Advances in Dendritic-Cell-Based Vaccines against Respiratory Fungal Infections
Abstract
1. Introduction
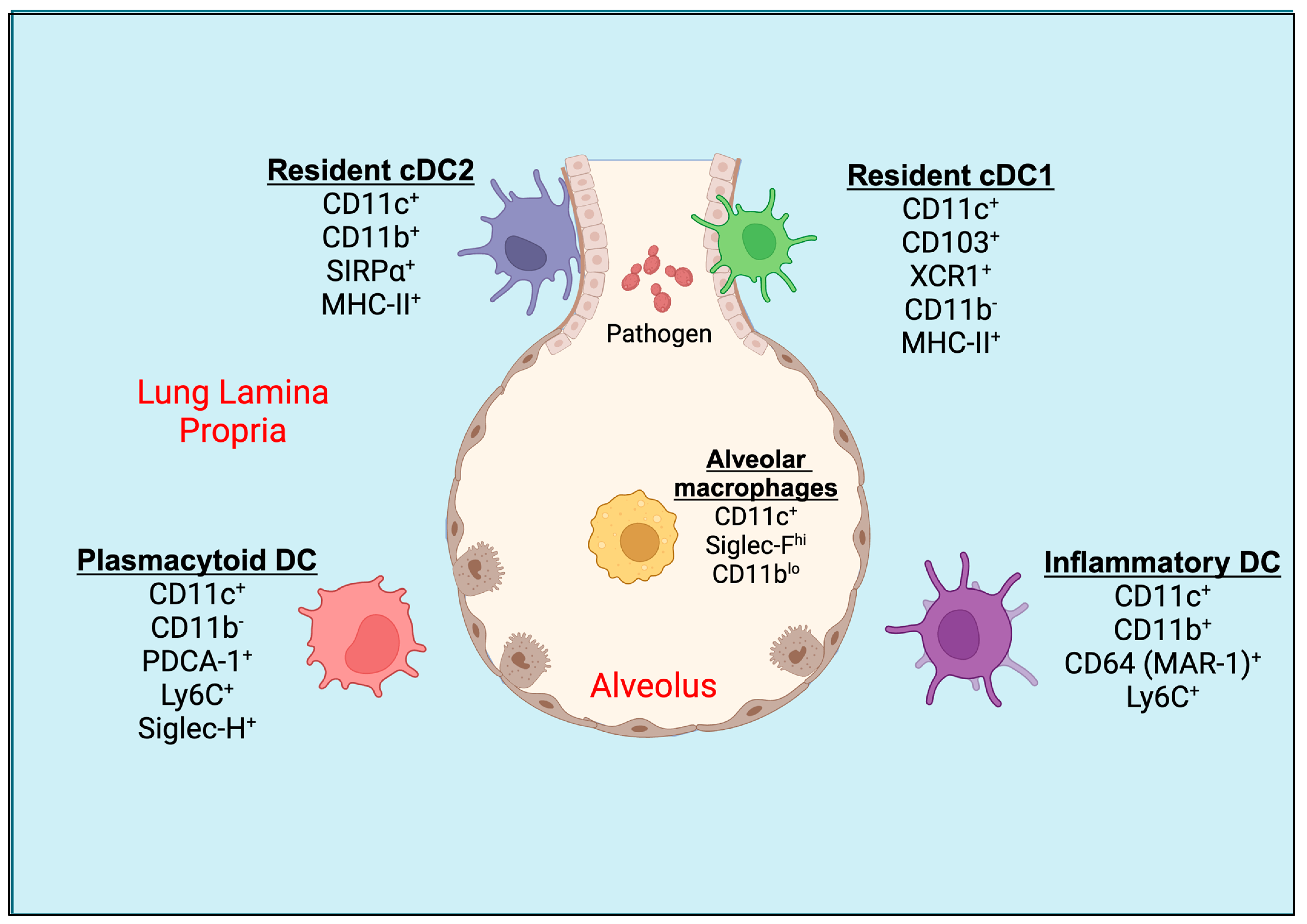
2. Pulmonary Dendritic Cells (DCs)
3. Origin and Development of Lung DCs
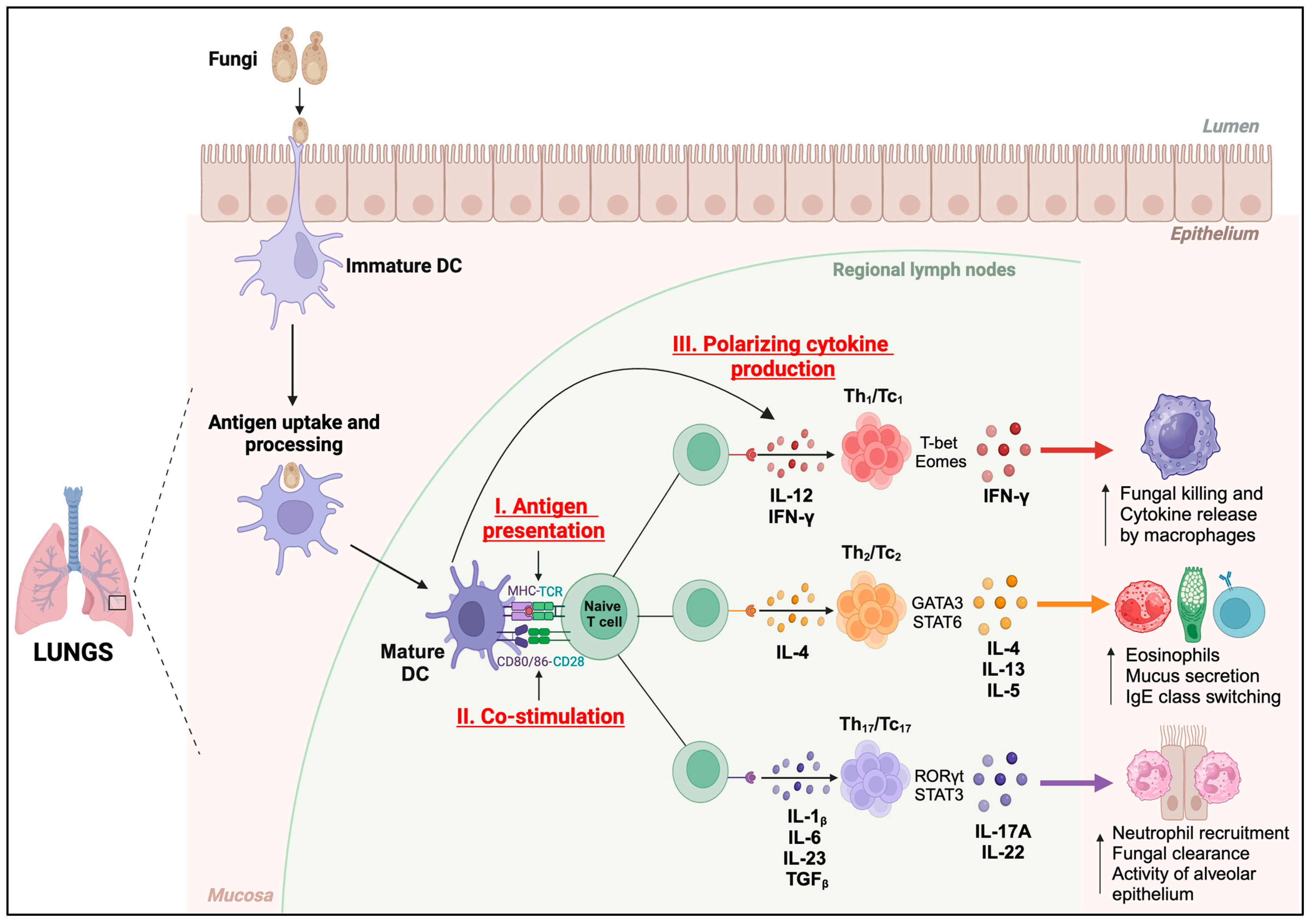
4. Functions of DC against Respiratory Fungal Pathogens
5. Dendritic-Cell-Based Experimental Fungal Vaccines
5.1. Aspergillus spp.
5.2. Coccidiodes spp.
5.3. Paracoccidioides spp.
5.4. Cryptococcus spp.
| Fungi | Vaccine Type | Methodology | Major Outcomes | References |
|---|---|---|---|---|
| Aspergillus spp. | RNA or live fungi/Crude antigen or subunit vaccine | Murine and human DC-pulsed (RNA complexed with DOTAP) | Enhanced DC’s MHC-II and co-stimulatory molecules expression, and increased Th1/Th2 response | [108] |
| Heat-killed fungi/Crude antigen vaccine | Human DC-pulsed | In vivo protective antigen-specific Th1 response (high IFN-γ and IL-10 production) | [109] | |
| Heat-inactivated fungi/viral transduction | Murine DC-pulsed and IL-12 gene therapy | Increased Th1 responses, improved survivability, and reduced fungal burden | [110] | |
| Coccidioides spp. | Ag2/Subunit vaccine (Ag2/PRA-cDNA transfected DC) | Murine transfected DCs | Reduced fungal burden, tissue injury in vaccinated mice, enhanced IgG levels, and increased IFN-γ, IL-4, and IL-17 production | [120,121,122,123] |
| Paracoccidioides spp. | Peptide vaccine (P10) P10 primary DC P10 primary monocyte derived-DC | P10-primed murine DCs | Reduced fungal burden in both immunocompetent and immunosuppressed mice, protection against intratracheal challenge, protective Th1 responses, activation and upregulation of MHC-II, CD80, and CD86 on the DCs, and induction of CD4+ and CD8+ T-cell proliferation. | [131,132,133] |
| Cryptococcus spp. | Heat-killed Cryptococcus gattii mutant ∆cap60 | Murine DC-pulsed | Protection and stimulation of tissue-resident memory Th17 cells in the lungs | [138,144] |
| Live or heat-killed Cryptococcus neoformans mutant | - | Protective Th1-type adaptive immune response Induction of trained immunity of DCs | [140] |
6. Future Perspectives of Fungal DC Vaccines
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Whitham, H.K.; Jackson, B.R. Economic Burden of Fungal Diseases in the United States. Open Forum Infect. Dis. 2022, 9, ofac097. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Data and Statistics on Fungal Diseases. Available online: https://www.cdc.gov/fungal/data-research/facts-stats/index.html (accessed on 8 July 2024).
- Robbins, N.; Wright, G.D.; Cowen, L.E. Antifungal Drugs: The Current Armamentarium and Development of New Agents. Microbiol. Spectr. 2016, 4, FUNK-0002-2016. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. Overcoming antifungal resistance. Drug Discov. Today Technol. 2014, 11, 65–71. [Google Scholar] [CrossRef]
- Thomas, C.M.; Shae, W.; Koestler, D.; DeFor, T.; Bahr, N.C.; Alpern, J.D. Antifungal drug price increases in the United States, 2000–2019. Mycoses 2022, 65, 859–865. [Google Scholar] [CrossRef]
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973, 137, 1142–1162. [Google Scholar] [CrossRef]
- Steinman, R.M. Dendritic cells and the control of immunity: Enhancing the efficiency of antigen presentation. Mt. Sinai J. Med. 2001, 68, 160–166. [Google Scholar]
- Guilliams, M.; Lambrecht, B.N.; Hammad, H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 2013, 6, 464–473. [Google Scholar] [CrossRef]
- Bosteels, C.; Neyt, K.; Vanheerswynghels, M.; van Helden, M.J.; Sichien, D.; Debeuf, N.; De Prijck, S.; Bosteels, V.; Vandamme, N.; Martens, L.; et al. Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection. Immunity 2020, 52, 1039–1056.E9. [Google Scholar] [CrossRef]
- Kawasaki, T.; Ikegawa, M.; Kawai, T. Antigen Presentation in the Lung. Front. Immunol. 2022, 13, 860915. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, B.; Rossi, S.A.; Santos, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Therapies and Vaccines Based on Nanoparticles for the Treatment of Systemic Fungal Infections. Front. Cell Infect. Microbiol. 2020, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Rodrigues, M.L.; Janbon, G. Extracellular Vesicles in Fungi: Past, Present, and Future Perspectives. Front. Cell Infect. Microbiol. 2020, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Mack, S.M.; Madl, A.K.; Pinkerton, K.E. Respiratory Health Effects of Exposure to Ambient Particulate Matter and Bioaerosols. Compr. Physiol. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- von Garnier, C.; Filgueira, L.; Wikstrom, M.; Smith, M.; Thomas, J.A.; Strickland, D.H.; Holt, P.G.; Stumbles, P.A. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J. Immunol. 2005, 175, 1609–1618. [Google Scholar] [CrossRef]
- Condon, T.V.; Sawyer, R.T.; Fenton, M.J.; Riches, D.W. Lung dendritic cells at the innate-adaptive immune interface. J. Leukoc. Biol. 2011, 90, 883–895. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Randall, T.D.; Silva-Sanchez, A. Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front. Immunol. 2016, 7, 258. [Google Scholar] [CrossRef]
- Lysen, A.; Gudjonsson, A.; Tesfaye, D.Y.; Bobic, S.; Bern, M.; Bogen, B.; Fossum, E. Intranasal delivery of a cDC1 targeted influenza vaccine with poly(I:C) enhances T cell responses and protects against influenza infection. Scand. J. Immunol. 2022, 95, e13128. [Google Scholar] [CrossRef]
- Guilliams, M.; Henri, S.; Tamoutounour, S.; Ardouin, L.; Schwartz-Cornil, I.; Dalod, M.; Malissen, B. From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. Eur. J. Immunol. 2010, 40, 2089–2094. [Google Scholar] [CrossRef]
- Bachem, A.; Guttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef]
- Desch, A.N.; Randolph, G.J.; Murphy, K.; Gautier, E.L.; Kedl, R.M.; Lahoud, M.H.; Caminschi, I.; Shortman, K.; Henson, P.M.; Jakubzick, C.V. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J. Exp. Med. 2011, 208, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Dammeijer, F.; Aerts, J.; Vroman, H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets? Front. Immunol. 2018, 9, 2804. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.M.; Chan, A.; Rock, K.L. Pathways of MHC I cross-presentation of exogenous antigens. Semin. Immunol. 2023, 66, 101729. [Google Scholar] [CrossRef]
- Raymond, M.; Rubio, M.; Fortin, G.; Shalaby, K.H.; Hammad, H.; Lambrecht, B.N.; Sarfati, M. Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of TH2-mediated allergic inflammation. J. Allergy Clin. Immunol. 2009, 124, 1333–1342.E1. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Dendritic cells and epithelial cells: Linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Roy, R.M.; Klein, B.S. Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe 2012, 11, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Ruffell, B. moDCs, Less Problems. Immunity 2018, 48, 6–8. [Google Scholar] [CrossRef]
- Banki, Z.; Werner, R.; Riepler, L.; Rossler, A.; Mullauer, B.; Hegen, V.; Bayer, W.; Verbeek, J.S.; Dittmer, U.; Stoiber, H. Fcgamma Receptor Type I (CD64)-Mediated Impairment of the Capacity of Dendritic Cells to Activate Specific CD8 T Cells by IgG-opsonized Friend Virus. Viruses 2019, 11, 145. [Google Scholar] [CrossRef]
- Tamoutounour, S.; Henri, S.; Lelouard, H.; de Bovis, B.; de Haar, C.; van der Woude, C.J.; Woltman, A.M.; Reyal, Y.; Bonnet, D.; Sichien, D.; et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 2012, 42, 3150–3166. [Google Scholar] [CrossRef]
- Langlet, C.; Tamoutounour, S.; Henri, S.; Luche, H.; Ardouin, L.; Gregoire, C.; Malissen, B.; Guilliams, M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J. Immunol. 2012, 188, 1751–1760. [Google Scholar] [CrossRef]
- Cheong, C.; Matos, I.; Choi, J.H.; Dandamudi, D.B.; Shrestha, E.; Longhi, M.P.; Jeffrey, K.L.; Anthony, R.M.; Kluger, C.; Nchinda, G.; et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010, 143, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.; Lopez-Bravo, M.; Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26, 519–531. [Google Scholar] [CrossRef]
- Wu, X.; Briseno, C.G.; Durai, V.; Albring, J.C.; Haldar, M.; Bagadia, P.; Kim, K.W.; Randolph, G.J.; Murphy, T.L.; Murphy, K.M. Mafb lineage tracing to distinguish macrophages from other immune lineages reveals dual identity of Langerhans cells. J. Exp. Med. 2016, 213, 2553–2565. [Google Scholar] [CrossRef]
- Backer, R.A.; Probst, H.C.; Clausen, B.E. Classical DC2 subsets and monocyte-derived DC: Delineating the developmental and functional relationship. Eur. J. Immunol. 2023, 53, e2149548. [Google Scholar] [CrossRef]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Victora, G.D.; Schwickert, T.A.; Guermonprez, P.; Meredith, M.M.; Yao, K.; Chu, F.F.; Randolph, G.J.; Rudensky, A.Y.; Nussenzweig, M. In vivo analysis of dendritic cell development and homeostasis. Science 2009, 324, 392–397. [Google Scholar] [CrossRef]
- Kopf, M.; Schneider, C.; Nobs, S.P. The development and function of lung-resident macrophages and dendritic cells. Nat. Immunol. 2015, 16, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.I.; Booth, J.L.; Duggan, E.S.; Cate, S.; White, V.L.; Hutchings, D.; Kovats, S.; Burian, D.M.; Dozmorov, M.; Metcalf, J.P. Transcriptional Classification and Functional Characterization of Human Airway Macrophage and Dendritic Cell Subsets. J. Immunol. 2017, 198, 1183–1201. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A., III; Dutertre, C.A.; Ginhoux, F.; Murphy, K.M. Genetic models of human and mouse dendritic cell development and function. Nat. Rev. Immunol. 2021, 21, 101–115. [Google Scholar] [CrossRef]
- Diao, J.; Winter, E.; Cantin, C.; Chen, W.; Xu, L.; Kelvin, D.; Phillips, J.; Cattral, M.S. In situ replication of immediate dendritic cell (DC) precursors contributes to conventional DC homeostasis in lymphoid tissue. J. Immunol. 2006, 176, 7196–7206. [Google Scholar] [CrossRef]
- Maraskovsky, E.; Brasel, K.; Teepe, M.; Roux, E.R.; Lyman, S.D.; Shortman, K.; McKenna, H.J. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: Multiple dendritic cell subpopulations identified. J. Exp. Med. 1996, 184, 1953–1962. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Schraml, B.U.; van Blijswijk, J.; Zelenay, S.; Whitney, P.G.; Filby, A.; Acton, S.E.; Rogers, N.C.; Moncaut, N.; Carvajal, J.J.; Reis e Sousa, C. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell 2013, 154, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Boonstra, A.; Paturel, C.; Antonenko, S.; Xu, X.L.; Trinchieri, G.; O’Garra, A.; Liu, Y.J. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 2002, 195, 953–958. [Google Scholar] [CrossRef]
- Adams, N.M.; Das, A.; Yun, T.J.; Reizis, B. Ontogeny and Function of Plasmacytoid Dendritic Cells. Annu. Rev. Immunol. 2024, 42, 347–373. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.; Tirard, A.; Villani, A.C. Plasmacytoid dendritic cells: Welcome back to the DC fold. Immunity 2022, 55, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ohteki, T.; Ginhoux, F.; Shortman, K.; Spits, H. Reclassification of plasmacytoid dendritic cells as innate lymphocytes is premature. Nat. Rev. Immunol. 2023, 23, 338–339. [Google Scholar] [CrossRef]
- Nagasawa, M.; Schmidlin, H.; Hazekamp, M.G.; Schotte, R.; Blom, B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur. J. Immunol. 2008, 38, 2389–2400. [Google Scholar] [CrossRef]
- Cisse, B.; Caton, M.L.; Lehner, M.; Maeda, T.; Scheu, S.; Locksley, R.; Holmberg, D.; Zweier, C.; den Hollander, N.S.; Kant, S.G.; et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008, 135, 37–48. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- Holt, P.G.; Haining, S.; Nelson, D.J.; Sedgwick, J.D. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J. Immunol. 1994, 153, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Liu, K.; Helft, J.; Bogunovic, M.; Greter, M.; Hashimoto, D.; Price, J.; Yin, N.; Bromberg, J.; Lira, S.A.; et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 2009, 206, 3115–3130. [Google Scholar] [CrossRef]
- Segura, E.; Villadangos, J.A. Antigen presentation by dendritic cells in vivo. Curr. Opin. Immunol. 2009, 21, 105–110. [Google Scholar] [CrossRef]
- McGinnis, M.R.; Tyring, S.K. Introduction to Mycology. In Medical Microbiology, 4th ed.; Baron, S., Ed.; The University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Bozza, S.; Gaziano, R.; Spreca, A.; Bacci, A.; Montagnoli, C.; di Francesco, P.; Romani, L. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 2002, 168, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; Pereira da Costa, M.; Reis e Sousa, C. Dendritic Cells Revisited. Annu. Rev. Immunol. 2021, 39, 131–166. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N. Spreading the load: Antigen transfer between migratory and lymph node-resident dendritic cells promotes T-cell priming. Eur. J. Immunol. 2017, 47, 1798–1801. [Google Scholar] [CrossRef]
- Xu, J.; Hissong, R.; Bareis, R.; Creech, A.; Goughenour, K.D.; Freeman, C.M.; Olszewski, M.A. Batf3-dependent orchestration of the robust Th1 responses and fungal control during cryptococcal infection, the role of cDC1. mBio 2024, 15, e0285323. [Google Scholar] [CrossRef]
- Guasconi, L.; Beccacece, I.; Volpini, X.; Burstein, V.L.; Mena, C.J.; Silvane, L.; Almeida, M.A.; Musri, M.M.; Cervi, L.; Chiapello, L.S. Pulmonary Conventional Type 1 Langerin-Expressing Dendritic Cells Play a Role in Impairing Early Protective Immune Response against Cryptococcus neoformans Infection in Mice. J. Fungi 2022, 8, 792. [Google Scholar] [CrossRef]
- Van Prooyen, N.; Henderson, C.A.; Hocking Murray, D.; Sil, A. CD103+ Conventional Dendritic Cells Are Critical for TLR7/9-Dependent Host Defense against Histoplasma capsulatum, an Endemic Fungal Pathogen of Humans. PLoS Pathog. 2016, 12, e1005749. [Google Scholar] [CrossRef]
- Wiesner, D.L.; Specht, C.A.; Lee, C.K.; Smith, K.D.; Mukaremera, L.; Lee, S.T.; Lee, C.G.; Elias, J.A.; Nielsen, J.N.; Boulware, D.R.; et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015, 11, e1004701. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Curtis, J.L.; Polak, T.; Ames, T.; Chen, G.H.; McDonald, R.; Huffnagle, G.B.; Toews, G.B. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J. Immunol. 2008, 181, 610–620. [Google Scholar] [CrossRef]
- Ersland, K.; Wuthrich, M.; Klein, B.S. Dynamic interplay among monocyte-derived, dermal, and resident lymph node dendritic cells during the generation of vaccine immunity to fungi. Cell Host Microbe 2010, 7, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kasahara, S.; Jhingran, A.; Tosini, N.L.; Zhai, B.; Aufiero, M.A.; Mills, K.A.M.; Gjonbalaj, M.; Espinosa, V.; Rivera, A.; et al. During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk. Cell Host Microbe 2020, 28, 104–116.E4. [Google Scholar] [CrossRef]
- Trautwein-Weidner, K.; Gladiator, A.; Kirchner, F.R.; Becattini, S.; Rulicke, T.; Sallusto, F.; LeibundGut-Landmann, S. Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis. PLoS Pathog. 2015, 11, e1005164. [Google Scholar] [CrossRef]
- Teitz-Tennenbaum, S.; Viglianti, S.P.; Roussey, J.A.; Levitz, S.M.; Olszewski, M.A.; Osterholzer, J.J. Autocrine IL-10 Signaling Promotes Dendritic Cell Type-2 Activation and Persistence of Murine Cryptococcal Lung Infection. J. Immunol. 2018, 201, 2004–2015. [Google Scholar] [CrossRef]
- Fei, M.; Bhatia, S.; Oriss, T.B.; Yarlagadda, M.; Khare, A.; Akira, S.; Saijo, S.; Iwakura, Y.; Fallert Junecko, B.A.; Reinhart, T.A.; et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5360–5365. [Google Scholar] [CrossRef]
- Huang, S.; Deepe, G.S., Jr. Notch regulates Histoplasma capsulatum clearance in mouse lungs during innate and adaptive immune response phases in primary infection. J. Leukoc. Biol. 2022, 112, 1137–1154. [Google Scholar] [CrossRef] [PubMed]
- Bacci, A.; Montagnoli, C.; Perruccio, K.; Bozza, S.; Gaziano, R.; Pitzurra, L.; Velardi, A.; d’Ostiani, C.F.; Cutler, J.E.; Romani, L. Dendritic cells pulsed with fungal RNA induce protective immunity to Candida albicans in hematopoietic transplantation. J. Immunol. 2002, 168, 2904–2913. [Google Scholar] [CrossRef] [PubMed]
- Montagnoli, C.; Bacci, A.; Bozza, S.; Gaziano, R.; Fiorucci, S.; Spreca, A.; Romani, L. The plasticity of dendritic cells at the host/fungal interface. Immunobiology 2001, 204, 582–589. [Google Scholar] [CrossRef]
- d’Ostiani, C.F.; Del Sero, G.; Bacci, A.; Montagnoli, C.; Spreca, A.; Mencacci, A.; Ricciardi-Castagnoli, P.; Romani, L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 2000, 191, 1661–1673. [Google Scholar] [CrossRef]
- Mezger, M.; Kneitz, S.; Wozniok, I.; Kurzai, O.; Einsele, H.; Loeffler, J. Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J. Infect. Dis. 2008, 197, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Hohl, T.M.; Collins, N.; Leiner, I.; Gallegos, A.; Saijo, S.; Coward, J.W.; Iwakura, Y.; Pamer, E.G. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 2011, 208, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Stephen-Victor, E.; Karnam, A.; Fontaine, T.; Beauvais, A.; Das, M.; Hegde, P.; Prakhar, P.; Holla, S.; Balaji, K.N.; Kaveri, S.V.; et al. Aspergillus fumigatus Cell Wall alpha-(1,3)-Glucan Stimulates Regulatory T-Cell Polarization by Inducing PD-L1 Expression on Human Dendritic Cells. J. Infect. Dis. 2017, 216, 1281–1294. [Google Scholar] [CrossRef]
- Rivera, A.; Van Epps, H.L.; Hohl, T.M.; Rizzuto, G.; Pamer, E.G. Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia. Infect. Immun. 2005, 73, 7170–7179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hohl, T.M.; Van Epps, H.L.; Rivera, A.; Morgan, L.A.; Chen, P.L.; Feldmesser, M.; Pamer, E.G. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005, 1, e30. [Google Scholar] [CrossRef]
- Heung, L.J.; Wiesner, D.L.; Wang, K.; Rivera, A.; Hohl, T.M. Immunity to fungi in the lung. Semin. Immunol. 2023, 66, 101728. [Google Scholar] [CrossRef]
- Salazar, F.; Brown, G.D. Antifungal Innate Immunity: A Perspective from the Last 10 Years. J. Innate Immun. 2018, 10, 373–397. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, Z.G.; Lee, C.K.; Wang, J.P.; Boon, L.; Specht, C.A.; Levitz, S.M. A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe 2011, 9, 415–424. [Google Scholar] [CrossRef]
- Loures, F.V.; Rohm, M.; Lee, C.K.; Santos, E.; Wang, J.P.; Specht, C.A.; Calich, V.L.; Urban, C.F.; Levitz, S.M. Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps. PLoS Pathog. 2015, 11, e1004643. [Google Scholar] [CrossRef]
- Araujo, E.F.; Medeiros, D.H.; Galdino, N.A.; Condino-Neto, A.; Calich, V.L.; Loures, F.V. Tolerogenic Plasmacytoid Dendritic Cells Control Paracoccidioides brasiliensis Infection by Inducting Regulatory T Cells in an IDO-Dependent Manner. PLoS Pathog. 2016, 12, e1006115. [Google Scholar] [CrossRef]
- Joo, H.; Upchurch, K.; Zhang, W.; Ni, L.; Li, D.; Xue, Y.; Li, X.H.; Hori, T.; Zurawski, S.; Liu, Y.J.; et al. Opposing Roles of Dectin-1 Expressed on Human Plasmacytoid Dendritic Cells and Myeloid Dendritic Cells in Th2 Polarization. J. Immunol. 2015, 195, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Albiston, A.L.; Wicks, I.P.; Chai, S.Y.; Villadangos, J.A. Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20377–20381. [Google Scholar] [CrossRef] [PubMed]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef]
- Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976, 143, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Yang, C.W.; Wang, D.W.; Wu-Hsieh, B.A. Dendritic cells cross-present exogenous fungal antigens to stimulate a protective CD8 T cell response in infection by Histoplasma capsulatum. J. Immunol. 2005, 174, 6282–6291. [Google Scholar] [CrossRef]
- Carvalho, A.; De Luca, A.; Bozza, S.; Cunha, C.; D’Angelo, C.; Moretti, S.; Perruccio, K.; Iannitti, R.G.; Fallarino, F.; Pierini, A.; et al. TLR3 essentially promotes protective class I-restricted memory CD8+ T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood 2012, 119, 967–977. [Google Scholar] [CrossRef]
- Backer, R.; van Leeuwen, F.; Kraal, G.; den Haan, J.M. CD8- dendritic cells preferentially cross-present Saccharomyces cerevisiae antigens. Eur. J. Immunol. 2008, 38, 370–380. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, A.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Ogasawara, M.; Miyashita, M.; Yamagishi, Y.; Ota, S. Phase I/II Pilot Study of Wilms’ Tumor 1 Peptide-Pulsed Dendritic Cell Vaccination Combined with Conventional Chemotherapy in Patients with Head and Neck Cancer. Ther. Apher. Dial. 2019, 23, 279–288. [Google Scholar] [CrossRef]
- Tanyi, J.L.; George, E. Personalized vaccination against ovarian cancer: What are the possibilities? Expert. Rev. Vaccines 2018, 17, 955–958. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Avivi, I.; Vasir, B.; Uhl, L.; Munshi, N.C.; Katz, T.; Dey, B.R.; Somaiya, P.; Mills, H.; Campigotto, F.; et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin. Cancer Res. 2013, 19, 3640–3648. [Google Scholar] [CrossRef]
- Van Tendeloo, V.F.; Van de Velde, A.; Van Driessche, A.; Cools, N.; Anguille, S.; Ladell, K.; Gostick, E.; Vermeulen, K.; Pieters, K.; Nijs, G.; et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl. Acad. Sci. USA 2010, 107, 13824–13829. [Google Scholar] [CrossRef]
- Tel, J.; Aarntzen, E.H.; Baba, T.; Schreibelt, G.; Schulte, B.M.; Benitez-Ribas, D.; Boerman, O.C.; Croockewit, S.; Oyen, W.J.; van Rossum, M.; et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013, 73, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Inacio, M.M.; Moreira, A.L.E.; Cruz-Leite, V.R.M.; Mattos, K.; Silva, L.O.S.; Venturini, J.; Ruiz, O.H.; Ribeiro-Dias, F.; Weber, S.S.; Soares, C.M.A.; et al. Fungal Vaccine Development: State of the Art and Perspectives Using Immunoinformatics. J. Fungi 2023, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Tacken, P.J.; Zeelenberg, I.S.; Cruz, L.J.; van Hout-Kuijer, M.A.; van de Glind, G.; Fokkink, R.G.; Lambeck, A.J.; Figdor, C.G. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood 2011, 118, 6836–6844. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.S.; Huang, H.; Levitz, S.M. Effect of differential N-linked and O-linked mannosylation on recognition of fungal antigens by dendritic cells. PLoS ONE 2007, 2, e1009. [Google Scholar] [CrossRef]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio 2010, 1, e00164-10. [Google Scholar] [CrossRef]
- Carter, R.W.; Thompson, C.; Reid, D.M.; Wong, S.Y.; Tough, D.F. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J. Immunol. 2006, 177, 2276–2284. [Google Scholar] [CrossRef]
- Dutta, O.; Masso-Silva, J.A.; Wang, K.; Rivera, A. Host response to pulmonary fungal infections: A highlight on cell-driven immunity to Cryptococcus species and Aspergillus fumigatus. Curr. Pharmacol. Rep. 2017, 3, 335–345. [Google Scholar] [CrossRef]
- Aimanianda, V.; Bayry, J.; Bozza, S.; Kniemeyer, O.; Perruccio, K.; Elluru, S.R.; Clavaud, C.; Paris, S.; Brakhage, A.A.; Kaveri, S.V.; et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009, 460, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-associated fungal infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 2004, 42, 4419–4431. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Lodge, J.; Xue, C. Harnessing the Immune Response to Fungal Pathogens for Vaccine Development. Annu. Rev. Microbiol. 2022, 76, 703–726. [Google Scholar] [CrossRef]
- Oliveira, L.V.N.; Wang, R.; Specht, C.A.; Levitz, S.M. Vaccines for human fungal diseases: Close but still a long way to go. NPJ Vaccines 2021, 6, 33. [Google Scholar] [CrossRef]
- Bozza, S.; Gaziano, R.; Lipford, G.B.; Montagnoli, C.; Bacci, A.; Di Francesco, P.; Kurup, V.P.; Wagner, H.; Romani, L. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 2002, 4, 1281–1290. [Google Scholar] [CrossRef]
- Bozza, S.; Perruccio, K.; Montagnoli, C.; Gaziano, R.; Bellocchio, S.; Burchielli, E.; Nkwanyuo, G.; Pitzurra, L.; Velardi, A.; Romani, L. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 2003, 102, 3807–3814. [Google Scholar] [CrossRef]
- Shao, C.; Qu, J.; He, L.; Zhang, Y.; Wang, J.; Zhou, H.; Wang, Y.; Liu, X. Dendritic cells transduced with an adenovirus vector encoding interleukin-12 are a potent vaccine for invasive pulmonary aspergillosis. Genes. Immun. 2005, 6, 103–114. [Google Scholar] [CrossRef]
- Perruccio, K.; Bozza, S.; Montagnoli, C.; Bellocchio, S.; Aversa, F.; Martelli, M.; Bistoni, F.; Velardi, A.; Romani, L. Prospects for dendritic cell vaccination against fungal infections in hematopoietic transplantation. Blood Cells Mol. Dis. 2004, 33, 248–255. [Google Scholar] [CrossRef]
- Awasthi, S. Dendritic cell-based vaccine against coccidioides infection. Ann. N. Y. Acad. Sci. 2007, 1111, 269–274. [Google Scholar] [CrossRef]
- Chechi, J.L.; da Costa, F.A.C.; Figueiredo, J.M.; de Souza, C.M.; Valdez, A.F.; Zamith-Miranda, D.; Camara, A.C.; Taborda, C.P.; Nosanchuk, J.D. Vaccine development for pathogenic fungi: Current status and future directions. Expert. Rev. Vaccines 2023, 22, 1136–1153. [Google Scholar] [CrossRef]
- Zou, G.; Wei, Y. World Health Organization’s first-ever release of a fungal priority pathogens list: A reply action proposal for the prevention and treatment of fungal pathogens. Eco-Environ. Health 2023, 2, 43–44. [Google Scholar] [CrossRef]
- Liu, W.; Li, R.Y. Enlightenment of World Health Organization fungal priority pathogens list. Zhonghua Liu Xing Bing Xue Za Zhi 2023, 44, 1984–1987. [Google Scholar] [CrossRef] [PubMed]
- Sondermeyer, G.L.; Lee, L.A.; Gilliss, D.; Vugia, D.J. Coccidioidomycosis-Associated Deaths in California, 2000–2013. Public Health Rep. 2016, 131, 531–535. [Google Scholar] [CrossRef]
- Loh, J.T.; Lam, K.P. Fungal infections: Immune defense, immunotherapies and vaccines. Adv. Drug Deliv. Rev. 2023, 196, 114775. [Google Scholar] [CrossRef] [PubMed]
- Peyclit, L.; Yousfi, H.; Rolain, J.M.; Bittar, F. Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi. Pharmaceuticals 2021, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Nicola, A.M.; Albuquerque, P.; Paes, H.C.; Fernandes, L.; Costa, F.F.; Kioshima, E.S.; Abadio, A.K.R.; Bocca, A.L.; Felipe, M.S. Antifungal drugs: New insights in research & development. Pharmacol. Ther. 2019, 195, 21–38. [Google Scholar] [CrossRef]
- Awasthi, S.; Awasthi, V.; Magee, D.M.; Coalson, J.J. Efficacy of antigen 2/proline-rich antigen cDNA-transfected dendritic cells in immunization of mice against Coccidioides posadasii. J. Immunol. 2005, 175, 3900–3906. [Google Scholar] [CrossRef]
- Awasthi, S.; Vilekar, P.; Conkleton, A.; Rahman, N. Dendritic cell-based immunization induces Coccidioides Ag2/PRA-specific immune response. Vaccine 2019, 37, 1685–1691. [Google Scholar] [CrossRef]
- Awasthi, S. Intranasal Antifungal Vaccination Using DNA-Transfected Dendritic Cells. Methods Mol. Biol. 2017, 1625, 75–83. [Google Scholar] [CrossRef]
- Vilekar, P.; Awasthi, V.; Lagisetty, P.; King, C.; Shankar, N.; Awasthi, S. In vivo trafficking and immunostimulatory potential of an intranasally-administered primary dendritic cell-based vaccine. BMC Immunol. 2010, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.B.R.; Taborda, C.P.; Nosanchuk, J.D. Advances in Fungal Peptide Vaccines. J. Fungi 2020, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.; Silva, M.B.; Laurenti, R.; Travassos, L.R.; Taborda, C.P. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: A review from 1996 to 2006. Memórias Do Inst. Oswaldo Cruz 2009, 104, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.S.; Bastos, K.R.; Russo, M.; Almeida, S.R. Interaction between Paracoccidioides brasiliensis and pulmonary dendritic cells induces interleukin-10 production and toll-like receptor-2 expression: Possible mechanisms of susceptibility. J. Infect. Dis. 2007, 196, 1108–1115. [Google Scholar] [CrossRef][Green Version]
- Silva, L.B.R.; Taira, C.L.; Cleare, L.G.; Martins, M.; Junqueira, M.; Nosanchuk, J.D.; Taborda, C.P. Identification of Potentially Therapeutic Immunogenic Peptides from Paracoccidioides lutzii Species. Front. Immunol. 2021, 12, 670992. [Google Scholar] [CrossRef]
- Travassos, L.R.; Taborda, C.P. Linear Epitopes of Paracoccidioides brasiliensis and Other Fungal Agents of Human Systemic Mycoses As Vaccine Candidates. Front. Immunol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Jannuzzi, G.P.; de Almeida, J.R.F.; Dos Santos, S.S.; de Almeida, S.R.; Ferreira, K.S. Notch Signaling is Required for Dendritic Cell Maturation and T Cell Expansion in Paracoccidioidomycosis. Mycopathologia 2018, 183, 739–749. [Google Scholar] [CrossRef]
- Travassos, L.R.; Taborda, C.P. New advances in the development of a vaccine against paracoccidioidomycosis. Front. Microbiol. 2012, 3, 212. [Google Scholar] [CrossRef]
- Magalhaes, A.; Ferreira, K.S.; Almeida, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis. Clin. Vaccine Immunol. 2012, 19, 23–29. [Google Scholar] [CrossRef]
- Silva, L.B.R.; Taira, C.L.; Dias, L.S.; Souza, A.C.O.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Experimental Therapy of Paracoccidioidomycosis Using P10-Primed Monocyte-Derived Dendritic Cells Isolated From Infected Mice. Front. Microbiol. 2019, 10, 1727. [Google Scholar] [CrossRef]
- Silva, L.B.R.; Dias, L.S.; Rittner, G.M.G.; Munoz, J.E.; Souza, A.C.O.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Dendritic Cells Primed with Paracoccidioides brasiliensis Peptide P10 Are Therapeutic in Immunosuppressed Mice with Paracoccidioidomycosis. Front. Microbiol. 2017, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, J.; Escandon, P.; Agudelo, C.I.; Firacative, C.; Meyer, W.; Castaneda, E. Retrospective study of the epidemiology and clinical manifestations of Cryptococcus gattii infections in Colombia from 1997–2011. PLoS Negl. Trop. Dis. 2014, 8, e3272. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Emergence of Cryptococcus gattii—Pacific Northwest, 2004–2010. Morb. Mortal. Wkly. Rep. (MMWR) 2010, 59, 865–868. [Google Scholar]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef]
- Smith, R.M.; Mba-Jonas, A.; Tourdjman, M.; Schimek, T.; DeBess, E.; Marsden-Haug, N.; Harris, J.R. Treatment and outcomes among patients with Cryptococcus gattii infections in the United States Pacific Northwest. PLoS ONE 2014, 9, e88875. [Google Scholar] [CrossRef]
- Ueno, K.; Kinjo, Y.; Okubo, Y.; Aki, K.; Urai, M.; Kaneko, Y.; Shimizu, K.; Wang, D.N.; Okawara, A.; Nara, T.; et al. Dendritic cell-based immunization ameliorates pulmonary infection with highly virulent Cryptococcus gattii. Infect. Immun. 2015, 83, 1577–1586. [Google Scholar] [CrossRef]
- Goughenour, K.D.; Nair, A.S.; Xu, J.; Olszewski, M.A.; Wozniak, K.L. Dendritic Cells: Multifunctional Roles in Host Defenses to Cryptococcus Infections. J. Fungi 2023, 9, 1050. [Google Scholar] [CrossRef]
- Hole, C.R.; Wager, C.M.L.; Castro-Lopez, N.; Campuzano, A.; Cai, H.; Wozniak, K.L.; Wang, Y.; Wormley, F.L., Jr. Induction of memory-like dendritic cell responses in vivo. Nat. Commun. 2019, 10, 2955. [Google Scholar] [CrossRef]
- Nami, S.; Mohammadi, R.; Vakili, M.; Khezripour, K.; Mirzaei, H.; Morovati, H. Fungal vaccines, mechanism of actions and immunology: A comprehensive review. Biomed. Pharmacother. 2019, 109, 333–344. [Google Scholar] [CrossRef]
- Caballero Van Dyke, M.C.; Wormley, F.L., Jr. A Call to Arms: Quest for a Cryptococcal Vaccine. Trends Microbiol. 2018, 26, 436–446. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Ravi, S.; Macias, S.; Young, M.L.; Olszewski, M.A.; Steele, C.; Wormley, F.L. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PLoS ONE 2009, 4, e6854. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Urai, M.; Sadamoto, S.; Shinozaki, M.; Takatsuka, S.; Abe, M.; Otani, Y.; Yanagihara, N.; Shimizu, K.; Iwakura, Y.; et al. A dendritic cell-based systemic vaccine induces long-lived lung-resident memory Th17 cells and ameliorates pulmonary mycosis. Mucosal Immunol. 2019, 12, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Chaze, T.; Miranda, K.; Roberson, R.W.; Gorgette, O.; Nimrichter, L.; Matondo, M.; Latge, J.P.; Beauvais, A.; Rodrigues, M.L. Characterization of Extracellular Vesicles Produced by Aspergillus fumigatus Protoplasts. mSphere 2020, 5, e00476-20. [Google Scholar] [CrossRef]
- Marina, C.L.; Burgel, P.H.; Agostinho, D.P.; Zamith-Miranda, D.; Las-Casas, L.O.; Tavares, A.H.; Nosanchuk, J.D.; Bocca, A.L. Nutritional Conditions Modulate C. neoformans Extracellular Vesicles’ Capacity to Elicit Host Immune Response. Microorganisms 2020, 8, 1815. [Google Scholar] [CrossRef] [PubMed]
- Joffe, L.S.; Nimrichter, L.; Rodrigues, M.L.; Del Poeta, M. Potential Roles of Fungal Extracellular Vesicles during Infection. mSphere 2016, 1, e00099-16. [Google Scholar] [CrossRef]
- Sandbrink, J.B.; Shattock, R.J. RNA Vaccines: A Suitable Platform for Tackling Emerging Pandemics? Front. Immunol. 2020, 11, 608460. [Google Scholar] [CrossRef]
- Oda, Y.; Kumagai, Y.; Kanai, M.; Iwama, Y.; Okura, I.; Minamida, T.; Yagi, Y.; Kurosawa, T.; Greener, B.; Zhang, Y.; et al. Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: A double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet Infect. Dis. 2024, 24, 351–360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, N.A.; Nanjappa, S.G. Advances in Dendritic-Cell-Based Vaccines against Respiratory Fungal Infections. Vaccines 2024, 12, 981. https://doi.org/10.3390/vaccines12090981
Kulkarni NA, Nanjappa SG. Advances in Dendritic-Cell-Based Vaccines against Respiratory Fungal Infections. Vaccines. 2024; 12(9):981. https://doi.org/10.3390/vaccines12090981
Chicago/Turabian StyleKulkarni, Nitish A., and Som G. Nanjappa. 2024. "Advances in Dendritic-Cell-Based Vaccines against Respiratory Fungal Infections" Vaccines 12, no. 9: 981. https://doi.org/10.3390/vaccines12090981
APA StyleKulkarni, N. A., & Nanjappa, S. G. (2024). Advances in Dendritic-Cell-Based Vaccines against Respiratory Fungal Infections. Vaccines, 12(9), 981. https://doi.org/10.3390/vaccines12090981






