Optimizing Microneutralization and IFN-γ ELISPOT Assays to Evaluate Mpox Immunity
Abstract
1. Introduction
2. Materials and Methods
3. Results
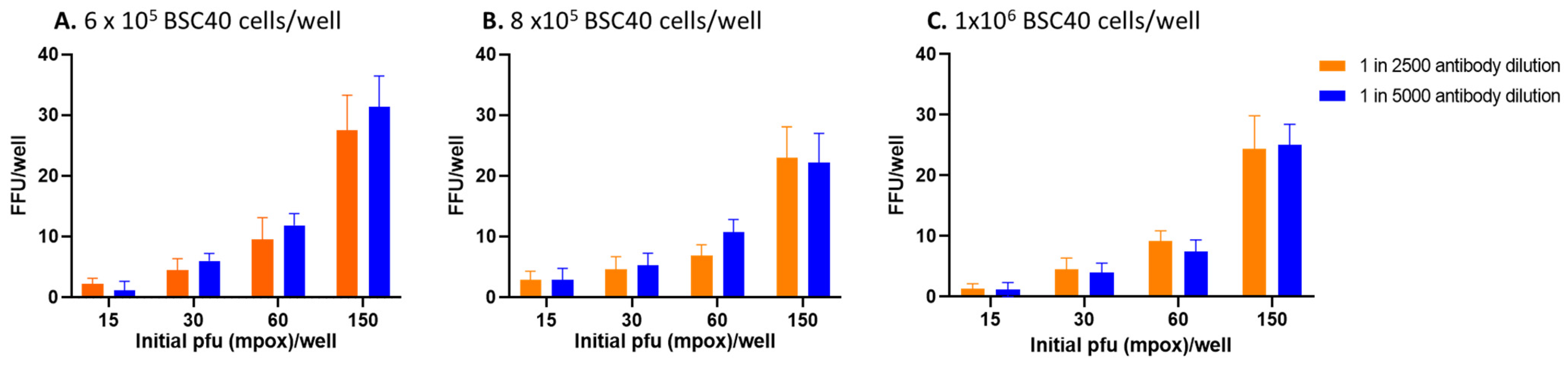
3.1. Effects of Number of BSC40 Cells, PFU, and Dilution of Primary Antibody in FRNT
3.2. Further Testing of Selected Parameters for FRNT
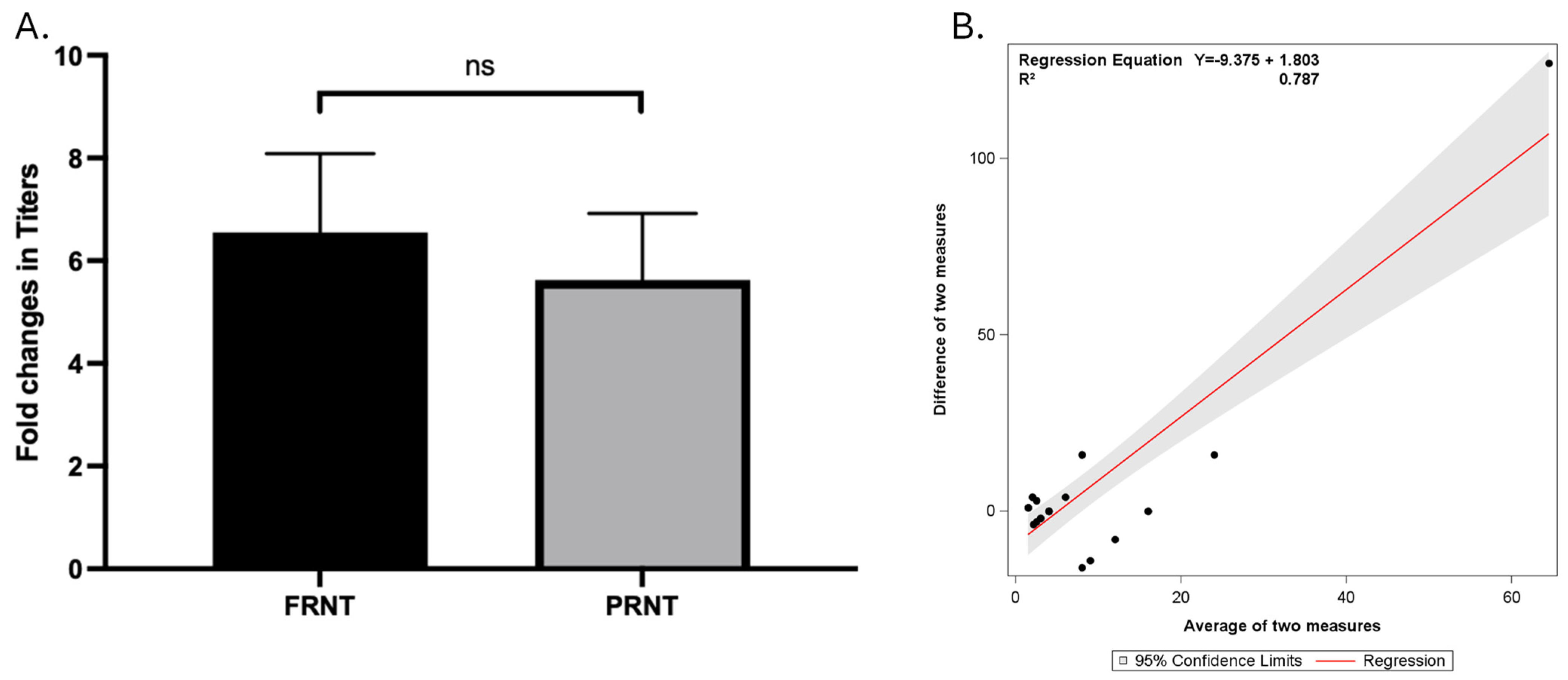
3.3. Comparing Different Cut-off Values to Interpret FRNT and PRNT Results
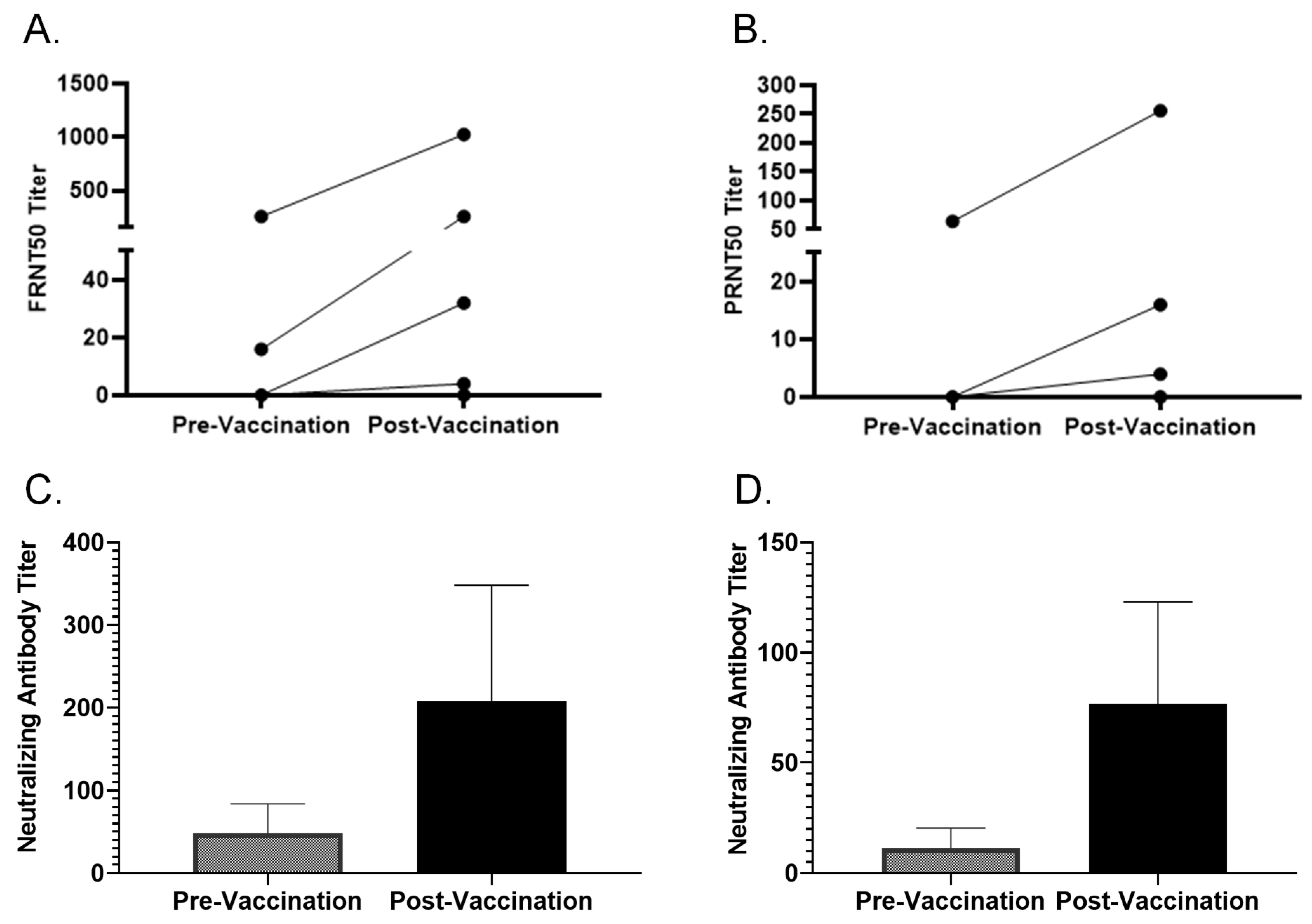
3.4. FRNT and PRNT on Multiple Paired Samples
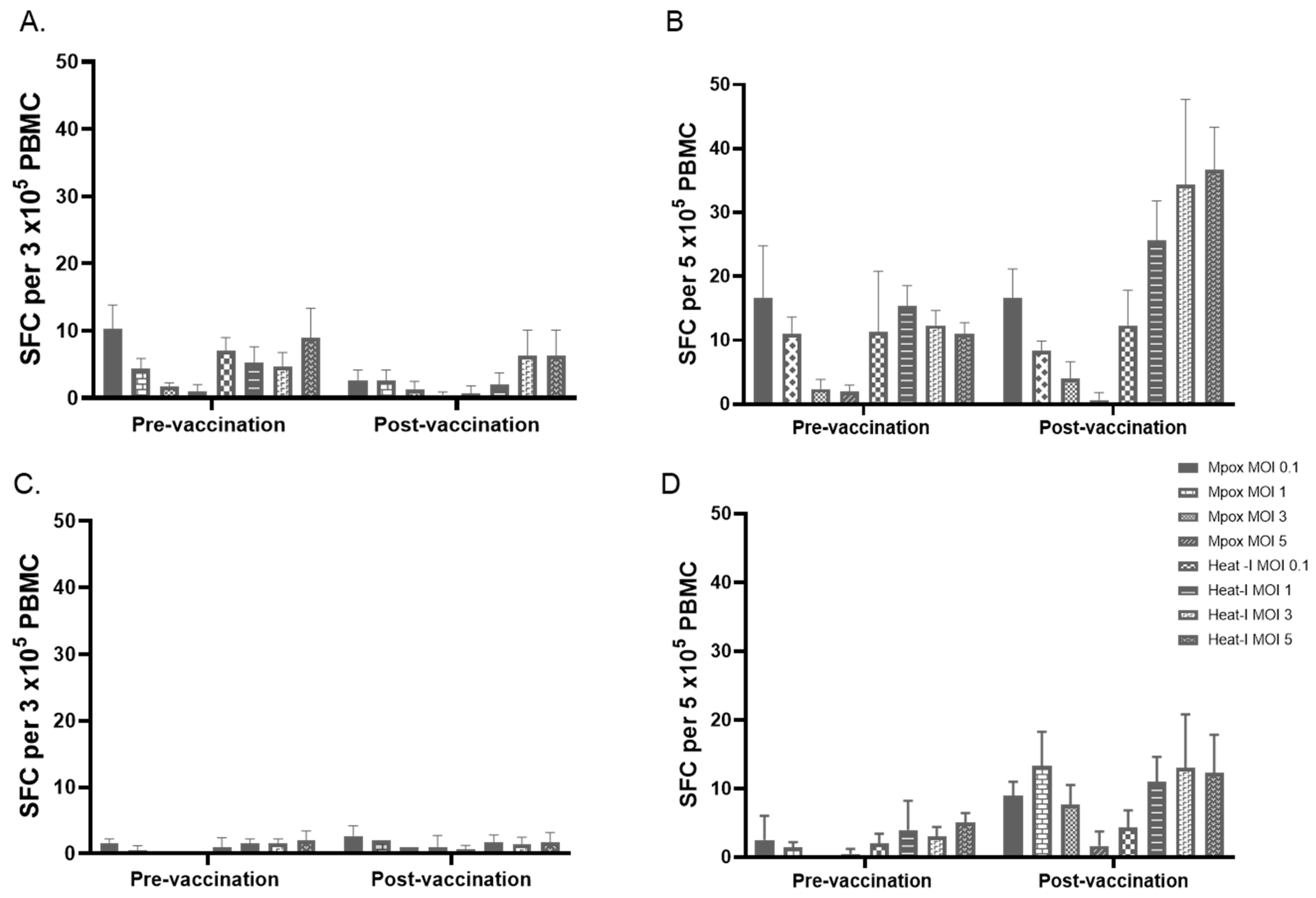
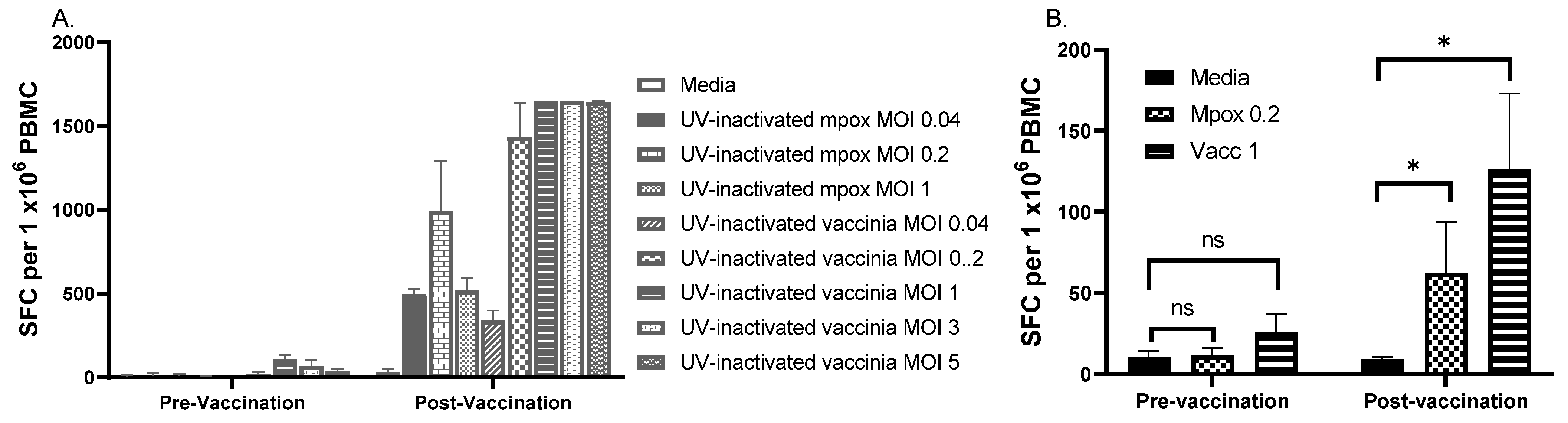
3.5. Effects of Number of PBMC, Antigen Preparations, and Concentration of Antigens on IFN-γ ELISPOT Results
3.6. Testing Selected MOIs on Paired Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tajudeen, Y.A.; Oladipo, H.J.; Muili, A.O.; Ikebuaso, J.G. Monkeypox: A review of a zoonotic disease of global public health concern. Health Promot. Perspect. 2023, 13, 1–9. [Google Scholar] [CrossRef]
- Americo, J.L.; Earl, P.L.; Moss, B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc. Natl. Acad. Sci. USA 2023, 120, e2220415120. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86 Pt 10, 2661–2672. [Google Scholar] [CrossRef]
- Breman, J.G.; Kalisa, R.; Steniowski, M.V.; Zanotto, E.; Gromyko, A.I.; Arita, I. Human monkeypox, 1970–1979. Bull. World Health Organ. 1980, 58, 165–182. [Google Scholar]
- Foster, S.O.; Brink, E.W.; Hutchins, D.L.; Pifer, J.M.; Lourie, B.; Moser, C.R.; Cummings, E.C.; Kuteyi, O.E.; Eke, R.E.A.; Titus, J.B.; et al. Human monkeypox. Bull. World Health Organ. 1972, 46, 569–576. [Google Scholar]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of human monkeypox in Nigeria in 2017-18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Davidson, W.B.; Curns, A.T.; Conover, C.S.; Huhn, G.; Davis, J.P.; Wegner, M.; Croft, D.R.; Newman, A.; Obiesie, N.N.; et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg. Infect. Dis. 2007, 13, 1332–1339. [Google Scholar] [CrossRef]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef]
- WHO. 2022–2024 Mpox (Monkeypox) Outbreak: Global Trends. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 31 October 2024).
- Africa-CDC. Event Based Surveillance Report, March 2024. Addis Ababa: ACDC. 2024. Available online: https://africacdc.org/download/africa-cdc-weekly-event-based-surveillance-report (accessed on 21 November 2024).
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; Van Royen, M.E.; Götz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef]
- Moschetta, N.; Raccagni, A.R.; Bianchi, M.; Diotallevi, S.; Lolatto, R.; Candela, C.; Foppa, C.U.; Gismondo, M.R.; Castagna, A.; Nozza, S.; et al. Mpox neutralising antibodies at 6 months from mpox infection or MVA-BN vaccination: A comparative analysis. Lancet Infect. Dis. 2023, 23, e455–e456. [Google Scholar] [CrossRef]
- Damon, I.K.; Davidson, W.B.; Hughes, C.M.; Olson, V.A.; Smith, S.K.; Holman, R.C.; Frey, S.E.; Newman, F.; Belshe, R.B.; Yan, L.; et al. Evaluation of smallpox vaccines using variola neutralization. J. Gen. Virol. 2009, 90 Pt 8, 1962–1966. [Google Scholar] [CrossRef] [PubMed]
- Newman, F.K.; Frey, S.E.; Blevins, T.P.; Mandava, M.; Bonifacio, A., Jr.; Yan, L.; Belshe, R.B. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J. Clin. Microbiol. 2003, 41, 3154–3157. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.B. The effect of the virus-serum incubation period upon vaccinia virus serum neutralization titers. J. Biol. Stand. 1987, 15, 389–392. [Google Scholar] [CrossRef]
- Stienlauf, S.; Shoresh, M.; Solomon, A.; Lublin-Tennenbaum, T.; Atsmon, Y.; Meirovich, Y.; Katz, E. Kinetics of formation of neutralizing antibodies against vaccinia virus following re-vaccination. Vaccine 1999, 17, 201–204. [Google Scholar] [CrossRef]
- Lansiaux, E.; Jain, N.; Laivacuma, S.; Reinis, A. The virology of human monkeypox virus (hMPXV): A brief overview. Virus Res. 2022, 322, 198932. [Google Scholar] [CrossRef]
- Sagdat, K.; Batyrkhan, A.; Kanayeva, D. Exploring monkeypox virus proteins and rapid detection techniques. Front. Cell Infect. Microbiol. 2024, 14, 1414224. [Google Scholar] [CrossRef]
- Shchelkunov, S.; Totmenin, A.; Safronov, P.; Mikheev, M.; Gutorov, V.; Ryazankina, O.; Petrov, N.; Babkin, I.; Uvarova, E.; Sandakhchiev, L.; et al. Analysis of the monkeypox virus genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef]
- Franceschi, V.; Parker, S.; Jacca, S.; Crump, R.W.; Doronin, K.; Hembrador, E.; Pompilio, D.; Tebaldi, G.; Estep, R.D.; Wong, S.W.; et al. BoHV-4-Based Vector Single Heterologous Antigen Delivery Protects STAT1(-/-) Mice from Monkeypoxvirus Lethal Challenge. PLoS Negl. Trop. Dis. 2015, 9, e0003850. [Google Scholar] [CrossRef]
- Cai, J.-P.; Chu, W.-M.; Tam, A.R.; Wang, K.; Han, Y.; Chen, L.-L.; Zhang, X.; Choi, C.Y.-K.; Cheng, V.C.-C.; Chan, K.-H.; et al. Determination of seroprevalence and kinetics of humoral response using mpox virus A29 protein. Commun. Med. 2023, 3, 168. [Google Scholar] [CrossRef]
- von Krempelhuber, A.; Vollmar, J.; Pokorny, R.; Rapp, P.; Wulff, N.; Petzold, B.; Handley, A.; Mateo, L.; Siersbol, H.; Kollaritsch, H.; et al. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine 2010, 28, 1209–1216. [Google Scholar] [CrossRef]
- Frey, S.E.; Wald, A.; Edupuganti, S.; Jackson, L.A.; Stapleton, J.T.; El Sahly, H.; El-Kamary, S.S.; Edwards, K.; Keyserling, H.; Winokur, P.; et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naive subjects. Vaccine 2015, 33, 5225–5234. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Newman, F.K.; Kennedy, J.S.; Sobek, V.; Ennis, F.A.; Hill, H.; Yan, L.K.; Chaplin, P.; Vollmar, J.; Chaitman, B.R.; et al. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine 2007, 25, 8562–8573. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Newman, F.K.; Frey, S.E.; Couch, R.B.; Treanor, J.J.; Tacket, C.O.; Yan, L. Dose-dependent neutralizing-antibody responses to vaccinia. J. Infect. Dis. 2004, 189, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Newman, F.K.; Kennedy, J.S.; Ennis, F.; Abate, G.; Hoft, D.F.; Monath, T.P. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000 and Dryvax in healthy vaccinia-naive adults. Vaccine 2009, 27, 1637–1644. [Google Scholar] [CrossRef]
- Talbot, T.R.; Stapleton, J.T.; Brady, R.C.; Winokur, P.L.; Bernstein, D.I.; Germanson, T.; Yoder, S.M.; Rock, M.T.; Crowe, J.E.; Edwards, K.M.; et al. Vaccination success rate and reaction profile with diluted and undiluted smallpox vaccine: A randomized controlled trial. JAMA 2004, 292, 1205–1212. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Gurwith, M.; Dekker, C.L.; Frey, S.E.; Edwards, K.M.; Kenner, J.; Greenberg, R.N. Safety and immunogenicity of LC16m8, an attenuated smallpox vaccine in vaccinia-naive adults. J. Infect. Dis. 2011, 204, 1395–1402. [Google Scholar] [CrossRef]
- Mazzotta, V.; Lepri, A.C.; Matusali, G.; Cimini, E.; Piselli, P.; Aguglia, C.; Lanini, S.; Colavita, F.; Notari, S.; Oliva, A.; et al. Immunogenicity and reactogenicity of modified vaccinia Ankara pre-exposure vaccination against mpox according to previous smallpox vaccine exposure and HIV infection: Prospective cohort study. EClinicalMedicine 2024, 68, 102420. [Google Scholar] [CrossRef]
- Manischewitz, J.; King, L.R.; Bleckwenn, N.A.; Shiloach, J.; Taffs, R.; Merchlinsky, M.; Eller, N.; Mikolajczyk, M.G.; Clanton, D.J.; Monath, T.; et al. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J. Infect. Dis. 2003, 188, 440–448. [Google Scholar] [CrossRef]
- Cosma, A.; Bühler, S.; Nagaraj, R.; Staib, C.; Hammarin, A.-L.; Wahren, B.; Goebel, F.D.; Erfle, V.; Sutter, G. Neutralization assay using a modified vaccinia virus Ankara vector expressing the green fluorescent protein is a high-throughput method to monitor the humoral immune response against vaccinia virus. Clin. Diagn. Lab. Immunol. 2004, 11, 406–410. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Moss, B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J. Virol. 2003, 77, 10684–10688. [Google Scholar] [CrossRef]
- Manenti, A.; Solfanelli, N.; Cantaloni, P.; Mazzini, L.; Leonardi, M.; Benincasa, L.; Piccini, G.; Marchi, S.; Boncioli, M.; Raffaelli, C.S.; et al. Evaluation of Monkeypox- and Vaccinia virus-neutralizing antibodies in human serum samples after vaccination and natural infection. Front. Public. Health 2023, 11, 1195674. [Google Scholar] [CrossRef] [PubMed]
- Grossegesse, M.; Stern, D.; Hofmann, N.; Surtees, R.; Kohl, C.; Michel, J.; Nitsche, A. Serological methods for the detection of antibodies against monkeypox virus applicable for laboratories with different biosafety levels. J. Med. Virol. 2023, 95, e29261. [Google Scholar] [CrossRef] [PubMed]
- Grüner, E.; Grossegesse, M.; Stern, D.; Ober, V.; Eser, T.M.; Reiling, G.; Roider, J. Mpox-specific immune responses elicited by vaccination or infection in people living with HIV. J. Infect. Dis. 2024, 230, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, J.C.; Cassaniti, I.; Ferrari, A.; Piralla, A.; Bergami, F.; Arena, F.A.; Paolucci, S.; Rovida, F.; Lilleri, D.; Percivalle, E.; et al. Characterization of immune response against monkeypox virus in cohorts of infected patients, historic and newly vaccinated subjects. J. Med. Virol. 2023, 95, e28778. [Google Scholar] [CrossRef]
- Lawrence, S.J.; Lottenbach, K.R.; Newman, F.K.; Buller RM, L.; Bellone, C.J.; Chen, J.J.; Cohen, G.H.; Eisenberg, R.J.; Belshe, R.B.; Stanley, S.L., Jr.; et al. Antibody responses to vaccinia membrane proteins after smallpox vaccination. J. Infect. Dis. 2007, 196, 220–229. [Google Scholar] [CrossRef]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef]
- Smith, G.L.; Vanderplasschen, A. Extracellular Enveloped Vaccinia Virus; Springer: Boston, MA, USA, 1998. [Google Scholar]
- Locker, J.K.; Kuehn, A.; Schleich, S.; Rutter, G.; Hohenberg, H.; Wepf, R.; Griffiths, G. Entry of the two infectious forms of vaccinia virus at the plasma membane is signaling-dependent for the IMV but not the EEV. Mol. Biol. Cell 2000, 11, 2497–2511. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Wiser, I.; Orr, N.; Smetana, Z.; Spungin-Bialik, A.; Mendelson, E.; Cohen, D. Alternative immunological markers to document successful multiple smallpox revaccinations. Clin. Infect. Dis. 2011, 52, 856–861. [Google Scholar] [CrossRef][Green Version]
- Collier, A.-R.Y.; McMahan, K.; Jacob-Dolan, C.; Liu, J.; Borducchi, E.N.; Moss, B.; Barouch, D.H. Decline of Mpox Antibody Responses After Modified Vaccinia Ankara-Bavarian Nordic Vaccination. JAMA 2024, 332, 1669–1672. [Google Scholar] [CrossRef]
- Matusali, G.; Petruccioli, E.; Cimini, E.; Colavita, F.; Bettini, A.; Tartaglia, E.; Maggi, F. Evaluation of Cross-Immunity to the Mpox Virus Due to Historic Smallpox Vaccination. Vaccines 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Medaglia, M.L.; Pereira Ade, C.; Freitas, T.R.; Damaso, C.R. Swinepox virus outbreak, Brazil, 2011. Emerg. Infect. Dis. 2011, 17, 1976–1978. [Google Scholar] [CrossRef] [PubMed]
- Ruedas-Torres, I.; Nga, B.T.T.; Salguero, F.J. Pathogenicity and virulence of African swine fever virus. Virulence 2024, 15, 2375550. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, T.R.; Shive, C.L.; Targoni, O.S.; Tary-Lehmann, M.; Lehmann, P.V. Increased per cell IFN-gamma productivity indicates recent in vivo activation of T cells. Cell Immunol. 2009, 258, 131–137. [Google Scholar] [CrossRef]
- Karulin, A.Y.; Caspell, R.; Dittrich, M.; Lehmann, P.V. Normal Distribution of CD8+ T-Cell-Derived ELISPOT Counts within Replicates Justifies the Reliance on Parametric Statistics for Identifying Positive Responses. Cells 2015, 4, 96–111. [Google Scholar] [CrossRef]
- Sundararaman, S.; Karulin, A.Y.; Ansari, T.; BenHamouda, N.; Gottwein, J.; Laxmanan, S.; Levine, S.M.; Loffredo, J.T.; McArdle, S.; Neudoerfl, C.; et al. High Reproducibility of ELISPOT Counts from Nine Different Laboratories. Cells 2015, 4, 21–39. [Google Scholar] [CrossRef]
- Barabas, S.; Spindler, T.; Kiener, R.; Tonar, C.; Lugner, T.; Batzilla, J.; Bendfeldt, H.; Rascle, A.; Asbach, B.; Wagner, R.; et al. An optimized IFN-gamma ELISpot assay for the sensitive and standardized monitoring of CMV protein-reactive effector cells of cell-mediated immunity. BMC Immunol. 2017, 18, 14. [Google Scholar] [CrossRef]
- Patterson, E.I.; Prince, T.; Anderson, E.R.; Casas-Sanchez, A.; Smith, S.L.; Cansado-Utrilla, C.; Hughes, G.L. Methods of Inactivation of SARS-CoV-2 for Downstream Biological Assays. J. Infect. Dis. 2020, 222, 1462–1467. [Google Scholar] [CrossRef]
- Elveborg, S.; Monteil, V.M.; Mirazimi, A. Methods of Inactivation of Highly Pathogenic Viruses for Molecular, Serology or Vaccine Development Purposes. Pathogens 2022, 11, 271. [Google Scholar] [CrossRef]
- Croft, S.; Wong, Y.C.; Smith, S.A.; Flesch, I.E.A.; Tscharke, D.C. Surprisingly Effective Priming of CD8(+) T Cells by Heat-Inactivated Vaccinia Virus Virions. J. Virol. 2020, 94, 10-1128. [Google Scholar] [CrossRef]
- Moraes-Cardoso, I.; Benet, S.; Carabelli, J.; Perez-Zsolt, D.; Mendoza, A.; Rivero, A.; Alemany, A.; Descalzo, V.; Alarcón-Soto, Y.; Grifoni, A.; et al. Immune responses associated with mpox viral clearance in men with and without HIV in Spain: A multisite, observational, prospective cohort study. Lancet Microbe 2024, 5, 100859. [Google Scholar] [CrossRef] [PubMed]
- Edghill-Smith, Y.; Golding, H.; Manischewitz, J.; King, L.R.; Scott, D.; Bray, M.; Nalca, A.; Hooper, J.W.; Whitehouse, C.A.; Schmitz, J.E.; et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005, 11, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.E.; Bray, M.; Whitehouse, C.A.; Miller, D.; Mucker, E.; Manischewitz, J.; King, L.R.; Guroff, M.R.; Hryniewicz, A.; Venzon, D.; et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect. Dis. 2005, 191, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.M.; Elrosasy, A.; Mahmoud, A.M.; Saed, S.A.A.; Moawad, W.A.E.; Hamouda, E.; Nguyen, D.; Tran, V.P.; Pham, H.T.; Sah, S.; et al. The effect of HIV and mpox co-infection on clinical outcomes: Systematic review and meta-analysis. HIV Med. 2024, 25, 897–909. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Meza, K.; Colbert, C.; Hoft, D.F.; Jaunarajs, A.; Blazevic, A.; Frey, S.E.; Abate, G. Optimizing Microneutralization and IFN-γ ELISPOT Assays to Evaluate Mpox Immunity. Vaccines 2025, 13, 27. https://doi.org/10.3390/vaccines13010027
Yu Y, Meza K, Colbert C, Hoft DF, Jaunarajs A, Blazevic A, Frey SE, Abate G. Optimizing Microneutralization and IFN-γ ELISPOT Assays to Evaluate Mpox Immunity. Vaccines. 2025; 13(1):27. https://doi.org/10.3390/vaccines13010027
Chicago/Turabian StyleYu, Yinyi, Krystal Meza, Chase Colbert, Daniel F. Hoft, Anna Jaunarajs, Azra Blazevic, Sharon E. Frey, and Getahun Abate. 2025. "Optimizing Microneutralization and IFN-γ ELISPOT Assays to Evaluate Mpox Immunity" Vaccines 13, no. 1: 27. https://doi.org/10.3390/vaccines13010027
APA StyleYu, Y., Meza, K., Colbert, C., Hoft, D. F., Jaunarajs, A., Blazevic, A., Frey, S. E., & Abate, G. (2025). Optimizing Microneutralization and IFN-γ ELISPOT Assays to Evaluate Mpox Immunity. Vaccines, 13(1), 27. https://doi.org/10.3390/vaccines13010027





