Immunogenicity and Protective Efficacy of a Multi-Antigen Mycobacterium tuberculosis Subunit Vaccine in Mice
Abstract
1. Introduction
2. Materials and Methods
3. Results
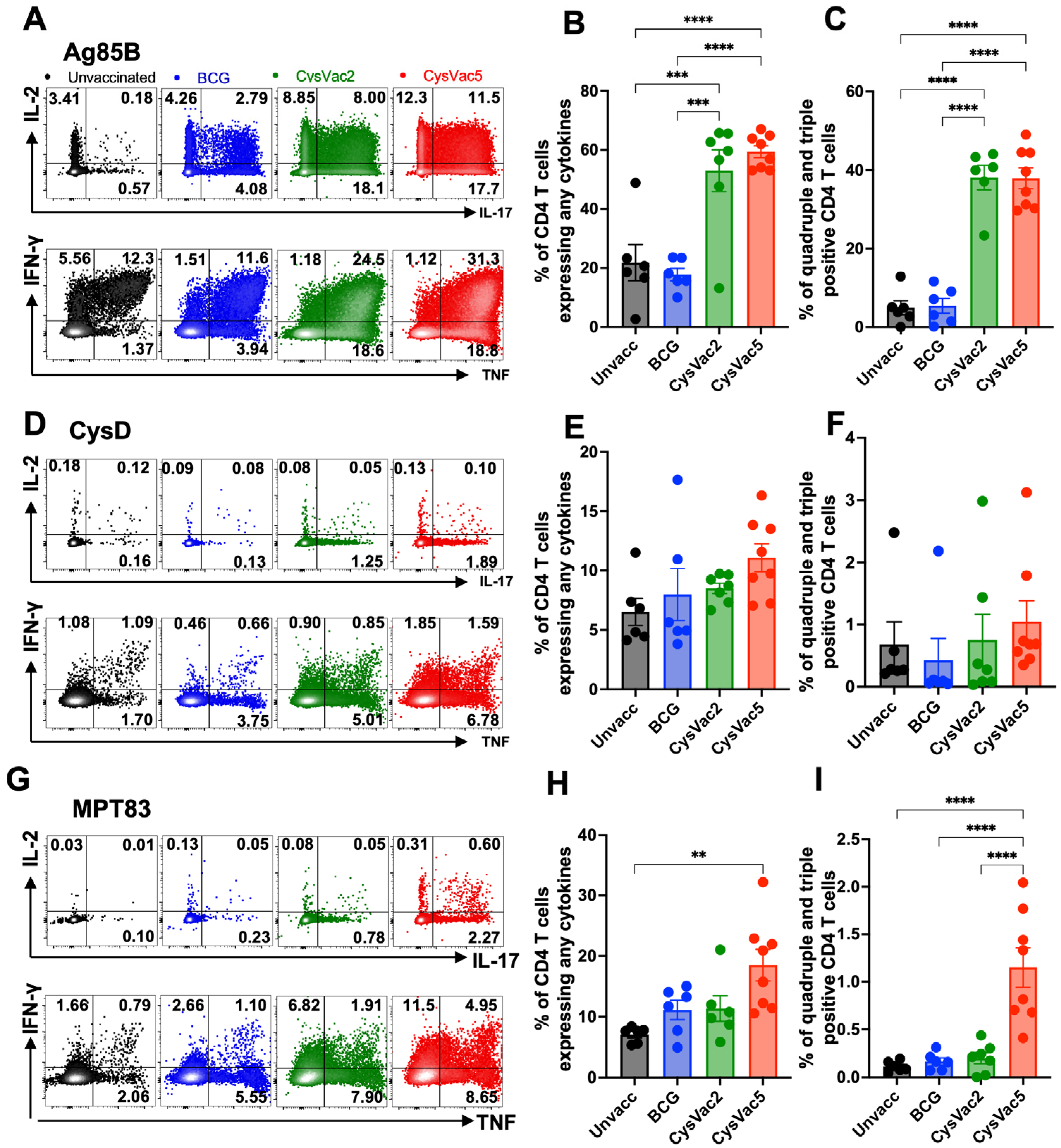
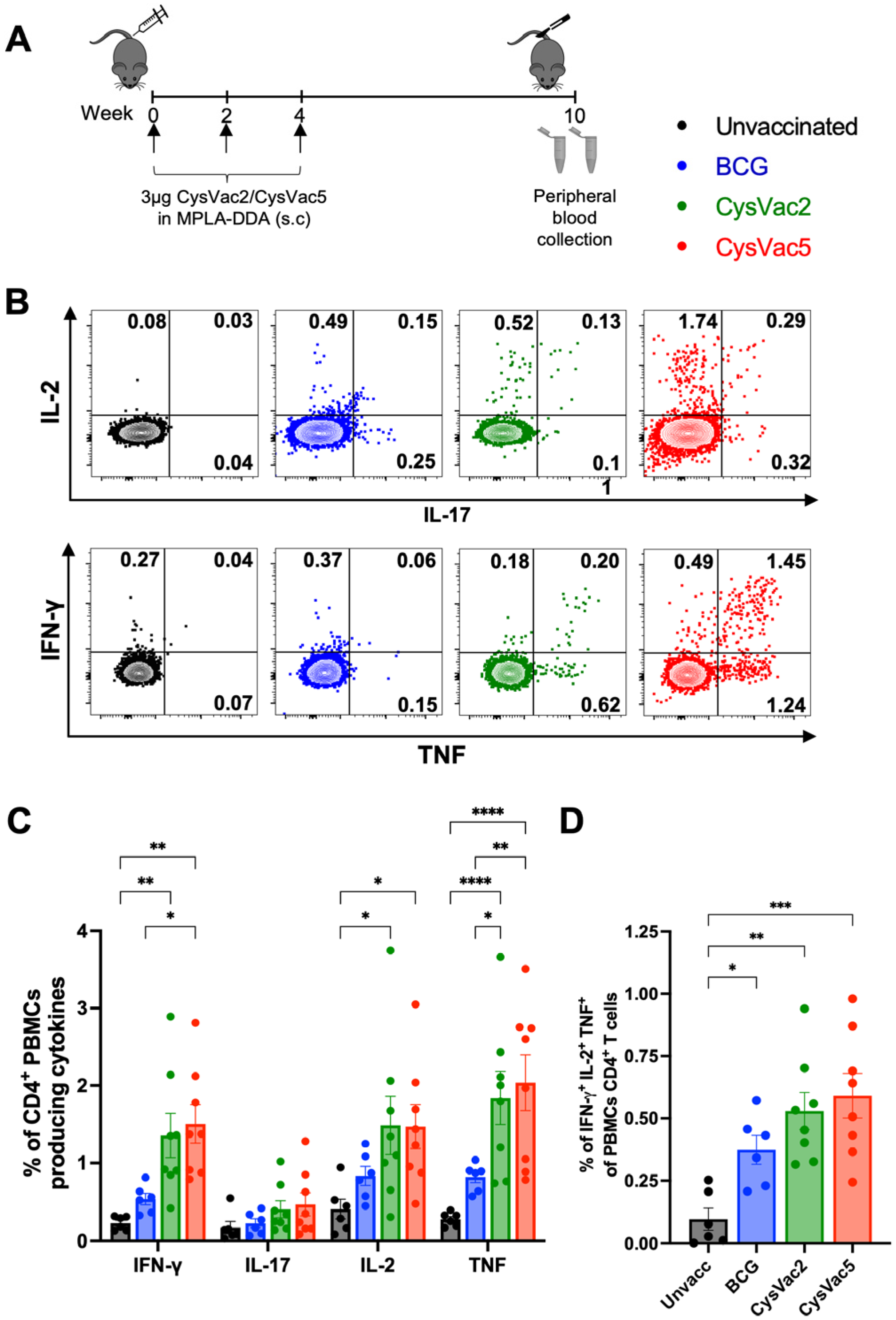
3.1. CysVac5-MPLA/DDA Vaccination Induces High Frequency of Circulating Multifunctional CD4+ T Cells
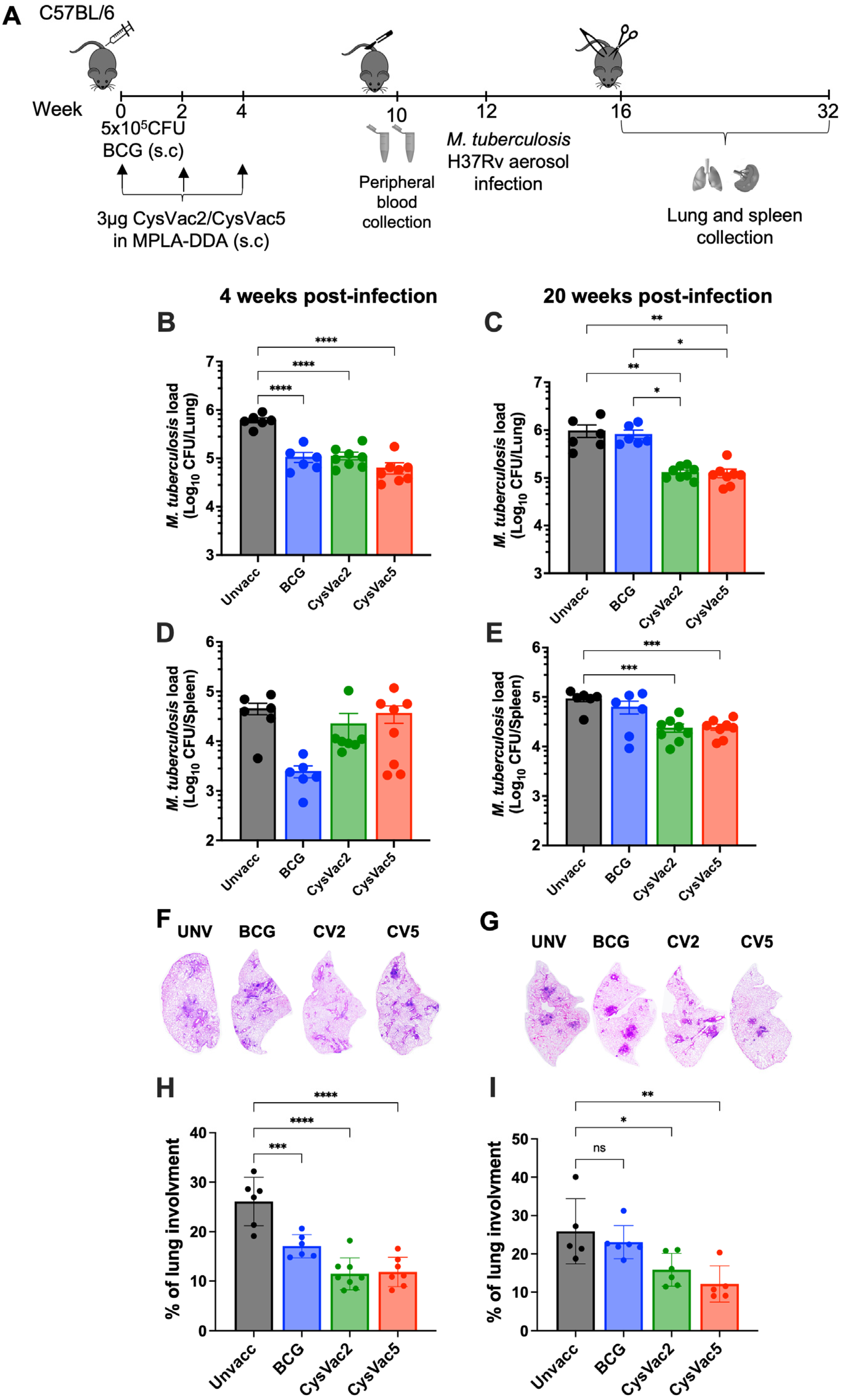
3.2. CysVac5-MPLA/DDA Reduced the Bacterial Burden and Pathology in M. tuberculosis-Infected Mice
3.3. CysVac5-MPLA/DDA Vaccination Increases the Number of Antigen-Specific Cytokine-Secreting Cells Following M. tuberculosis Infection
3.4. CysVac5-MPLA/DDA Induces Protective Immunity across Different MHC Haplotypes

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 7 May 2024).
- Bagcchi, S. WHO’s global tuberculosis report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E. Vaccine development against tuberculosis before and after COVID-19. Front. Immunol. 2023, 14, 1273938. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J.; Ahmed, R. Vaccines: Effector and memory T-cell differentiation: Implications for vaccine development. Nat. Rev. Immunol. 2002, 2, nri778. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.M.; Dietrich, J.; Billeskov, R. Tuberculosis subunit vaccines: From basic science to clinical testing. Expert. Opin. Biol. Ther. 2007, 7, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Bellini, C.; Horváti, K. Recent advances in the development of protein-and peptide-based subunit vaccines against tuberculosis. Cells 2020, 9, 2673. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Scriba, T.J. Moving tuberculosis vaccines from theory to practice. Nat. Rev. Immunol. 2019, 19, 550–562. [Google Scholar] [CrossRef]
- Tait, D.R.; Hatherill, M.; Van Der Meeren, O.; Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.J.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final Analysis of a Trial of M72/AS01(E) Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef]
- Dijkman, K.; Lindenstrom, T.; Rosenkrands, I.; Soe, R.; Woodworth, J.S.; Lindestam Arlehamn, C.S.; Mortensen, R. A protective, single-visit TB vaccination regimen by co-administration of a subunit vaccine with BCG. NPJ Vaccines 2023, 8, 66. [Google Scholar] [CrossRef]
- Woodworth, J.S.; Clemmensen, H.S.; Battey, H.; Dijkman, K.; Lindenstrom, T.; Laureano, R.S.; Taplitz, R.; Morgan, J.; Aagaard, C.; Rosenkrands, I.; et al. A Mycobacterium tuberculosis-specific subunit vaccine that provides synergistic immunity upon co-administration with Bacillus Calmette-Guerin. Nat. Commun. 2021, 12, 6658. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Tang, Q.X.; Britton, W.J.; Leyh, T.S.; Triccas, J.A. The Mycobacterium tuberculosis cysD and cysNC genes form a stress-induced operon that encodes a tri-functional sulfate-activating complex. Microbiology 2004, 150, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.; Leotta, L.; Shanahan, E.R.; West, N.P.; Leyh, T.S.; Britton, W.; Triccas, J.A. Host Cell—Induced Components of the Sulfate Assimilation Pathway Are Major Protective Antigens of Mycobacterium tuberculosis. J. Infect. Dis. 2012, 207, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Hill-Cawthorne, G.A.; Feng, C.G.; Britton, W.J.; Triccas, J.A. Mycobacterium tuberculosis components expressed during chronic infection of the lung contribute to long-term control of pulmonary tuberculosis in mice. NPJ Vaccines 2016, 1, 16012. [Google Scholar] [CrossRef]
- Counoupas, C.; Ferrell, K.C.; Ashhurst, A.; Bhattacharyya, N.D.; Nagalingam, G.; Stewart, E.L.; Feng, C.G.; Petrovsky, N.; Britton, W.J.; Triccas, J.A. Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis. NPJ Vaccines 2020, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Britton, W.J.; Triccas, J.A. Protective efficacy of recombinant BCG over-expressing protective, stage-specific antigens of Mycobacterium tuberculosis. Vaccine 2018, 36, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Sander, P. Mycobacterium tuberculosis lipoproteins in virulence and immunity–fighting with a double-edged sword. FEBS Lett. 2016, 590, 3800–3819. [Google Scholar] [CrossRef]
- Kao, F.F.; Mahmuda, S.; Pinto, R.; Triccas, J.A.; West, N.P.; Britton, W.J. The secreted lipoprotein, MPT83, of Mycobacterium tuberculosis is recognized during human tuberculosis and stimulates protective immunity in mice. PLoS ONE 2012, 7, e34991. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, M.; Chen, H.; Liu, S.; Wu, X.; Cui, Z.; Yang, H.; Liu, H.; Ge, B. Mycobacterium tuberculosis Lipoprotein MPT83 Induces Apoptosis of Infected Macrophages by Activating the TLR2/p38/COX-2 Signaling Pathway. J. Immunol. 2017, 198, 4772–4780. [Google Scholar] [CrossRef]
- Nusbaum, R.J.; Calderon, V.E.; Huante, M.B.; Sutjita, P.; Vijayakumar, S.; Lancaster, K.L.; Hunter, R.L.; Actor, J.K.; Cirillo, J.D.; Aronson, J. Pulmonary tuberculosis in humanized mice infected with HIV-1. Sci. Rep. 2016, 6, 21522. [Google Scholar] [CrossRef]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247–258. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Lange, C.; Rao, M.; Balaji, K.N.; Lotze, M.; Schito, M.; Zumla, A.I.; Maeurer, M. Progress in tuberculosis vaccine development and host-directed therapies—A state of the art review. Lancet Respir. Med. 2014, 2, 301–320. [Google Scholar] [CrossRef]
- Martin, C.; Aguilo, N.; Marinova, D.; Gonzalo-Asensio, J. Update on TB vaccine pipeline. Appl. Sci. 2020, 10, 2632. [Google Scholar] [CrossRef]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Delta inulin-based adjuvants promote the generation of polyfunctional CD4(+) T cell responses and protection against Mycobacterium tuberculosis infection. Sci. Rep. 2017, 7, 8582. [Google Scholar] [CrossRef]
- Ogongo, P.; Tezera, L.B.; Ardain, A.; Nhamoyebonde, S.; Ramsuran, D.; Singh, A.; Ng’oepe, A.; Karim, F.; Naidoo, T.; Khan, K.; et al. Tissue-resident-like CD4+ T cells secreting IL-17 control Mycobacterium tuberculosis in the human lung. J. Clin. Investig. 2021, 131, e142014. [Google Scholar] [CrossRef]
- Wang, W.; Deng, G.; Zhang, G.; Yu, Z.; Yang, F.; Chen, J.; Cai, Y.; Werz, O.; Chen, X. Genetic polymorphism rs8193036 of IL17A is associated with increased susceptibility to pulmonary tuberculosis in Chinese Han population. Cytokine 2020, 127, 154956. [Google Scholar] [CrossRef]
- Smith, S.G.; Zelmer, A.; Blitz, R.; Fletcher, H.A.; Dockrell, H.M. Polyfunctional CD4 T-cells correlate with in vitro mycobacterial growth inhibition following Mycobacterium bovis BCG-vaccination of infants. Vaccine 2016, 34, 5298–5305. [Google Scholar] [CrossRef]
- Lewinsohn, D.A.; Lewinsohn, D.M.; Scriba, T.J. Polyfunctional CD4(+) T Cells As Targets for Tuberculosis Vaccination. Front. Immunol. 2017, 8, 1262. [Google Scholar] [CrossRef]
- Carpenter, S.M.; Lu, L.L. Leveraging Antibody, B Cell and Fc Receptor Interactions to Understand Heterogeneous Immune Responses in Tuberculosis. Front. Immunol. 2022, 13, 830482. [Google Scholar] [CrossRef]
- Weinrich Olsen, A.; van Pinxteren, L.A.; Meng Okkels, L.; Birk Rasmussen, P.; Andersen, P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect. Immun. 2001, 69, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Azad, A.K.; Chowdhury, P.A.; Wakayama, M. Computational Identification and Characterization of a Promiscuous T-Cell Epitope on the Extracellular Protein 85B of Mycobacterium spp. for Peptide-Based Subunit Vaccine Design. Biomed. Res. Int. 2017, 2017, 4826030. [Google Scholar] [CrossRef]
- Mahanty, S.; Prigent, A.; Garraud, O. Immunogenicity of infectious pathogens and vaccine antigens. BMC Immunol. 2015, 16, 31. [Google Scholar] [CrossRef]
- Di Pietrantonio, T.; Correa, J.A.; Orlova, M.; Behr, M.A.; Schurr, E. Joint effects of host genetic background and mycobacterial pathogen on susceptibility to infection. Infect. Immun. 2011, 79, 2372–2378. [Google Scholar] [CrossRef][Green Version]
- Abel, L.; El-Baghdadi, J.; Bousfiha, A.A.; Casanova, J.-L.; Schurr, E. Human genetics of tuberculosis: A long and winding road. Phil. Trans. R. Soc. B 2014, 369, 20130428. [Google Scholar] [CrossRef]
- Creissen, E.; Izzo, L.; Dawson, C.; Izzo, A.A. Guinea Pig Model of Mycobacterium tuberculosis Infection. Curr. Protoc. 2021, 1, e312. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisa, A.; Pinto, R.; Britton, W.J.; Triccas, J.A.; Counoupas, C. Immunogenicity and Protective Efficacy of a Multi-Antigen Mycobacterium tuberculosis Subunit Vaccine in Mice. Vaccines 2024, 12, 997. https://doi.org/10.3390/vaccines12090997
Nisa A, Pinto R, Britton WJ, Triccas JA, Counoupas C. Immunogenicity and Protective Efficacy of a Multi-Antigen Mycobacterium tuberculosis Subunit Vaccine in Mice. Vaccines. 2024; 12(9):997. https://doi.org/10.3390/vaccines12090997
Chicago/Turabian StyleNisa, Annuurun, Rachel Pinto, Warwick J. Britton, James A. Triccas, and Claudio Counoupas. 2024. "Immunogenicity and Protective Efficacy of a Multi-Antigen Mycobacterium tuberculosis Subunit Vaccine in Mice" Vaccines 12, no. 9: 997. https://doi.org/10.3390/vaccines12090997
APA StyleNisa, A., Pinto, R., Britton, W. J., Triccas, J. A., & Counoupas, C. (2024). Immunogenicity and Protective Efficacy of a Multi-Antigen Mycobacterium tuberculosis Subunit Vaccine in Mice. Vaccines, 12(9), 997. https://doi.org/10.3390/vaccines12090997





