Safety and Immunogenicity of Trivalent Oral Polio Vaccine in Vaccinated Children and Vaccine-Naïve Infants: A Phase 4 Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Vaccine and Immunization Procedures
2.3. Study Assessments
2.4. Immunogenicity Assessments
2.5. Safety Assessments
2.6. Statistical Analysis
2.7. Ethics
3. Results
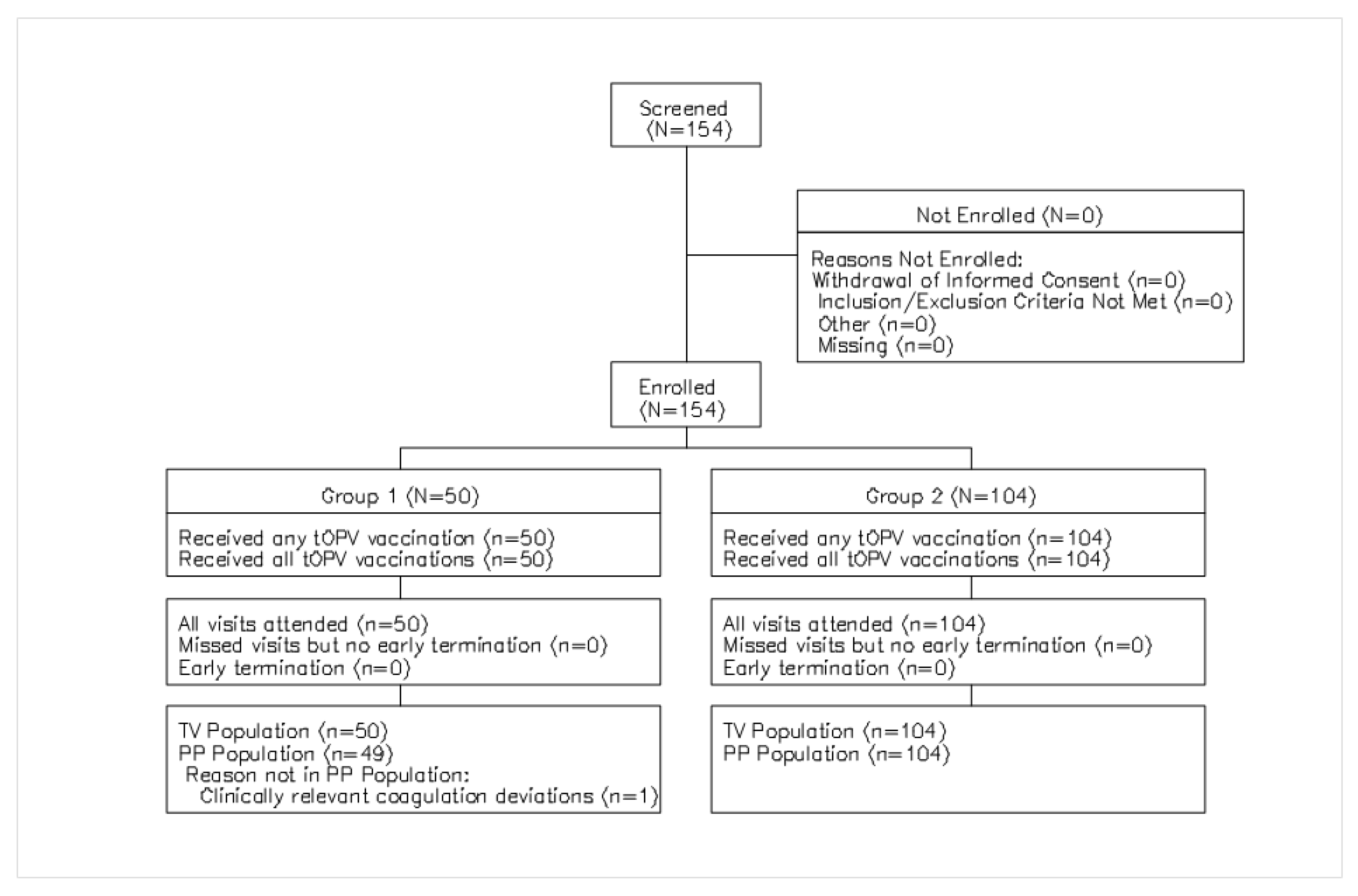
3.1. Study Subjects
3.1.1. Safety Results
3.1.2. Immunogenicity Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Polio Eradication Initiative. Available online: https://polioeradication.org/polio-today/history-of-polio/ (accessed on 8 April 2024).
- World Health Organization. Polio Eradication Strategy 2022–2026: Delivering on a Promise; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Fomban Leke, R.G.; King, A.; Pallansch, M.A.; Tangermann, R.H.; Mkanda, P.; Chunsuttiwat, S.; Jack, A.; Kaboré, B.J.; Kane, I.; Khomo, N.E.; et al. Certifying the Interruption of Wild Poliovirus Transmission in the WHO African Region on the Turbulent Journey to a Polio-Free World. Lancet Glob. Health 2020, 8, e1345–e1351. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Kumar, R.; Menabde, N.; Thapa, A.; McFarland, J.; Swezy, V.; Tangermann, R.H.; Jafari, H.S.; Elsner, L.; Wassilak, S.G.F.; et al. Polio-Free Certification and Lessons Learned—South-East Asia Region, March 2014. MMWR Morb. Mortal. Wkly Rep. 2014, 63, 941–946. [Google Scholar] [PubMed]
- World Health Organization. Countries Reaffirm Commitment to Ending Polio at Launch of New Eradication Strategy. Available online: https://www.who.int/news/item/10-06-2021-countries-reaffirm-commitment-to-ending-polio-at-launch-of-new-eradication-strategy (accessed on 6 June 2024).
- World Health Organization. Polio Vaccines: WHO Position Paper. Wkly. Epidemiol. Rec. 2016, 91, 145–168. [Google Scholar]
- Moffett, D.B.; Llewellyn, A.; Singh, H.; Saxentoff, E.; Partridge, J.; Boualam, L.; Pallansch, M.; Wassilak, S.; Asghar, H.; Roesel, S.; et al. Progress Toward Poliovirus Containment Implementation—Worldwide, 2019–2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Modlin, J.F.; Wenger, J.; Gast, C. Immunogenicity of New Primary Immunization Schedules with Inactivated Poliovirus Vaccine and Bivalent Oral Polio Vaccine for the Polio Endgame: A Review. Clin. Infect. Dis. 2018, 67 (Suppl. 1), S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Global Polio Eradication Initiative. Key-Points-about-Poliovirus-Containment. 2023. Available online: https://polioeradication.org/polio-today/preparing-for-a-polio-free-world/containment/#:~:text=Containment%20includes%20biosafety%20and%20biosecurity,being%20released%20into%20the%20community. (accessed on 30 July 2024).
- Bigouette, J.P.; Henderson, E.; Traoré, M.A.; Wassilak, S.G.F.; Jorba, J.; Mahoney, F.; Bolu, O.; Diop, O.M.; Burns, C.C. Update on Vaccine-Derived Poliovirus Outbreaks—Worldwide, January 2021–December 2022. Morb. Mortal. Wkly. Rep. 2023, 72, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, V.; Quddus, A.; Pallansch, M.; Zipursky, S.; Woods, D.; Ottosen, A.; Vertefeuille, J.; Lewis, I. Global Oral Poliovirus Vaccine Stockpile Management as an Essential Preparedness and Response Mechanism for Type 2 Poliovirus Outbreaks Following Global Oral Poliovirus Vaccine Type 2 Withdrawal. Vaccine 2023, 41, A70–A78. [Google Scholar] [CrossRef] [PubMed]
- Blake, I.M.; Pons-Salort, M.; Molodecky, N.A.; Diop, O.M.; Chenoweth, P.; Bandyopadhyay, A.S.; Zaffran, M.; Sutter, R.W.; Grassly, N.C. Type 2 Poliovirus Detection after Global Withdrawal of Trivalent Oral Vaccine. N. Engl. J. Med. 2018, 379, 834–845. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). First Ever Vaccine Listed under WHO Emergency Use. Available online: https://www.who.int/news/item/13-11-2020-first-ever-vaccine-listed-under-who-emergency-use (accessed on 11 August 2024).
- Konopka-Anstadt, J.L.; Campagnoli, R.; Vincent, A.; Shaw, J.; Wei, L.; Wynn, N.T.; Smithee, S.E.; Bujaki, E.; Te Yeh, M.; Laassri, M.; et al. Development of a New Oral Poliovirus Vaccine for the Eradication End Game Using Codon Deoptimization. NPJ Vaccines 2020, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.T.; Smith, M.; Carlyle, S.; Konopka-Anstadt, J.L.; Burns, C.C.; Konz, J.; Andino, R.; Macadam, A. Genetic Stabilization of Attenuated Oral Vaccines against Poliovirus Types 1 and 3. Nature 2023, 619, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Llorens, X.; Bandyopadhyay, A.S.; Gast, C.; Leon, T.D.; DeAntonio, R.; Jimeno, J.; Caballero, M.I.; Aguirre, G.; Oberste, M.S.; Weldon, W.C.; et al. Safety and Immunogenicity of Two Novel Type 2 Oral Poliovirus Vaccine Candidates Compared with a Monovalent Type 2 Oral Poliovirus Vaccine in Children and Infants: Two Clinical Trials. Lancet 2021, 397, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Costa Clemens, S.A.; Santos, G.M.L.; Gonzalez, I.; Clemens, R. The Role of a Genetically Stable, Novel Oral Type 2 Poliovirus Vaccine in the Poliomyelitis Endgame. Rev. Panam. De Salud Pública 2023, 47, e99. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.E.; Asturias, E.J.; Balakrishnan, M.R.; Halsey, N.A.; Law, B.; Zuber, P.L.F. Assessment of Causality of Individual Adverse Events Following Immunization (AEFI): A WHO Tool for Global Use. Vaccine 2013, 31, 5041–5046. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yang, Y.; Huang, L.; Wang, L.; Jiang, Z.; Gong, J.; Wang, W.; Wang, H.; Guo, S.; Li, C.; et al. Immunogenicity and Safety Evaluation of Bivalent Types 1 and 3 Oral Poliovirus Vaccine by Comparing Different Poliomyelitis Vaccination Schedules in China: A Randomized Controlled Non-Inferiority Clinical Trial. Hum. Vaccin. Immunother. 2017, 13, 1359–1368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patriarca, P.A.; Wright, P.F.; John, T.J. Factors Affecting the Immunogenicity of Oral Poliovirus Vaccine in Developing Countries: Review. Rev. Infect Dis. 1991, 13, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, S.; Kim, D.R.; Haldar, B.; Snider, C.; Nalavade, U.; Kim, S.A.; Park, J.Y.; Sinha, A.; Mallick, A.H.; Manna, B.; et al. Comparison of IPV to TOPV Week 39 Boost of Primary OPV Vaccination in Indian Infants: An Open Labelled Randomized Controlled Trial. Heliyon 2017, 3, e00223. [Google Scholar] [CrossRef] [PubMed]
- Modlin, J.F.; Bandyopadhyay, A.S.; Sutter, R. Immunization Against Poliomyelitis and the Challenges to Worldwide Poliomyelitis Eradication. J. Infect Dis. 2021, 224 (Suppl. 4), S398–S404. [Google Scholar] [CrossRef] [PubMed]
- PATH. Advancing Novel Oral Polio Vaccines: Planning for Long–Term Protection against Polio Resurgence. PATH: Seattle January 2024. Available online: https://www.path.org/our-impact/resources/advancing-novel-oral-polio-vaccines-planning-for-long-term-protection-against-polio-resurgence/ (accessed on 6 June 2024).
- Containment Advisory Group. Report of CAG TC3 on NOPV2 Candidate Vaccines and S19- Poliovirus Type 2 Strains 7 June 2018. 2018. Available online: https://polioeradication.org/wp-content/uploads/2017/08/CAG-TC3-20180630-EN.pdf (accessed on 7 May 2024).
| Characteristic | Children (Group 1) | Infants (Group 2) | ||
|---|---|---|---|---|
| (N = 50) | (N = 104) | |||
| Gender | Male | n (%) | 27 (54.0) | 48 (46.2) |
| Female | n (%) | 23 (46.0) | 56 (53.8) | |
| Age in weeks | Mean (SD) | 148.3 (59.62) | 5.8 (0.80) | |
| Median (Q1, Q3) | 153.0 (101.0, 193.0) | 6.0 (5.0, 6.0) | ||
| Range (min, max) | (53, 257) | (5, 8) | ||
| Ethnicity | Hispanic | n (%) | 50 (100) | 104 (100) |
| Body weight [kg] | N | 50 | 104 | |
| Mean (SD) | 14.1 (3.02) | 4.9 (0.51) | ||
| Median (Q1, Q3) | 14.2 (12.2, 16.4) | 4.9 (4.5, 5.3) | ||
| Range (min, max) | (8.1, 20.0) | (3.7, 6.3) | ||
| Solicited AE | Causally Associated 1 | Severity 2,3 | Group 1 Days 0–7 (N = 50) | Group 2 Days 0–7 (N = 104) | Group 2 Days 28–35 (N = 104) | Group 2 Days 56–63 (N = 104) | Group 2 Total (N = 104) | |
|---|---|---|---|---|---|---|---|---|
| All | Total | Any | n (%) m | 7 (14.0) 9 | 27 (26.0) 43 | 18 (17.3) 29 | 7 (6.7) 15 | 39 (37.5) 87 |
| Causally associated | Any | n (%) m | 0 (0.0) 0 | 7 (6.7) 8 | 4 (3.8) 6 | 3 (2.9) 4 | 12 (11.5) 18 | |
| Loss of appetite | Total | Any | n (%) m | 2 (4.0) 2 | 5 (4.8) 5 | 1 (1.0) 1 | 2 (1.9) 2 | 7 (6.7) 8 |
| Severe | n (%) m | 0 (0.0) 0 | 1 (1.0) 1 | 0 (0.0) 0 | 0 (0.0) 0 | 1 (1.0) 1 | ||
| Causally associated | Any | n (%) m | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | |
| Severe | n (%) m | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | ||
| Abnormal Crying | Total | Any | n (%) m | 1 (2.0) 1 | 7 (6.7) 7 | 6 (5.8) 8 | 5 (4.8) 5 | 15 (14.4) 20 |
| Severe | n (%) m | 0 (0.0) 0 | 1 (1.0) 1 | 0 (0.0) 0 | 1 (1.0) 1 | 2 (1.9) 2 | ||
| Causally associated | Any | n (%) m | 0 (0.0) 0 | 2 (1.9) 2 | 3 (2.9) 4 | 2 (1.9) 2 | 6 (5.8) 8 | |
| Severe | n (%) m | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 1 (1.0) 1 | 1 (1.0) 1 | ||
| Drowsiness | Total | Any | n (%) m | 0 (0.0) 0 | 2 (1.9) 2 | 1 (1.0) 1 | 2 (1.9) 2 | 4 (3.8) 5 |
| Severe | n (%) m | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | ||
| Causally associated | Any | n (%) m | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 1 (1.0) 1 | 1 (1.0) 1 | |
| Severe | n (%) m | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | ||
| Fever | Total | Any | n (%) m | 5 (10.0) 5 | 5 (4.8) 6 | 8 (7.7) 9 | 2 (1.9) 2 | 13 (12.5) 17 |
| Severe | n (%) m | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | ||
| Causally associated | Any | n (%) m | 0 (0.0) 0 | 1 (1.0) 1 | 1 (1.0) 1 | 0 (0.0) 0 | 2 (1.9) 2 | |
| Severe | n (%) m | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | ||
| Irritability | Total | Any | n (%) m | 0 (0.0) 0 | 6 (5.8) 6 | 3 (2.9) 3 | 3 (2.9) 3 | 9 (8.7) 12 |
| Severe | n (%) m | 0 (0.0) 0 | 2 (1.9) 2 | 0 (0.0) 0 | 0 (0.0) 0 | 2 (1.9) 2 | ||
| Causally associated | Any | n (%) m | 0 (0.0) 0 | 1 (1.0) 1 | 1 (1.0) 1 | 0 (0.0) 0 | 2 (1.9) 2 | |
| Severe | n (%) m | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | 0 (0.0) 0 | ||
| Vomiting | Total | Any | n (%) m | 1 (2.0) 1 | 16 (15.4) 17 | 5 (4.8) 7 | 1 (1.0) 1 | 17 (16.3) 25 |
| Severe | n (%) m | 0 (0.0) 0 | 2 (1.9) 2 | 0 (0.0) 0 | 0 (0.0) 0 | 2 (1.9) 2 | ||
| Causally associated | Any | n (%) m | 0 (0.0) 0 | 4 (3.8) 4 | 0 (0.0) 0 | 1 (1.0) 1 | 4 (3.8) 5 | |
| Severe | n (%) m | 0 (0.0) 0 | 1 (1.0) 1 | 0 (0.0) 0 | 0 (0.0) 0 | 1 (1.0) 1 | ||
| Day | Type | Group 1 | Group 2 | |
|---|---|---|---|---|
| (N = 49) | (N = 104) | |||
| 0 | 1 | N | 49 | 104 |
| Median (log2) (LCI, UCI) | 8.2 (7.2, 9.8) | 4.2 (3.2, 5.5) | ||
| GMT (LCI, UCI) | 200.9 (116.2, 343.8) | 35.9 (24.7, 53.2) | ||
| 2 | N | 49 | 104 | |
| Median (log2) (LCI, UCI) | 7.8 (7.2, 8.8) | 3.8 (3.2, 4.2) | ||
| GMT (LCI, UCI) | 251.3 (174.0, 360.4) | 16.4 (13.2, 20.6) | ||
| 3 | N | 49 | 104 | |
| Median (log2) (LCI, UCI) | 6.5 (5.8, 7.5) | 2.5 (2.5, 2.5) | ||
| GMT (LCI, UCI) | 77.5 (51.2, 116.8) | 8.5 (7.3, 10.0) | ||
| 28 | 1 | N | 49 | 0 |
| Median (log2) (LCI, UCI) | 10.5 (9.17, 10.50) | NC (NC, NC) | ||
| GMT (LCI, UCI) | 445.5 (272.7, 710.6) | NC (NC, NC) | ||
| 2 | N | 49 | 0 | |
| Median (log2) (LCI, UCI) | 9.8 (9.5, 10.5) | NC (NC, NC) | ||
| GMT (LCI, UCI) | 681.2 (511.1, 882.9) | NC (NC, NC) | ||
| 3 | N | 49 | 0 | |
| Median (log2) (LCI, UCI) | 8.5 (7.8, 9.5) | NC (NC, NC) | ||
| GMT (LCI, UCI) | 306.3 (201.7, 452.0) | NC (NC, NC) | ||
| 84 | 1 | N | 0 | 104 |
| Median (log2) (LCI, UCI) | NC (NC, NC) | 10.5 (10.5, 10.5) | ||
| GMT (LCI, UCI) | NC (NC, NC) | 650.8 (473.7, 870.6) | ||
| 2 | N | 0 | 104 | |
| Median (log2) (LCI, UCI) | NC (NC, NC) | 10.2 (9.8, 10.2) | ||
| GMT (LCI, UCI) | NC (NC, NC) | 712.2 (594.4, 840.3) | ||
| 3 | N | 0 | 104 | |
| Median (log2) (LCI, UCI) | NC (NC, NC) | 8.8 (8.5, 9.5) | ||
| GMT (LCI, UCI) | NC (NC, NC) | 352.1 (263.9, 462.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía, L.R.; Mendez, L.P.; Rüttimann, R.W.; Gast, C.; Bandyopadhyay, A.S. Safety and Immunogenicity of Trivalent Oral Polio Vaccine in Vaccinated Children and Vaccine-Naïve Infants: A Phase 4 Study. Vaccines 2024, 12, 953. https://doi.org/10.3390/vaccines12090953
Mejía LR, Mendez LP, Rüttimann RW, Gast C, Bandyopadhyay AS. Safety and Immunogenicity of Trivalent Oral Polio Vaccine in Vaccinated Children and Vaccine-Naïve Infants: A Phase 4 Study. Vaccines. 2024; 12(9):953. https://doi.org/10.3390/vaccines12090953
Chicago/Turabian StyleMejía, Luis Rivera, Lourdes Peña Mendez, Ricardo W. Rüttimann, Chris Gast, and Ananda Sankar Bandyopadhyay. 2024. "Safety and Immunogenicity of Trivalent Oral Polio Vaccine in Vaccinated Children and Vaccine-Naïve Infants: A Phase 4 Study" Vaccines 12, no. 9: 953. https://doi.org/10.3390/vaccines12090953
APA StyleMejía, L. R., Mendez, L. P., Rüttimann, R. W., Gast, C., & Bandyopadhyay, A. S. (2024). Safety and Immunogenicity of Trivalent Oral Polio Vaccine in Vaccinated Children and Vaccine-Naïve Infants: A Phase 4 Study. Vaccines, 12(9), 953. https://doi.org/10.3390/vaccines12090953






