Follicular Immune Landscaping Reveals a Distinct Profile of FOXP3hiCD4hi T Cells in Treated Compared to Untreated HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Material
2.2. Tissue Processing
2.3. Confocal Imaging Assays
2.3.1. Tissue Staining and Data Acquisition
2.3.2. Quantitative Imaging Analysis (Histo-Cytometry)
2.3.3. Data Analysis–Neighboring Analysis
2.3.4. Viral Load Measurement
2.4. Statistical Analysis
3. Results
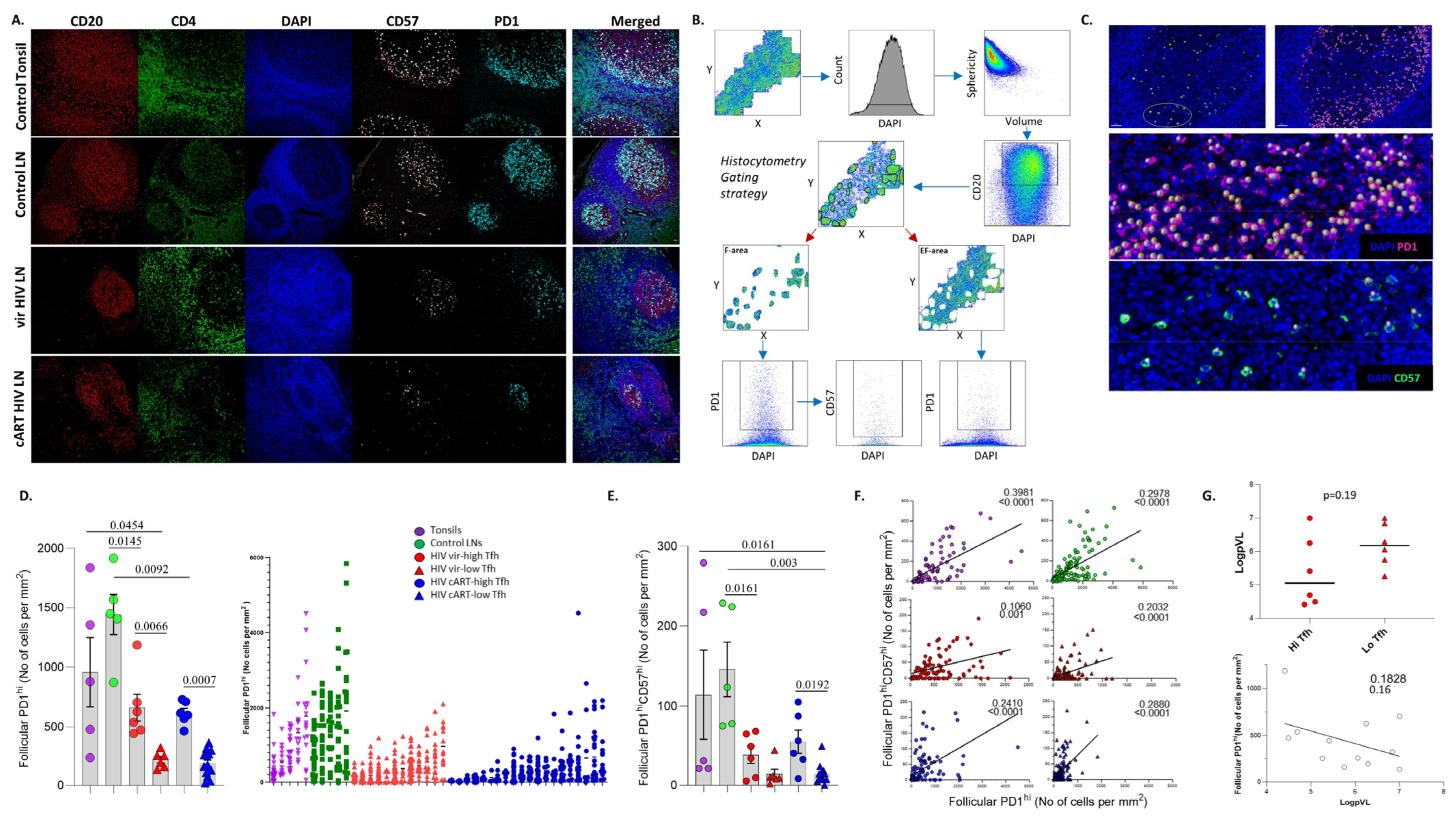
3.1. Similar Profiles of Follicular Helper CD4hi T Cell Densities in Viremic and cART HIV LNs
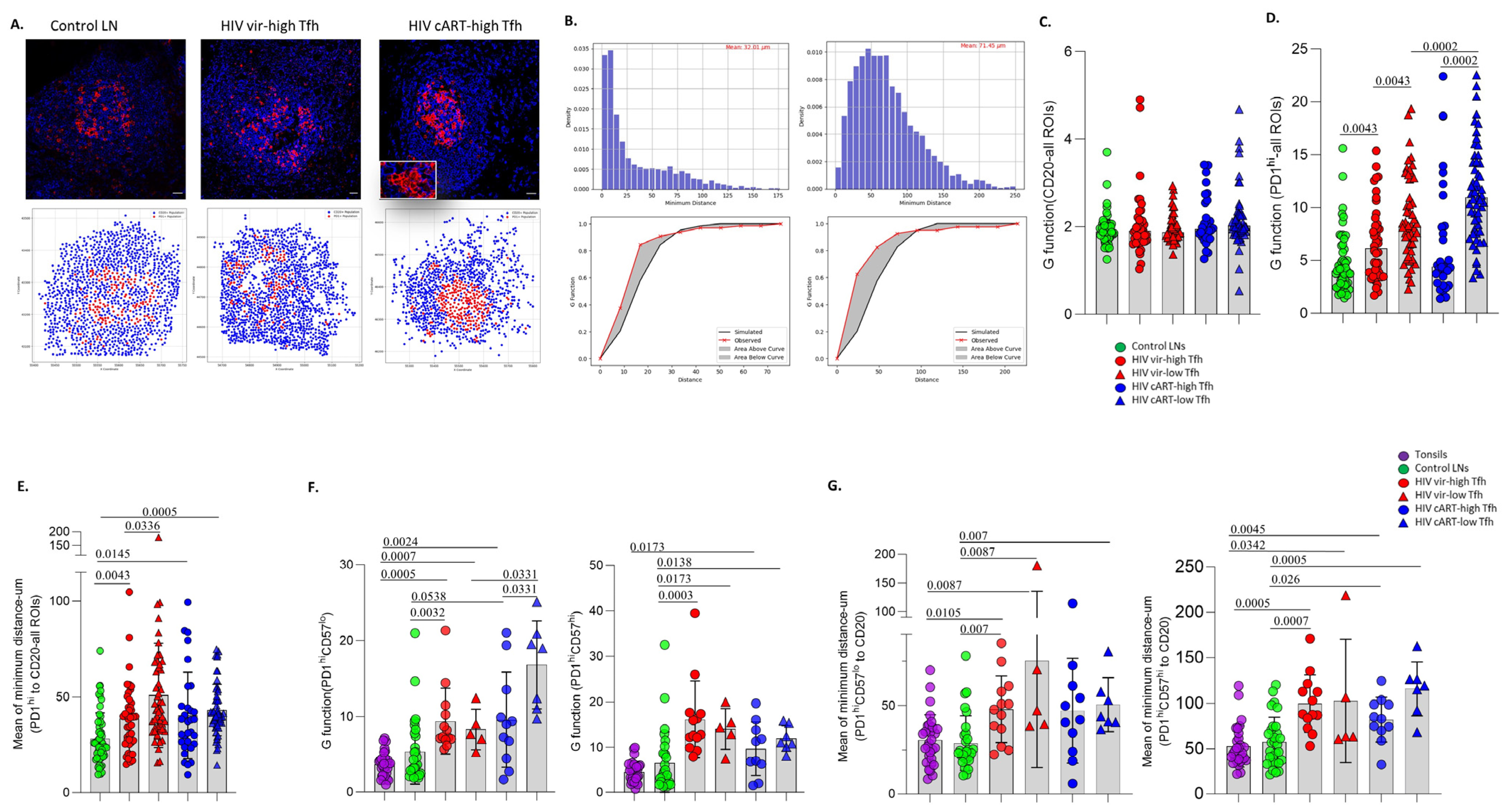
3.2. A Distinct Positioning Profile of TFH Cells in HIV-Infected Compared to Non-Infected Tissues
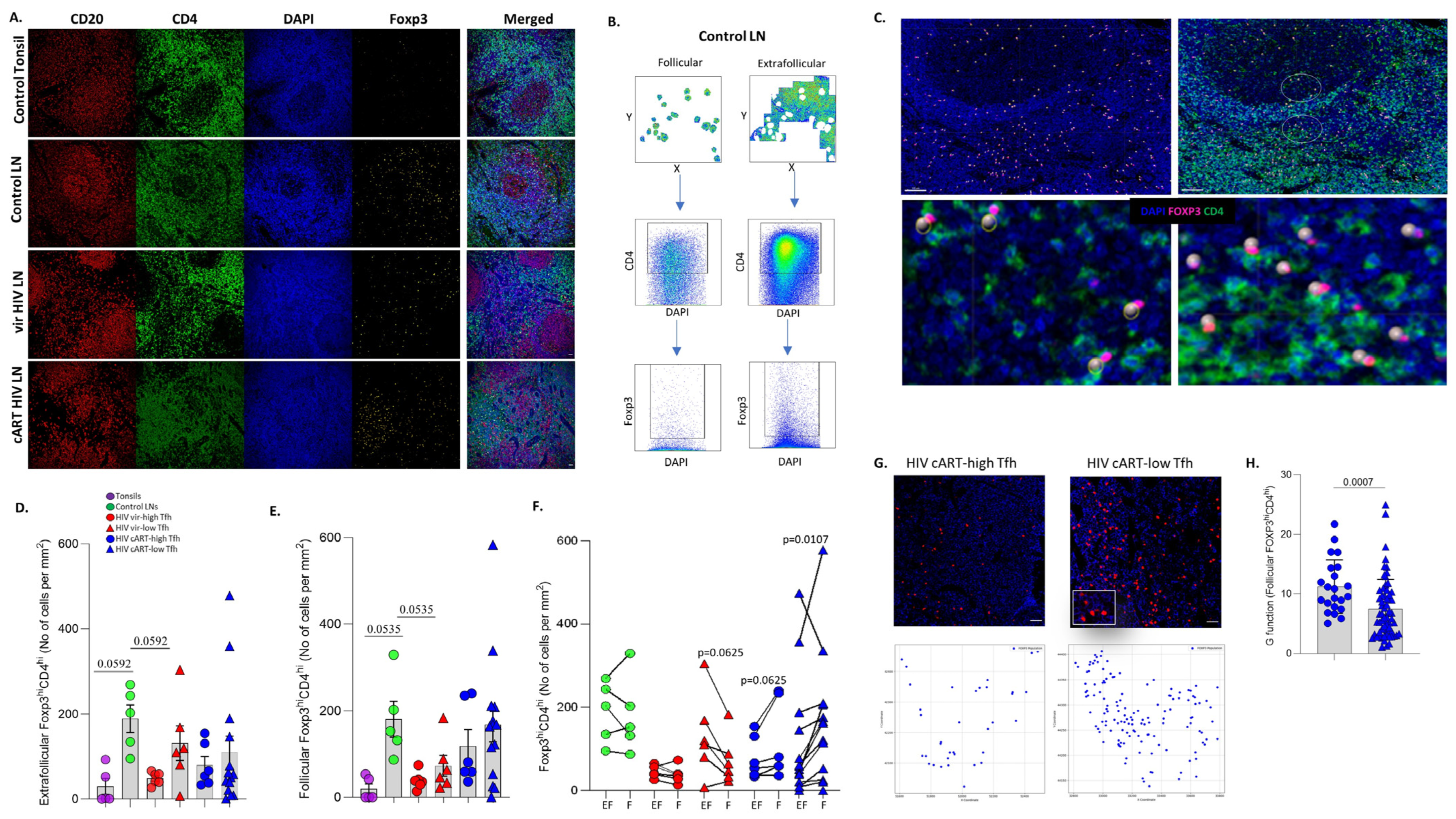
3.3. Significant Accumulation of Follicular Compared to Extrafollicular FOXP3hiCD4hi T Cells in cART Low-TFH LNs
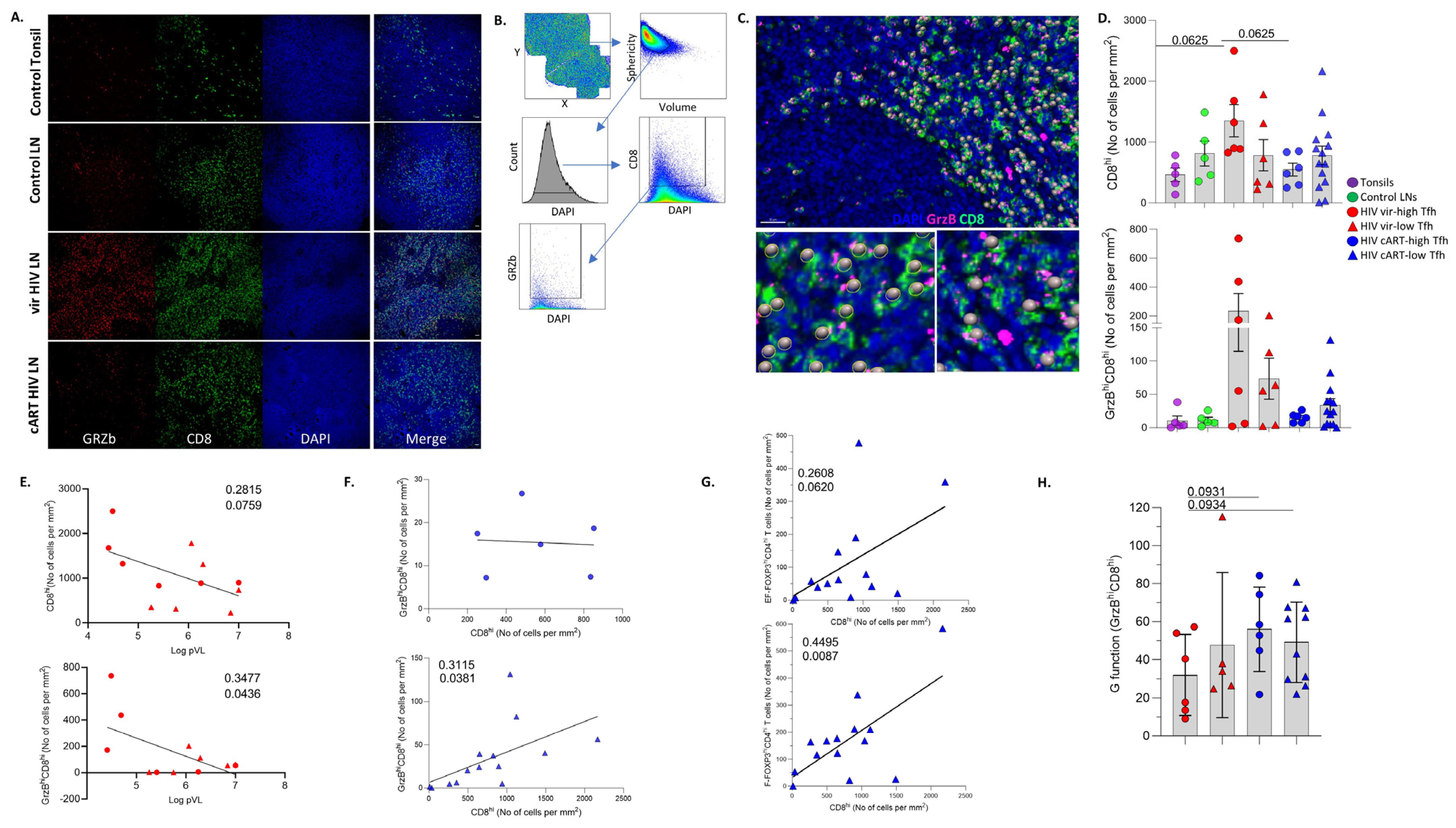
3.4. LN GrzBhiCD8hi T Cells Are Negatively Associated with Blood Viral Load
3.5. Differential Modulation of Innate Immune Cell Subsets by cART
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dey, B.; Berger, E.A. Towards an HIV cure based on targeted killing of infected cells: Different approaches against acute versus chronic infection. Curr. Opin. HIV AIDS 2015, 10, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Maldarelli, F. The role of HIV integration in viral persistence: No more whistling past the proviral graveyard. J. Clin. Investig. 2016, 126, 438–447. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; Serra, D.; Basso, C.; Zanacchi, P. Mechanistic aspects of chromium carcinogenicity. Arch. Toxicol. Suppl. 1989, 13, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Ruiz, M.J.; Pagliuzza, A.; Richard, C.; Shahid, A.; Fromentin, R.; Ponte, R.; Cattin, A.; Wiche Salinas, T.R.; Salahuddin, S.; et al. Near full-length HIV sequencing in multiple tissues collected postmortem reveals shared clonal expansions across distinct reservoirs during ART. Cell Rep. 2023, 42, 113053. [Google Scholar] [CrossRef]
- Estes, J.D. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol. Rev. 2013, 254, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Mudd, P.A.; Minervina, A.A.; Pogorelyy, M.V.; Turner, J.S.; Kim, W.; Kalaidina, E.; Petersen, J.; Schmitz, A.J.; Lei, T.; Haile, A.; et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell 2022, 185, 603–613.e15. [Google Scholar] [CrossRef] [PubMed]
- Noto, A.; Suffiotti, M.; Joo, V.; Mancarella, A.; Procopio, F.A.; Cavet, G.; Leung, Y.; Corpataux, J.M.; Cavassini, M.; Riva, A.; et al. The deficiency in Th2-like Tfh cells affects the maturation and quality of HIV-specific B cell response in viremic infection. Front. Immunol. 2022, 13, 960120. [Google Scholar] [CrossRef] [PubMed]
- Aid, M.; Dupuy, F.P.; Moysi, E.; Moir, S.; Haddad, E.K.; Estes, J.D.; Sekaly, R.P.; Petrovas, C.; Ribeiro, S.P. Follicular CD4 T Helper Cells As a Major HIV Reservoir Compartment: A Molecular Perspective. Front. Immunol. 2018, 9, 895. [Google Scholar] [CrossRef]
- Perreau, M.; Savoye, A.L.; De Crignis, E.; Corpataux, J.M.; Cubas, R.; Haddad, E.K.; De Leval, L.; Graziosi, C.; Pantaleo, G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2013, 210, 143–156. [Google Scholar] [CrossRef]
- Petrovas, C.; Yamamoto, T.; Gerner, M.Y.; Boswell, K.L.; Wloka, K.; Smith, E.C.; Ambrozak, D.R.; Sandler, N.G.; Timmer, K.J.; Sun, X.; et al. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Investig. 2012, 122, 3281–3294. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Malam, N.; Lackner, A.A.; Veazey, R.S. Persistent Simian Immunodeficiency Virus Infection Causes Ultimate Depletion of Follicular Th Cells in AIDS. J. Immunol. 2015, 195, 4351–4357. [Google Scholar] [CrossRef] [PubMed]
- Petrovas, C.; Ferrando-Martinez, S.; Gerner, M.Y.; Casazza, J.P.; Pegu, A.; Deleage, C.; Cooper, A.; Hataye, J.; Andrews, S.; Ambrozak, D.; et al. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci. Transl. Med. 2017, 9, eaag2285. [Google Scholar] [CrossRef] [PubMed]
- Graff-Dubois, S.; Rouers, A.; Moris, A. Impact of Chronic HIV/SIV Infection on T Follicular Helper Cell Subsets and Germinal Center Homeostasis. Front. Immunol. 2016, 7, 501. [Google Scholar] [CrossRef] [PubMed]
- Miles, B.; Miller, S.M.; Folkvord, J.M.; Kimball, A.; Chamanian, M.; Meditz, A.L.; Arends, T.; McCarter, M.D.; Levy, D.N.; Rakasz, E.G.; et al. Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nat. Commun. 2015, 6, 8608. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Martinez, S.; Moysi, E.; Pegu, A.; Andrews, S.; Nganou Makamdop, K.; Ambrozak, D.; McDermott, A.B.; Palesch, D.; Paiardini, M.; Pavlakis, G.N.; et al. Accumulation of follicular CD8+ T cells in pathogenic SIV infection. J. Clin. Investig. 2018, 128, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, W.; Tu, B.; Hong, W.G.; Zhang, Z.; Chen, W.W.; Zhao, M. Alterations of the frequency and functions of follicular regulatory T cells and related mechanisms in HIV infection. J. Infect. 2020, 81, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 2002, 14, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chan, C.N.; Rovira-Clave, X.; Chen, H.; Bai, Y.; Zhu, B.; McCaffrey, E.; Greenwald, N.F.; Liu, C.; Barlow, G.L.; et al. Combined protein and nucleic acid imaging reveals virus-dependent B cell and macrophage immunosuppression of tissue microenvironments. Immunity 2022, 55, 1118–1134.e8. [Google Scholar] [CrossRef] [PubMed]
- Banga, R.; Procopio, F.A.; Lana, E.; Gladkov, G.T.; Roseto, I.; Parsons, E.M.; Lian, X.; Armani-Tourret, M.; Bellefroid, M.; Gao, C.; et al. Lymph node dendritic cells harbor inducible replication-competent HIV despite years of suppressive ART. Cell Host Microbe 2023, 31, 1714–1731.e9. [Google Scholar] [CrossRef]
- Board, N.L.; Moskovljevic, M.; Wu, F.; Siliciano, R.F.; Siliciano, J.D. Engaging innate immunity in HIV-1 cure strategies. Nat. Rev. Immunol. 2022, 22, 499–512. [Google Scholar] [CrossRef]
- Neidleman, J.; Luo, X.; Frouard, J.; Xie, G.; Hsiao, F.; Ma, T.; Morcilla, V.; Lee, A.; Telwatte, S.; Thomas, R.; et al. Phenotypic analysis of the unstimulated in vivo HIV CD4 T cell reservoir. Elife 2020, 9, e60933. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M. Keeping the ill at ease. Postgrad. Med. 1990, 88, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Szabo, P.A.; Levitin, H.M.; Miron, M.; Snyder, M.E.; Senda, T.; Yuan, J.; Cheng, Y.L.; Bush, E.C.; Dogra, P.; Thapa, P.; et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat. Commun. 2019, 10, 4706. [Google Scholar] [CrossRef] [PubMed]
- Moysi, E.; Del Rio Estrada, P.M.; Torres-Ruiz, F.; Reyes-Teran, G.; Koup, R.A.; Petrovas, C. In Situ Characterization of Human Lymphoid Tissue Immune Cells by Multispectral Confocal Imaging and Quantitative Image Analysis; Implications for HIV Reservoir Characterization. Front. Immunol. 2021, 12, 683396. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lovell, D.P. Biological importance and statistical significance. J. Agric. Food Chem. 2013, 61, 8340–8348. [Google Scholar] [CrossRef]
- Miles, B.; Connick, E. TFH in HIV Latency and as Sources of Replication-Competent Virus. Trends Microbiol. 2016, 24, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Havenar-Daughton, C.; Lee, J.H.; Crotty, S. Tfh cells and HIV bnAbs, an immunodominance model of the HIV neutralizing antibody generation problem. Immunol. Rev. 2017, 275, 49–61. [Google Scholar] [CrossRef]
- Biberfeld, P.; Ost, A.; Porwit, A.; Sandstedt, B.; Pallesen, G.; Bottiger, B.; Morfelt-Mansson, L.; Biberfeld, G. Histopathology and immunohistology of HTLV-III/LAV related lymphadenopathy and AIDS. Acta Pathol. Microbiol. Immunol. Scand. A 1987, 95, 47–65. [Google Scholar] [CrossRef]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Rott, L.S.; Clark-Lewis, I.; Campbell, D.J.; Wu, L.; Butcher, E.C. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 2001, 193, 1373–1381. [Google Scholar] [CrossRef]
- Padhan, K.; Moysi, E.; Noto, A.; Chassiakos, A.; Ghneim, K.; Perra, M.M.; Shah, S.; Papaioannou, V.; Fabozzi, G.; Ambrozak, D.R.; et al. Acquisition of optimal TFH cell function is defined by specific molecular, positional, and TCR dynamic signatures. Proc. Natl. Acad. Sci. USA 2021, 118, e2016855118. [Google Scholar] [CrossRef]
- Parra, E.R. Methods to Determine and Analyze the Cellular Spatial Distribution Extracted From Multiplex Immunofluorescence Data to Understand the Tumor Microenvironment. Front. Mol. Biosci. 2021, 8, 668340. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Lee, S.A.; Siedner, M.J. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J. Infect. Dis. 2016, 214 (Suppl. S2), S44–S50. [Google Scholar] [CrossRef]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef] [PubMed]
- Yeap, W.H.; Wong, K.L.; Shimasaki, N.; Teo, E.C.; Quek, J.K.; Yong, H.X.; Diong, C.P.; Bertoletti, A.; Linn, Y.C.; Wong, S.C. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci. Rep. 2016, 6, 34310. [Google Scholar] [CrossRef]
- McKenna, E.; Mhaonaigh, A.U.; Wubben, R.; Dwivedi, A.; Hurley, T.; Kelly, L.A.; Stevenson, N.J.; Little, M.A.; Molloy, E.J. Neutrophils: Need for Standardized Nomenclature. Front. Immunol. 2021, 12, 602963. [Google Scholar] [CrossRef]
- Boritz, E.A.; Darko, S.; Swaszek, L.; Wolf, G.; Wells, D.; Wu, X.; Henry, A.R.; Laboune, F.; Hu, J.; Ambrozak, D.; et al. Multiple Origins of Virus Persistence during Natural Control of HIV Infection. Cell 2016, 166, 1004–1015. [Google Scholar] [CrossRef]
- Doitsh, G.; Cavrois, M.; Lassen, K.G.; Zepeda, O.; Yang, Z.; Santiago, M.L.; Hebbeler, A.M.; Greene, W.C. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 2010, 143, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Smith, A.J.; Wietgrefe, S.W.; Southern, P.J.; Schacker, T.W.; Reilly, C.S.; Estes, J.D.; Burton, G.F.; Silvestri, G.; Lifson, J.D.; et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J. Clin. Investig. 2011, 121, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Schuler, T.; Cavert, W.; Notermans, D.W.; Gebhard, K.; Henry, K.; Havlir, D.V.; Gunthard, H.F.; Wong, J.K.; Little, S.; et al. Reversibility of the pathological changes in the follicular dendritic cell network with treatment of HIV-1 infection. Proc. Natl. Acad. Sci. USA 1999, 96, 5169–5172. [Google Scholar] [CrossRef] [PubMed]
- Galvez, C.; Urrea, V.; Dalmau, J.; Jimenez, M.; Clotet, B.; Monceaux, V.; Huot, N.; Leal, L.; Gonzalez-Soler, V.; Gonzalez-Cao, M.; et al. Extremely low viral reservoir in treated chronically HIV-1-infected individuals. EBioMedicine 2020, 57, 102830. [Google Scholar] [CrossRef]
- Zeng, M.; Southern, P.J.; Reilly, C.S.; Beilman, G.J.; Chipman, J.G.; Schacker, T.W.; Haase, A.T. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012, 8, e1002437. [Google Scholar] [CrossRef] [PubMed]
- Baumjohann, D.; Preite, S.; Reboldi, A.; Ronchi, F.; Ansel, K.M.; Lanzavecchia, A.; Sallusto, F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013, 38, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Sayin, I.; Radtke, A.J.; Vella, L.A.; Jin, W.; Wherry, E.J.; Buggert, M.; Betts, M.R.; Herati, R.S.; Germain, R.N.; Canaday, D.H. Spatial distribution and function of T follicular regulatory cells in human lymph nodes. J. Exp. Med. 2018, 215, 1531–1542. [Google Scholar] [CrossRef]
- Chowdhury, A.; Del Rio Estrada, P.M.; Tharp, G.K.; Trible, R.P.; Amara, R.R.; Chahroudi, A.; Reyes-Teran, G.; Bosinger, S.E.; Silvestri, G. Decreased T Follicular Regulatory Cell/T Follicular Helper Cell (TFH) in Simian Immunodeficiency Virus-Infected Rhesus Macaques May Contribute to Accumulation of TFH in Chronic Infection. J. Immunol. 2015, 195, 3237–3247. [Google Scholar] [CrossRef]
- Amodio, D.; Cotugno, N.; Macchiarulo, G.; Rocca, S.; Dimopoulos, Y.; Castrucci, M.R.; De Vito, R.; Tucci, F.M.; McDermott, A.B.; Narpala, S.; et al. Quantitative Multiplexed Imaging Analysis Reveals a Strong Association between Immunogen-Specific B Cell Responses and Tonsillar Germinal Center Immune Dynamics in Children after Influenza Vaccination. J. Immunol. 2018, 200, 538–550. [Google Scholar] [CrossRef]
- Jacobsen, J.T.; Hu, W.; TB, R.C.; Solem, S.; Galante, A.; Lin, Z.; Allon, S.J.; Mesin, L.; Bilate, A.M.; Schiepers, A.; et al. Expression of Foxp3 by T follicular helper cells in end-stage germinal centers. Science 2021, 373, eabe5146. [Google Scholar] [CrossRef]
- Brainard, D.M.; Tager, A.M.; Misdraji, J.; Frahm, N.; Lichterfeld, M.; Draenert, R.; Brander, C.; Walker, B.D.; Luster, A.D. Decreased CXCR3+ CD8 T cells in advanced human immunodeficiency virus infection suggest that a homing defect contributes to cytotoxic T-lymphocyte dysfunction. J. Virol. 2007, 81, 8439–8450. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.R.; Gaiha, G.D.; Walker, B.D. CD8+ T cells in HIV control, cure and prevention. Nat. Rev. Immunol. 2020, 20, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Policicchio, B.B.; Cardozo-Ojeda, E.F.; Xu, C.; Ma, D.; He, T.; Raehtz, K.D.; Sivanandham, R.; Kleinman, A.J.; Perelson, A.S.; Apetrei, C.; et al. CD8+ T cells control SIV infection using both cytolytic effects and non-cytolytic suppression of virus production. Nat. Commun. 2023, 14, 6657. [Google Scholar] [CrossRef] [PubMed]
- Strongin, Z.; Raymond Marchand, L.; Deleage, C.; Pampena, M.B.; Cardenas, M.A.; Beusch, C.M.; Hoang, T.N.; Urban, E.A.; Gourves, M.; Nguyen, K.; et al. Distinct SIV-specific CD8+ T cells in the lymph node exhibit simultaneous effector and stem-like profiles and are associated with limited SIV persistence. Nat. Immunol. 2024, 25, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Miles, B.; Miller, S.M.; Folkvord, J.M.; Levy, D.N.; Rakasz, E.G.; Skinner, P.J.; Connick, E. Follicular Regulatory CD8 T Cells Impair the Germinal Center Response in SIV and Ex Vivo HIV Infection. PLoS Pathog. 2016, 12, e1005924. [Google Scholar] [CrossRef]
- Di Pilato, M.; Palomino-Segura, M.; Mejias-Perez, E.; Gomez, C.E.; Rubio-Ponce, A.; D’Antuono, R.; Pizzagalli, D.U.; Perez, P.; Kfuri-Rubens, R.; Benguria, A.; et al. Neutrophil subtypes shape HIV-specific CD8 T-cell responses after vaccinia virus infection. NPJ Vaccines 2021, 6, 52. [Google Scholar] [CrossRef]

| ID (cART-HIV) | Age | Gender | Anatomic Location | CD4hi Counts (Cells/μL) | Log pVL (Copies/mL) | Years HIV+ | Treatment Duration (Years) | Current ART Regimen |
|---|---|---|---|---|---|---|---|---|
| cART-LN1 | 52 | M | Inguinal | 708 | ND | 6.5 | 5 | EVG/COBI/3TC/TAF |
| cART-LN2 | 60 | M | Inguinal | 864 | ND | 22 | 22 | DTG/DESCOVY |
| cART-LN3 | 56 | F | Inguinal | 437 | ND | 23 | 15 | DTG/DESCOVY |
| cART-LN4 | 67 | F | Inguinal | 1168 | ND | 10 | 7.5 | Biktarvy |
| cART-LN5 | 53 | F | Inguinal | 454 | ND | 26 | 11 | Triumeq |
| cART-LN6 | 53 | M | Inguinal | 271 | ND | 9 | 14 | DVC/COBI/TRV |
| cART-LN7 | 50 | M | Inguinal | 849 | ND | 32 | 15 | Genvova |
| cART-LN8 | 59 | M | Inguinal | 587 | ND | 10 | 10 | Atripla |
| cART-LN9 | 63 | M | Inguinal | 276 | <40 | 14 | 4 | RPV/DRV/RTV/DTG |
| cART-LN10 | 52 | M | Inguinal | 459 | ND | 13 | 1 | ABC/3TC/DTG |
| cART-LN11 | 46 | M | Inguinal | 493 | ND | 3 | 3 | EVT/COBI/3TC/TDF |
| cART-LN12 | 65 | M | Inguinal | 476 | ND | 26 | 25 | FPV/RTV/3TC/TDF/MVC |
| cART-LN13 | 54 | F | Inguinal | 459 | ND | 13 | 1 | ABC/3TC/DTG |
| cART-LN14 | 44 | M | Inguinal | 545 | ND | 16 | 7 | ATV/RTV/FTC/TDF |
| cART-LN15 | 33 | M | Inguinal | 1121 | ND | 3y 5mo | 2y 1mo | EVG/COBI/FTC/TDF |
| cART-LN16 | 51 | M | Inguinal | 805 | ND | 17 | 10 | DTG/FTC/TDF |
| cART-LN17 | 37 | M | Inguinal | 662 | ND | 5 | 4 | EFV/FTC/TDF |
| cART-LN18 | 53 | M | Inguinal | 441 | ND | 22 | 10 | DTG/FTC/TDF |
| cART-LN19 | 57 | M | Inguinal | 500 | <40 | 29 | 17 | DRV/RTV/ABC/FTC/TDF |
| cART-LN20 | 58 | M | Inguinal | 636 | ND | 19 | 15 | DRV/RTV/FTC/TDF |
| ID (Viremic-HIV) | Age | Gender | Anatomic Location | CD4hi Counts (cells/μL) | CD8hi Counts (cells/μL) | Treatment Duration (years) | Log pVL (copies/mL) | |
| HIV-LN1 | 27 | M | Cervical | 520 | 175 | No treatment | 5.26 | |
| HIV-LN2 | 20 | M | Cervical | 290 | 881 | No treatment | 7 | |
| HIV-LN3 | 29 | M | Inguinal | 545 | 830 | No treatment | 5.41 | |
| HIV-LN4 | 22 | M | Cervical | 476 | 465 | No treatment | 5.75 | |
| HIV-LN5 | 37 | M | Inguinal | 391 | 1158 | No treatment | 6.25 | |
| HIV-LN6 | 24 | M | Inguinal | 453 | 3623 | No treatment | 6.84 | |
| HIV-LN7 | 29 | M | Cervical | 347 | 756 | No treatment | 7 | |
| HIV-LN8 | 33 | M | Cervical | 499 | 2211 | No treatment | 6.06 | |
| HIV-LN9 | 31 | M | Inguinal | 281 | 2142 | No treatment | 6.29 | |
| HIV-LN10 | 35 | M | Cervical | 491 | 2284 | No treatment | 4.49 | |
| HIV-LN11 | 27 | M | Cervical | 719 | 1831 | No treatment | 4.69 | |
| HIV-LN12 | 28 | M | Cervical | 777 | 2195 | No treatment | 4.49 | |
| ID (Reactive LNs) | Age | Gender | Anatomic Location | |||||
| LN1 | 74 | M | Axillary | |||||
| LN2 | 41 | M | Cervical | |||||
| LN3 | 21 | F | Cervical | |||||
| LN4 | 49 | M | Inguinal | |||||
| LN5 | 25 | F | Inguinal | |||||
| PD1hi TFH | PD1hiCD57hi TFH | FOXP3hiCD4hi (Follicular) | CD8hi (Entire LN) | GrzBhiCD8hi (Entire LN) | CD68hiCD163lo (Entire LN) | CD68loCD163hi (Entire LN) | CD15hiCD16lo (Entire LN) | CD15loCD16hi (Entire LN) | |
|---|---|---|---|---|---|---|---|---|---|
| Tonsils | H | H | VL | L | VL | L | H | VL | VL |
| Control LNs | VH | H | H | L | VL | L | H | VL | VL |
| High-TFH viremic | H | L | L | H | L | L | H | L | L |
| Low-TFH viremic | L | L | L | L | L | L | H | VL | VL |
| High-TFH cART | H | L | L | L | VL | L | L | VL | VL |
| Low-TFH cART | L | L | H | L | VL | L | L | VL | VL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgakis, S.; Orfanakis, M.; Brenna, C.; Burgermeister, S.; Del Rio Estrada, P.M.; González-Navarro, M.; Torres-Ruiz, F.; Reyes-Terán, G.; Avila-Rios, S.; Luna-Villalobos, Y.A.; et al. Follicular Immune Landscaping Reveals a Distinct Profile of FOXP3hiCD4hi T Cells in Treated Compared to Untreated HIV. Vaccines 2024, 12, 912. https://doi.org/10.3390/vaccines12080912
Georgakis S, Orfanakis M, Brenna C, Burgermeister S, Del Rio Estrada PM, González-Navarro M, Torres-Ruiz F, Reyes-Terán G, Avila-Rios S, Luna-Villalobos YA, et al. Follicular Immune Landscaping Reveals a Distinct Profile of FOXP3hiCD4hi T Cells in Treated Compared to Untreated HIV. Vaccines. 2024; 12(8):912. https://doi.org/10.3390/vaccines12080912
Chicago/Turabian StyleGeorgakis, Spiros, Michail Orfanakis, Cloe Brenna, Simon Burgermeister, Perla M. Del Rio Estrada, Mauricio González-Navarro, Fernanda Torres-Ruiz, Gustavo Reyes-Terán, Santiago Avila-Rios, Yara Andrea Luna-Villalobos, and et al. 2024. "Follicular Immune Landscaping Reveals a Distinct Profile of FOXP3hiCD4hi T Cells in Treated Compared to Untreated HIV" Vaccines 12, no. 8: 912. https://doi.org/10.3390/vaccines12080912
APA StyleGeorgakis, S., Orfanakis, M., Brenna, C., Burgermeister, S., Del Rio Estrada, P. M., González-Navarro, M., Torres-Ruiz, F., Reyes-Terán, G., Avila-Rios, S., Luna-Villalobos, Y. A., Chén, O. Y., Pantaleo, G., Koup, R. A., & Petrovas, C. (2024). Follicular Immune Landscaping Reveals a Distinct Profile of FOXP3hiCD4hi T Cells in Treated Compared to Untreated HIV. Vaccines, 12(8), 912. https://doi.org/10.3390/vaccines12080912







