodies, and the design for epitope-focused and self-assembly nanoparticle vaccines [43,45,46,47,48].
3.7. Computational Tools for T Cell Epitope-Based Vaccines
In vaccine design, the T cell response is one of the critical factors since T cell activation facilitates B cell activation [49,50,51]. A series of immunoinformatics tools, such as iVAX, an integrated toolkit, is available for computational vaccine design from genome sequencing data by identifying antigens and predicting T cell epitopes, epitope density, and immunogenic potential [52,53]). The concept and workflow have been validated in retrospective and prospective studies with influenza virus, Mycobacterium tuberculosis, Burkholderia mallei and pseudomallei, and Coxiella burnetii [52].
3.8. Vaccine Platforms and Technologies
Current vaccine platforms include recombinant DNA and proteins, outer membrane vesicles (OMVs), glycoconjugates, viral vectors, and nucleic acid (DNA/RNA) technologies [54,55,56]. Several promising new platforms have been discussed that could prove suitable for ESKAPE vaccines. For example, the versatile GSK GMMA platform relies on outer membrane vehicles (OMVs) produced by engineered E. coli [55,57,58]. OMVs can include multiple antigens in their natural conformation, provide self-adjuvating activity, and have a high yielding manufacturing process at a relatively low cost. In contrast, glycoconjugation technologies rely on bacterial polysaccharides conjugated to a carrier protein, and more easily manufactured alternatives, such as bioconjugated and multiple antigen-presenting systems (MAPSs), were discussed, some of which have already been tested and developed for select ESKAPE pathogens [54,59,60,61].
4. Concluding Remarks
During the workshop, valuable opportunities were identified to advance ESKAPE antigen discovery, develop medical countermeasures, and close scientific and technological gaps. First, research into longitudinal cohort studies and associated biobanks for select human bacterial infections is essential. These resources could be made available to the scientific community to enhance our understanding of pathogenesis, human host immune responses, treatment, and clinical outcomes. Second, utilizing samples and information from longitudinal cohorts could significantly expand human system immunology and system vaccinology studies aimed at identifying molecular signatures, immune markers, and correlates associated with clinical outcomes. Third, research to identify both T cell and B cell antigens and epitopes for ESKAPE bacteria is critical for the development of effective vaccines, which can elicit a robust and long-lasting immune response and help to overcome bacterial evasion strategies. Lastly, the integration of emerging technologies and approaches, such as systems biology and multi-scale methods incorporating computational biology, artificial intelligence, and predictive techniques, can be a pivotal moment to advance the discovery of ESKAPE bacterial epitopes and antigens. These advancements and newly identified antigens and epitopes could lay the foundation for the future development of vaccines, therapeutics, and diagnostics, leveraging newly identified antigens and epitopes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vaccines13010087/s1: Workshop Details S1: The list of speakers, panelists, and moderators.
Author Contributions
Conceptualization and workshop organization: C.H., I.S., K.K., K.T., L.B., T.S. and L.Z.; Original writing: C.H., I.S., K.K., K.T., L.Z. and T.S.; Revisions: I.S., K.K. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
The workshop was sponsored by NIAID.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This report summarizes the workshop, unlike a research or review article, no data was generated, and no software source code was developed. Detailed information about the workshop is provided in the Supplementary Materials.
Acknowledgments
We thank all the workshop moderators, speakers and participants for their expertise, insights, contributions, and valuable discussions regarding the identification of key approaches and scientific and technological gaps to advance ESKAPE antigen discovery. The workshop moderators, speakers and panelists were Alter, Galit (Moderna, MA, USA), Aman, M. Javad (AbVacc, MD, USA), Bubeck-Wardenburg, Juliane (Washington University, MO, USA), Cooke, Timothy (Omniose, MO, USA), Creech, C. Buddy (Vanderbilt University, TN, USA), Fowler, Vance (Duke University, NC, USA), Haltaufderhyde, Kirk (EpiVax Inc, RI), Impens, Francis (Ghent University, Belgium), Liu, George Y. (University of California, San Diego, CA, USA), Malley, Richard (Harvard University, MA, USA), McClean, Siobhán (University College Dublin, UK), Meiler, Jens (University Leipzig Medical School, Germany & Vanderbilt University, TN, USA), Montgomery, Christopher (The Ohio State University, OH), Peters, Bjoern (La Jolla Institute for Immunology, CA, USA), Pizza, Mariagrazia (Imperial College London, UK), Pulendran, Bali (Stanford University, CA, USA), Roggensack, Sara (GlaxoSmithKline, MA, USA), Rouphael, Nadine (Emory University, GA, USA), Savchenko, Alexei (University of Calgary, Alberto, Canada), Sellman, Bret (AstraZeneca, MD, USA), Silverman, Gregg (New York University, NY, USA), Talan, David (University of California, Los Angeles, CA, USA), van Opijnen, Tim (The Broad Institute, MA, USA) and Walters, Maroya (CDC, GA, USA). We also thank Joseph Breen and Timothy Gondre-Lewis for their initial discussion on searching for workshop speakers.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf (accessed on 13 November 2019).
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 27 February 2017).
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Creech, C.B.; Sheldon, E.A.; Seiden, D.J.; Kankam, M.K.; Baber, J.; Zito, E.; Hubler, R.; Eiden, J.; Severs, J.M.; et al. Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): Results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine 2017, 35, 375–384. [Google Scholar] [CrossRef]
- Wong Fok Lung, T.; Chan, L.C.; Prince, A.; Yeaman, M.R.; Archer, N.K.; Aman, M.J.; Proctor, R.A. Staphylococcus aureus adaptive evolution: Recent insights on how immune evasion, immunometabolic subversion and host genetics impact vaccine development. Front. Cell. Infect. Microbiol. 2022, 12, 1060810. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Bacterial Vaccines in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2022.
- Frost, I.; Sati, H.; Garcia-Vello, P.; Hasso-Agopsowicz, M.; Lienhardt, C.; Gigante, V.; Beyer, P. The role of bacterial vaccines in the fight against antimicrobial resistance: An analysis of the preclinical and clinical development pipeline. Lancet Microbe 2023, 4, e113–e125. [Google Scholar] [CrossRef] [PubMed]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report. 2022. Available online: https://stacks.cdc.gov/view/cdc/119025 (accessed on 30 June 2022).
- Rhee, C.; Kadri, S.S.; Dekker, J.P.; Danner, R.L.; Chen, H.C.; Fram, D.; Zhang, F.; Wang, R.; Klompas, M.; CDC Prevention Epicenters Program. Prevalence of Antibiotic-Resistant Pathogens in Culture-Proven Sepsis and Outcomes Associated with Inadequate and Broad-Spectrum Empiric Antibiotic Use. JAMA Netw. Open 2020, 3, e202899. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.E.; Hatfield, K.M.; Wolford, H.; Samore, M.H.; Scott, R.D.; Reddy, S.C.; Olubajo, B.; Paul, P.; Jernigan, J.A.; Baggs, J. National Estimates of Healthcare Costs Associated with Multidrug-Resistant Bacterial Infections Among Hospitalized Patients in the United States. Clin. Infect. Dis. 2021, 72 (Suppl. 1), S17–S26. [Google Scholar] [CrossRef]
- Leuzzi, R.; Bodini, M.; Thomsen, I.P.; Soldaini, E.; Bartolini, E.; Muzzi, A.; Clemente, B.; Galletti, B.; Manetti, A.G.O.; Giovani, C.; et al. Dissecting the Human Response to Staphylococcus aureus Systemic Infections. Front. Immunol. 2021, 12, 749432. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.M.; Horswill, A.R. Staphylococcus aureus bloodstream infections: Pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol. 2020, 53, 51–60. [Google Scholar] [CrossRef]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Fowler, V.G.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153. [Google Scholar] [CrossRef]
- Muthukrishnan, G.; Masters, E.A.; Daiss, J.L.; Schwarz, E.M. Mechanisms of Immune Evasion and Bone Tissue Colonization That Make Staphylococcus aureus the Primary Pathogen in Osteomyelitis. Curr. Osteoporos. Rep. 2019, 17, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Vale de Macedo, G.H.R.; Costa, G.D.E.; Oliveira, E.R.; Damasceno, G.V.; Mendonca, J.S.P.; Silva, L.D.S.; Chagas, V.L.; Bazan, J.M.N.; Alianca, A.; Miranda, R.C.M.; et al. Interplay between ESKAPE Pathogens and Immunity in Skin Infections: An Overview of the Major Determinants of Virulence and Antibiotic Resistance. Pathogens 2021, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, P.; Vasudevan, S.; David, H.; Shaktivel, A.; Shanmugam, K.; Neelakantan, P.; Solomon, A.P. Revisiting ESKAPE Pathogens: Virulence, resistance, and combating strategies focusing on quorum sensing. Front. Cell. Infect. Microbiol. 2023, 13, 1159798. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian-Ding, I. Challenges for Clinical Development of Vaccines for Prevention of Hospital-Acquired Bacterial Infections. Front. Immunol. 2020, 11, 1755. [Google Scholar] [CrossRef]
- Proctor, R. Respiration and Small Colony Variants of Staphylococcus aureus. Microbiol. Spectr. 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cain, A.K.; Barquist, L.; Goodman, A.L.; Paulsen, I.T.; Parkhill, J.; van Opijnen, T. A decade of advances in transposon-insertion sequencing. Nat. Rev. Genet. 2020, 21, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Leshchiner, D.; Rosconi, F.; Sundaresh, B.; Rudmann, E.; Ramirez, L.M.N.; Nishimoto, A.T.; Wood, S.J.; Jana, B.; Bujan, N.; Li, K.; et al. A genome-wide atlas of antibiotic susceptibility targets and pathways to tolerance. Nat. Commun. 2022, 13, 3165. [Google Scholar] [CrossRef] [PubMed]
- Rosconi, F.; Rudmann, E.; Li, J.; Surujon, D.; Anthony, J.; Frank, M.; Jones, D.S.; Rock, C.; Rosch, J.W.; Johnston, C.D.; et al. A bacterial pan-genome makes gene essentiality strain-dependent and evolvable. Nat. Microbiol. 2022, 7, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Rosini, R.; Nicchi, S.; Pizza, M.; Rappuoli, R. Vaccines Against Antimicrobial Resistance. Front. Immunol. 2020, 11, 1048. [Google Scholar] [CrossRef]
- Donnelly, J.; Medini, D.; Boccadifuoco, G.; Biolchi, A.; Ward, J.; Frasch, C.; Moxon, E.R.; Stella, M.; Comanducci, M.; Bambini, S.; et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 19490–19495. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.M.; Adu-Bobie, J.; Comanducci, M.; Arico, B.; Savino, S.; Santini, L.; Brunelli, B.; Bambini, S.; Biolchi, A.; Capecchi, B.; et al. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 2006, 103, 10834–10839. [Google Scholar] [CrossRef]
- Masignani, V.; Pizza, M.; Moxon, E.R. The Development of a Vaccine Against Meningococcus B Using Reverse Vaccinology. Front. Immunol. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Moriel, D.G.; Bertoldi, I.; Spagnuolo, A.; Marchi, S.; Rosini, R.; Nesta, B.; Pastorello, I.; Corea, V.A.; Torricelli, G.; Cartocci, E.; et al. Identification of protective and broadly conserved vaccine antigens from the genome of extraintestinal pathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 2010, 107, 9072–9077. [Google Scholar] [CrossRef] [PubMed]
- Pizza, M.; Scarlato, V.; Masignani, V.; Giuliani, M.M.; Arico, B.; Comanducci, M.; Jennings, G.T.; Baldi, L.; Bartolini, E.; Capecchi, B.; et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 2000, 287, 1816–1820. [Google Scholar] [CrossRef]
- Rojas-Lopez, M.; Martinelli, M.; Brandi, V.; Jubelin, G.; Polticelli, F.; Soriani, M.; Pizza, M.; Desvaux, M.; Rosini, R. Identification of lipid A deacylase as a novel, highly conserved and protective antigen against enterohemorrhagic Escherichia coli. Sci. Rep. 2019, 9, 17014. [Google Scholar] [CrossRef]
- Bassani-Sternberg, M.; Pletscher-Frankild, S.; Jensen, L.J.; Mann, M. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol. Cell. Proteom. 2015, 14, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.L.; Verbeke, R.; Asselman, C.; Aernout, I.; Gul, A.; Eggermont, D.; Boucher, K.; Thery, F.; Maia, T.M.; Demol, H.; et al. Immunopeptidomics-based design of mRNA vaccine formulations against Listeria monocytogenes. Nat. Commun. 2022, 13, 6075. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.; Cooke, M.F.; Huffman, A.; Xiang, Z.; Wong, M.U.; Wang, H.; Seetharaman, M.; Valdez, N.; He, Y. Vaxign2: The second generation of the first Web-based vaccine design program using reverse vaccinology and machine learning. Nucleic Acids Res. 2021, 49, W671–W678. [Google Scholar] [CrossRef] [PubMed]
- da Silva Antunes, R.; Garrigan, E.; Quiambao, L.G.; Dhanda, S.K.; Marrama, D.; Westernberg, L.; Wang, E.; Sutherland, A.; Armstrong, S.K.; Brickman, T.J.; et al. Genome-wide characterization of T cell responses to Bordetella pertussis reveals broad reactivity and similar polarization irrespective of childhood vaccination profiles. bioRxiv 2023. [CrossRef]
- Callaway, E. What’s next for AlphaFold and the AI protein-folding revolution. Nature 2022, 604, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.C.; Afonine, P.V.; Liebschner, D.; Croll, T.I.; McCoy, A.J.; Oeffner, R.D.; Williams, C.J.; Poon, B.K.; Richardson, J.S.; Read, R.J.; et al. Accelerating crystal structure determination with iterative AlphaFold prediction. Acta Crystallogr. Sect. D Struct. Biol. 2023, 79 Pt 3, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Anishchenko, I.; Pellock, S.J.; Chidyausiku, T.M.; Ramelot, T.A.; Ovchinnikov, S.; Hao, J.; Bafna, K.; Norn, C.; Kang, A.; Bera, A.K.; et al. De novo protein design by deep network hallucination. Nature 2021, 600, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Dauparas, J.; Anishchenko, I.; Bennett, N.; Bai, H.; Ragotte, R.J.; Milles, L.F.; Wicky, B.I.M.; Courbet, A.; de Haas, R.J.; Bethel, N.; et al. Robust deep learning-based protein sequence design using ProteinMPNN. Science 2022, 378, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Schoeder, C.T.; Gilchuk, P.; Sangha, A.K.; Ledwitch, K.V.; Malherbe, D.C.; Zhang, X.; Binshtein, E.; Williamson, L.E.; Martina, C.E.; Dong, J.; et al. Epitope-focused immunogen design based on the ebolavirus glycoprotein HR2-MPER region. PLoS Pathog. 2022, 18, e1010518. [Google Scholar] [CrossRef]
- Schoeder, C.T.; Schmitz, S.; Adolf-Bryfogle, J.; Sevy, A.M.; Finn, J.A.; Sauer, M.F.; Bozhanova, N.G.; Mueller, B.K.; Sangha, A.K.; Bonet, J.; et al. Modeling Immunity with Rosetta: Methods for Antibody and Antigen Design. Biochemistry 2021, 60, 825–846. [Google Scholar] [CrossRef]
- Correia, B.E.; Bates, J.T.; Loomis, R.J.; Baneyx, G.; Carrico, C.; Jardine, J.G.; Rupert, P.; Correnti, C.; Kalyuzhniy, O.; Vittal, V.; et al. Proof of principle for epitope-focused vaccine design. Nature 2014, 507, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; Schmitz, E.A.; Crowe, J.E., Jr.; Meiler, J. The human antibody sequence space and structural design of the V, J regions, and CDRH3 with Rosetta. mAbs 2022, 14, 2068212. [Google Scholar] [CrossRef] [PubMed]
- Sevy, A.M.; Panda, S.; Crowe, J.E., Jr.; Meiler, J.; Vorobeychik, Y. Integrating linear optimization with structural modeling to increase HIV neutralization breadth. PLoS Comput. Biol. 2018, 14, e1005999. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Finn, J.A.; Briney, B.; Sapparapu, G.; Singh, V.; King, H.; LaBranche, C.C.; Montefiori, D.C.; Meiler, J.; Crowe, J.E., Jr. Long antibody HCDR3s from HIV-naive donors presented on a PG9 neutralizing antibody background mediate HIV neutralization. Proc. Natl. Acad. Sci. USA 2016, 113, 4446–4451. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Haltaufderhyde, K.; Srikiatkhachorn, A.; Green, S.; Macareo, L.; Park, S.; Kalayanarooj, S.; Rothman, A.L.; Mathew, A. Activation of Peripheral T Follicular Helper Cells During Acute Dengue Virus Infection. J. Infect. Dis. 2018, 218, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Vardhana, S.; Baldo, L.; Morice, W.G., 2nd; Wherry, E.J. Understanding T cell responses to COVID-19 is essential for informing public health strategies. Sci. Immunol. 2022, 7, eabo1303. [Google Scholar] [CrossRef]
- De Groot, A.S.; Moise, L.; Terry, F.; Gutierrez, A.H.; Hindocha, P.; Richard, G.; Hoft, D.F.; Ross, T.M.; Noe, A.R.; Takahashi, Y.; et al. Better Epitope Discovery, Precision Immune Engineering, and Accelerated Vaccine Design Using Immunoinformatics Tools. Front. Immunol. 2020, 11, 442. [Google Scholar] [CrossRef]
- Moise, L.; Gutierrez, A.; Kibria, F.; Martin, R.; Tassone, R.; Liu, R.; Terry, F.; Martin, B.; De Groot, A.S. iVAX: An integrated toolkit for the selection and optimization of antigens and the design of epitope-driven vaccines. Hum. Vaccin. Immunother. 2015, 11, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Rappuoli, R. From empiricism to rational design: A personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014, 14, 505–514. [Google Scholar] [CrossRef]
- Gerke, C.; Colucci, A.M.; Giannelli, C.; Sanzone, S.; Vitali, C.G.; Sollai, L.; Rossi, O.; Martin, L.B.; Auerbach, J.; Di Cioccio, V.; et al. Production of a Shigella sonnei Vaccine Based on Generalized Modules for Membrane Antigens (GMMA), 1790GAHB. PLoS ONE 2015, 10, e0134478. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. Publisher Correction: A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 129. [Google Scholar] [CrossRef]
- Kapulu, M.C.; Nakakana, U.; Scire, A.S.; Sarakinou, E.; Conti, V.; Rossi, O.; Acquaviva, A.; Necchi, F.; Obiero, C.W.; Martin, L.B.; et al. Complement-mediated serum bactericidal activity of antibodies elicited by the Shigella sonnei GMMA vaccine in adults from a shigellosis-endemic country: Exploratory analysis of a Phase 2a randomized study. Front. Immunol. 2022, 13, 971866. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Nakakana, U.N.; Berlanda Scorza, F. Towards a Four-Component GMMA-Based Vaccine against Shigella. Vaccines 2022, 10, 328. [Google Scholar] [CrossRef]
- Boerth, E.M.; Gong, J.; Roffler, B.; Thompson, C.M.; Song, B.; Malley, S.F.; Hirsch, A.; MacLennan, C.A.; Zhang, F.; Malley, R.; et al. Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A. Vaccines 2023, 11, 1671. [Google Scholar] [CrossRef]
- Chichili, G.R.; Smulders, R.; Santos, V.; Cywin, B.; Kovanda, L.; Van Sant, C.; Malinoski, F.; Sebastian, S.; Siber, G.; Malley, R. Phase 1/2 study of a novel 24-valent pneumococcal vaccine in healthy adults aged 18 to 64 years and in older adults aged 65 to 85 years. Vaccine 2022, 40, 4190–4198. [Google Scholar] [CrossRef]
- Martin, P.; Alaimo, C. The Ongoing Journey of a Shigella Bioconjugate Vaccine. Vaccines 2022, 10, 212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).