Using Vaccine Safety Data to Demonstrate the Potential of Pooled Data Analysis
Abstract
1. Introduction
Our Approach
2. Materials and Methods
2.1. Scenario Assumptions
2.2. Analyses for Other Specific Scenarios
3. Results
3.1. Scenario 1: Myocarditis
3.2. Scenario 2: Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT)
4. Discussion
4.1. Limitations
4.2. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Government of Canada. Canadian Immunization Registry Functional Standards 2020 to 2024: Recommendations from the Canadian Immunization Registry and Coverage Network. 2023. Available online: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/canadian-immunization-registry-functional-standards-2020-2024.html (accessed on 22 January 2024).
- Government of Canada SC. Profile Table, Census Profile, 2021 Census of Population—Canada. 2022. Available online: https://www12.statcan.gc.ca/census-recensement/2021/dp-pd/prof/index.cfm?Lang=E (accessed on 22 January 2024).
- Government of Canada. Highlights from the 2021 Childhood National Immunization Coverage Survey (cNICS). 2023. Available online: https://www.canada.ca/en/public-health/services/immunization-vaccines/vaccination-coverage/2021-highlights-childhood-national-immunization-coverage-survey.html (accessed on 22 January 2024).
- Government of Canada. Vaccine Uptake in Canadian Adults 2021. 2022. Available online: https://www.canada.ca/en/public-health/services/immunization-vaccines/vaccination-coverage/highlights-2020-2021-seasonal-influenza-survey/full-report.html (accessed on 22 January 2024).
- Government of Canada. Seasonal Influenza Vaccination Coverage in Canada, 2021–2022: Full Report. 2022. Available online: https://www.canada.ca/en/public-health/services/immunization-vaccines/vaccination-coverage/seasonal-influenza-survey-results-2021-2022/full-report.html (accessed on 22 January 2024).
- Ontario Agency for Health Protection and Promotion (Public Health Ontario). Myocarditis and Pericarditis after COVID-19 mRNA Vaccines. Queen’s Printer for Ontario. 2022. Available online: https://www.publichealthontario.ca/-/media/documents/ncov/vaccines/2021/11/myocarditis-pericarditis-mrna-vaccines.pdf?sc_lang=en (accessed on 22 January 2024).
- Husby, A.; Gulseth, H.L.; Hovi, P.; Hansen, J.V.; Pihlström, N.; Gunnes, N.; Härkänen, T.; Dahl, J.; Karlstad, Ø.; Heliö, T.; et al. Clinical outcomes of myocarditis after SARS-CoV-2 mRNA vaccination in four Nordic countries: Population based cohort study. BMJ Med. 2023, 2, e000373. [Google Scholar] [CrossRef] [PubMed]
- Farrington, P.; Whitaker, H.; Weldeselassie, Y.G. Self-Controlled Case Series Studies: A Modelling Guide with R, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Whitaker, H.J.; Paddy Farrington, C.; Spiessens, B.; Musonda, P. Tutorial in biostatistics: The self-controlled case series method. Stat. Med. 2006, 25, 1768–1797. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Chan, B.; Stall, N.M.; Grill, A.; Ivers, N.; Maltsev, A.; Miller, K.J.; Odutayo, A.; Razak, F.; Schull, M.; et al. Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) Following Adenovirus Vector COVID-19 Vaccination. Ontario COVID-19 Science Advisory Table. Sci. Briefs Ont. COVID-19 Sci. Advis. Table 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Higgins, H.; Andrews, N.; Stowe, J.; Amirthalingam, G.; Ramsay, M.; Bahra, G.; Hackett, A.; Breen, K.A.; Desborough, M.; Khan, D.; et al. Risk of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination prior to the recognition of vaccine-induced thrombocytopenia and thrombosis: A self-controlled case series study in England. Res. Pract. Thromb. Haemost. 2022, 6, e12698. [Google Scholar] [CrossRef] [PubMed]
- Weldeselassie, Y.; Whitaker, H.; Farrington, P. SCCS: The Self-Controlled Case-Series Method. Available online: https://CRAN.R-project.org/package=SCCS (accessed on 22 January 2024).
- Hawken, S. Time to Detect a Safety Signal in an SCCS Surveillance Analysis. 2023. Available online: https://stevenhawken.shinyapps.io/sccs_time_to_detect/ (accessed on 22 January 2024).
- Xavier-Carter, B. Ontario Tops 50,000 Vaccine Doses Administered on One Day for First Time. Toronto Star. 2021. Available online: https://www.thestar.com/news/gta/ontario-tops-50-000-vaccine-doses-administered-on-one-day-for-first-time/article_ac657ec9-a366-5bc4-b213-4ffe2daa9b48.html (accessed on 22 January 2024).
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Fritzell, B. Detection of Adverse Events: What are the Current Sensitivity Limits during Clinical Development? Vaccine 2001, 20, S47–S48. [Google Scholar] [CrossRef]
- Naveed, Z.; Li, J.; Spencer, M.; Wilton, J.; Naus, M.; García, H.A.V.; Otterstatter, M.; Janjua, N.Z. Observed versus expected rates of myocarditis after SARS-CoV-2 vaccination: A population-based cohort study. CMAJ 2022, 194, E1529–E1536. [Google Scholar] [CrossRef] [PubMed]
- News CBC. “Mild Risk” Prompts Ontario to Recommend Pfizer over Moderna for Those Aged 18–24 | CBC News. CBC. 2021. Available online: https://www.cbc.ca/news/canada/toronto/covid-19-ontario-september-29-moore-briefing-update-1.6193455 (accessed on 22 January 2024).
- Barker CIS, Snape MD. Pandemic influenza A H1N1 vaccines and narcolepsy: Vaccine safety surveillance in action. Lancet Infect. Diseases 2014, 14, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Cauchemez, S.; Hayden, F.G. “Prepandemic” Immunization for Novel Influenza Viruses, “Swine Flu” Vaccine, Guillain-Barré Syndrome, and the Detection of Rare Severe Adverse Events. J. Infect. Dis. 2009, 200, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; Vasa, P.P.; Campitelli, M.A.; Hawken, S.; Wilson, K.; Rosella, L.C.; Stukel, T.A.; Crowcroft, N.S.; McGeer, A.J.; Zinman, L.; et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: A self-controlled study. Lancet Infect. Dis. 2013, 13, 769–776. [Google Scholar] [CrossRef] [PubMed]
- CNODES | About CNODES. Available online: https://www.cnodes.ca/about/ (accessed on 22 January 2024).
- Home | Global Vaccine Data Network. Available online: https://www.globalvaccinedatanetwork.org/ (accessed on 22 January 2024).
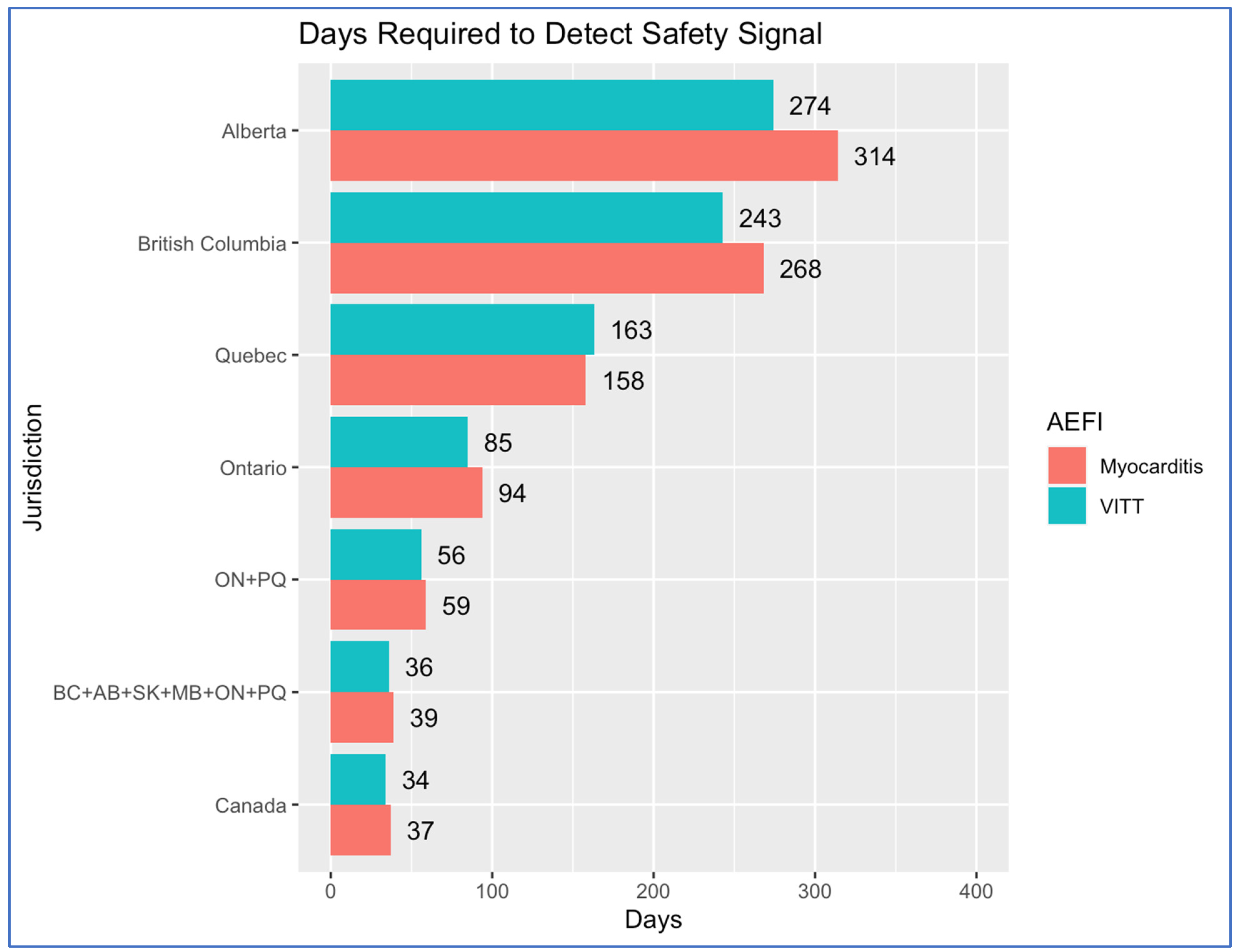
| Province/Territory | 2021 Population | Throughput (Vaccinations/Day) | Events/Day 1/100,000 Risk | Days to Accrue Necessary Events |
|---|---|---|---|---|
| Ontario | 14,223,942 | 50,000 | 0.50 | 94 |
| Quebec | 8,501,833 | 29,885 | 0.30 | 158 |
| British Columbia | 5,000,879 | 17,579 | 0.18 | 268 |
| Alberta | 4,262,635 | 14,984 | 0.15 | 314 |
| Manitoba | 1,342,153 | 4717 | 0.05 | 997 |
| Saskatchewan | 1,132,505 | 3980 | 0.04 | 1181 |
| Nova Scotia | 969,383 | 3407 | 0.03 | 1380 |
| New Brunswick | 775,610 | 2726 | 0.03 | 1725 |
| Newfoundland and Labrador | 510,550 | 1794 | 0.02 | 2620 |
| Prince Edward Island | 154,331 | 542 | 0.01 | 8672 |
| ON + PQ | 22,725,775 | 79,885 | 0.80 | 59 |
| BC + AB + SK + MB + ON + PQ | 34,463,947 | 121,145 | 1.21 | 39 |
| Canada | 36,991,981 | 130,028 | 1.30 | 37 |
| Province/Territory | 2021 Population (Aged 18–39) | Throughput (Vaccinations/Day) | Events/Day 1.5/100,000 Risk | Days to Accrue Necessary Events |
|---|---|---|---|---|
| Ontario | 4,517,570 | 15,811 | 0.24 | 85 |
| Quebec | 2,337,880 | 8182 | 0.12 | 163 |
| British Columbia | 1,568,044 | 5488 | 0.08 | 243 |
| Alberta | 1,395,268 | 4883 | 0.07 | 274 |
| Manitoba | 429,186 | 1502 | 0.02 | 888 |
| Saskatchewan | 351,071 | 1228 | 0.02 | 1086 |
| Nova Scotia | 273,295 | 956 | 0.01 | 1395 |
| New Brunswick | 199,309 | 697 | 0.01 | 1913 |
| Newfoundland and Labrador | 126,163 | 441 | <0.01 | 3024 |
| Prince Edward Island | 47,631 | 166 | <0.01 | 8033 |
| ON + PQ | 6,855,450 | 23,993 | 0.36 | 56 |
| BC + AB + SK + MB + ON + PQ | 10,599,019 | 37,094 | 0.56 | 36 |
| Canada | 11,287,640 | 39,506 | 0.59 | 34 |
| Province/Territory | Days to Accrue Necessary Events | |
|---|---|---|
| 1/1,000,000 Event Rate | 1/10,000 Event Rate | |
| Ontario | 940 | 10 |
| Quebec | 1573 | 16 |
| British Columbia | 2674 | 27 |
| Alberta | 3137 | 32 |
| Manitoba | 9964 | 100 |
| Saskatchewan | 11,810 | 119 |
| Nova Scotia | 13,796 | 138 |
| New Brunswick | 17,242 | 173 |
| Newfoundland and Labrador | 26,199 | 262 |
| Prince Edward Island | 86,716 | 868 |
| ON/PQ | 589 | 6 |
| BC/AB/SK/MB/ON | 516 | 6 |
| Canada | 362 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawken, S.; Wilson, L.A.; Wilson, K. Using Vaccine Safety Data to Demonstrate the Potential of Pooled Data Analysis. Vaccines 2024, 12, 1052. https://doi.org/10.3390/vaccines12091052
Hawken S, Wilson LA, Wilson K. Using Vaccine Safety Data to Demonstrate the Potential of Pooled Data Analysis. Vaccines. 2024; 12(9):1052. https://doi.org/10.3390/vaccines12091052
Chicago/Turabian StyleHawken, Steven, Lindsay A. Wilson, and Kumanan Wilson. 2024. "Using Vaccine Safety Data to Demonstrate the Potential of Pooled Data Analysis" Vaccines 12, no. 9: 1052. https://doi.org/10.3390/vaccines12091052
APA StyleHawken, S., Wilson, L. A., & Wilson, K. (2024). Using Vaccine Safety Data to Demonstrate the Potential of Pooled Data Analysis. Vaccines, 12(9), 1052. https://doi.org/10.3390/vaccines12091052






