Immunity to Sexually Transmitted Bacterial Infections of the Female Genital Tract: Toward Effective Vaccines
Abstract
1. Introduction
2. Bacterial STIs of the Female Genital Tract
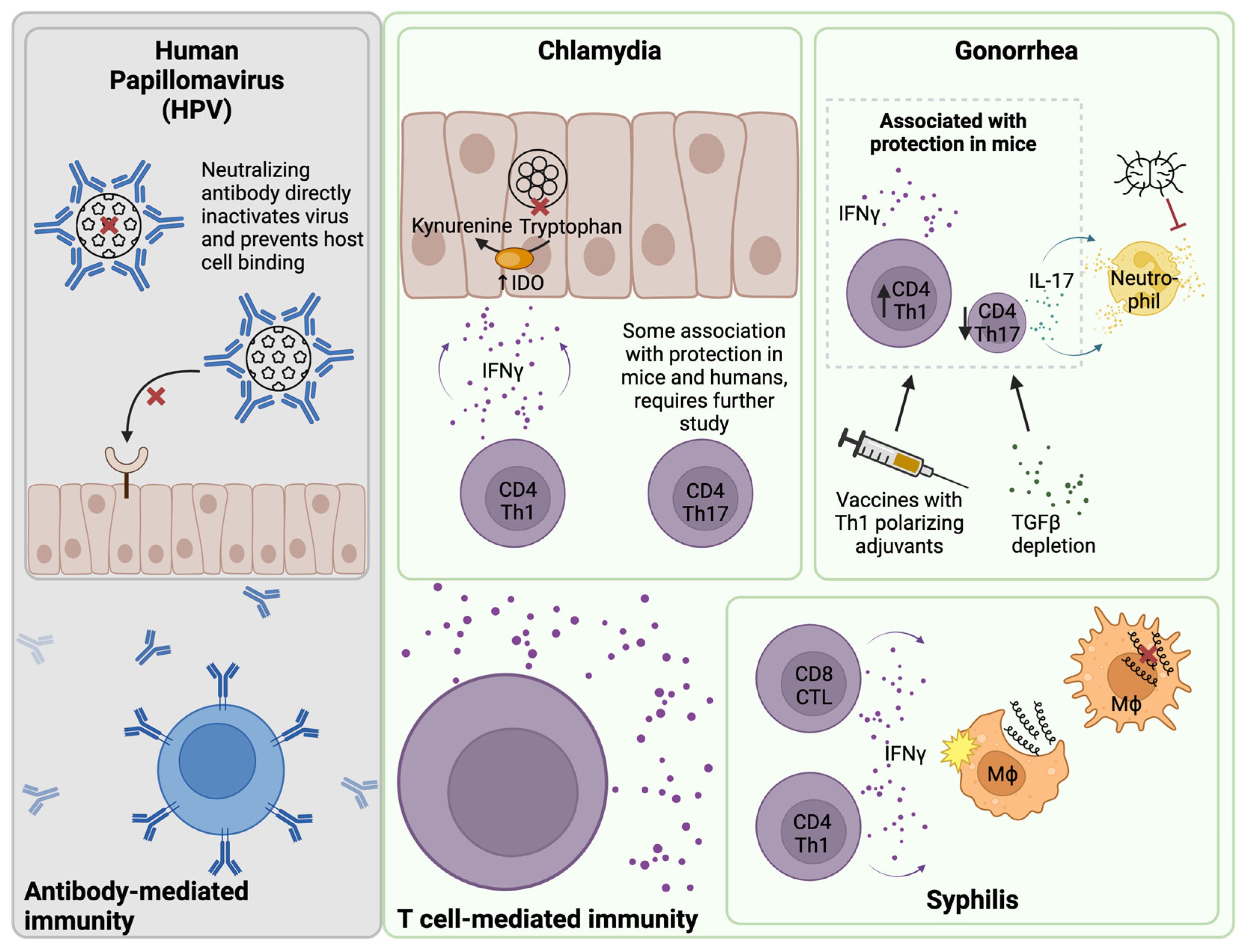
2.1. Chlamydia
2.2. Gonorrhea
2.3. Syphilis
3. B Cells and Antibody-Mediated Immunity
3.1. IgG and Chlamydia Infection
3.1.1. Neutralization
3.1.2. Opsonophagocytosis
3.1.3. Antibody-Dependent Cellular Cytotoxicity (ADCC)
3.1.4. FcRn Transcytosis
3.2. IgA and Chlamydia Infection
3.3. Chlamydia Antibody in Humans
3.4. Antibody in Gonorrhea Infection
3.5. Antibody in Syphilis Infection
4. T Cell-Mediated Immunity
4.1. T Cells and Chlamydia Infection
4.2. T Cells and Gonorrhea Infection
4.3. T Cells and Syphilis Infection
4.4. Resident Memory T Cells in the Female Genital Tract
5. Innate Lymphocyte-Mediated Immunity
5.1. NK Cells
5.2. Helper Innate Lymphoid Cells (ILCs)
5.3. Mucosal-Associated Invariant T (MAIT) Cells
6. Implications for Vaccine Design
6.1. Chlamydia Vaccines
6.2. Gonorrhea Vaccines
6.3. Syphilis Vaccines
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, Y.; Yu, Q.; Lin, Y.; Zhou, Y.; Lan, L.; Yang, S.; Wu, J. Global Burden and Trends of Sexually Transmitted Infections from 1990 to 2019: An Observational Trend Study. Lancet Infect. Dis. 2022, 22, 541–551. [Google Scholar] [CrossRef]
- Centers for Disease Control. Incidence, Prevalence, and Cost of Sexually Transmitted Infections in the United States, 2018; NCHHSTP Newsroom; CDC: Atlanta, GA, USA, 2018. [Google Scholar]
- Nelson, R. Syphilis Rates Soar in the USA Amid Penicillin Shortage. Lancet 2023, 402, 515. [Google Scholar] [CrossRef]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial Intracellular Survival Strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef]
- Rajeeve, K.; Das, S.; Prusty, B.K.; Rudel, T. Chlamydia Trachomatis Paralyses Neutrophils to Evade the Host Innate Immune Response. Nat. Microbiol. 2018, 3, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.N.; Zheng, X.; O’Connell, C.M.; Taylor, B.D.; Wiesenfeld, H.C.; Hillier, S.L.; Zhong, W.; Darville, T. Analysis of Factors Driving Incident and Ascending Infection and the Role of Serum Antibody in Chlamydia trachomatis Genital Tract Infection. J. Infect. Dis. 2016, 213, 523–531. [Google Scholar] [CrossRef]
- Liu, C.; Hufnagel, K.; O’Connell, C.M.; Goonetilleke, N.; Mokashi, N.; Waterboer, T.; Tollison, T.S.; Peng, X.; Wiesenfeld, H.C.; Hillier, S.L.; et al. Reduced Endometrial Ascension and Enhanced Reinfection Associated with Immunoglobulin G Antibodies to Specific Chlamydia trachomatis Proteins in Women at Risk for Chlamydia. J. Infect. Dis. 2022, 225, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Yount, K.S.; Kollipara, A.; Liu, C.; Zheng, X.; O’Connell, C.M.; Bagwell, B.; Wiesenfeld, H.C.; Hillier, S.L.; Darville, T. Unique T Cell Signatures Are Associated with Reduced Chlamydia trachomatis Reinfection in a Highly Exposed Cohort. bioRxiv 2023. bioRxiv: 2023.08.02.551709. [Google Scholar]
- Beatty, W.L.; Belanger, T.A.; Desai, A.A.; Morrison, R.P.; Byrne, G.I. Tryptophan Depletion as a Mechanism of Gamma Interferon-Mediated Chlamydial Persistence. Infect. Immun. 1994, 62, 3705–3711. [Google Scholar] [CrossRef] [PubMed]
- Rank, R.G.; Whittum-Hudson, J.A. Protective Immunity to Chlamydial Genital Infection: Evidence from Animal Studies. J. Infect. Dis. 2010, 201 (Suppl. S2), S168–S177. [Google Scholar] [CrossRef]
- Clercq, E.D.; Kalmar, I.; Vanrompay, D. Animal Models for Studying Female Genital Tract Infection with Chlamydia Trachomatis. Infect. Immun. 2013, 81, 3060–3067. [Google Scholar] [CrossRef]
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and Immunogenicity of the Chlamydia Vaccine Candidate Cth522 Adjuvanted with Caf01 Liposomes or Aluminium Hydroxide: A First-in-Human, Randomised, Double-Blind, Placebo-Controlled, Phase 1 Trial. Lancet Infect. Dis. 2019, 19, 1091–1100. [Google Scholar] [CrossRef]
- Olsen, A.W.; Rosenkrands, I.; Jacobsen, C.S.; Cheeseman, H.M.; Kristiansen, M.P.; Dietrich, J.; Shattock, R.J.; Follmann, F. Immune Signature of Chlamydia Vaccine Cth522/Caf®01 Translates from Mouse-to-Human and Induces Durable Protection in Mice. Nat. Commun. 2024, 15, 1665. [Google Scholar] [CrossRef]
- Pollock, K.M.; Borges, Á.H.; Cheeseman, H.M.; Rosenkrands, I.; Schmidt, K.L.; Søndergaard, R.E.; Day, S.; Evans, A.; McFarlane, L.R.; Joypooranachandran, J.; et al. An Investigation of Trachoma Vaccine Regimens by the Chlamydia Vaccine Cth522 Administered with Cationic Liposomes in Healthy Adults (Chlm-02): A Phase 1, Double-Blind Trial. Lancet Infect. Dis. 2024, 24, 829–844. [Google Scholar] [CrossRef]
- de la Maza, L.M.; Darville, T.L.; Pal, S. Chlamydia Trachomatis Vaccines for Genital Infections: Where Are We and How Far Is There to Go? Expert. Rev. Vaccines 2021, 20, 421–435. [Google Scholar] [CrossRef]
- Borges, Á.H.; Follmann, F.; Dietrich, J. Chlamydia Trachomatis Vaccine Development—A View on the Current Challenges and How to Move Forward. Expert. Rev. Vaccines 2022, 21, 1555–1567. [Google Scholar] [CrossRef]
- Poston, T.B. Advances in Vaccine Development for Chlamydia trachomatis. Pathog. Dis. 2024, ftae017. [Google Scholar] [CrossRef]
- Stern, A.; Brown, M.; Nickel, P.; Meyer, T.F. Opacity Genes in Neisseria Gonorrhoeae: Control of Phase and Antigenic Variation. Cell 1986, 47, 61–71. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Russell, M.W. Suppression of Host Adaptive Immune Responses by Neisseria Gonorrhoeae: Role of Interleukin 10 and Type 1 Regulatory T Cells. Mucosal Immunol. 2014, 7, 165–176. [Google Scholar] [CrossRef]
- Kim, J.J.; Zhou, D.; Mandrell, R.E.; Griffiss, J.M. Effect of Exogenous Sialylation of the Lipooligosaccharide of Neisseria Gonorrhoeae on Opsonophagocytosis. Infect. Immun. 1992, 60, 4439–4442. [Google Scholar] [CrossRef]
- Ngampasutadol, J.; Ram, S.; Gulati, S.; Agarwal, S.; Li, C.; Visintin, A.; Monks, B.; Madico, G.; Rice, P.A. Human Factor H Interacts Selectively with Neisseria Gonorrhoeae and Results in Species-Specific Complement Evasion. J. Immunol. 2008, 180, 3426–3435. [Google Scholar] [CrossRef]
- Pohlner, J.; Halter, R.; Beyreuther, K.; Meyer, T.F. Gene Structure and Extracellular Secretion of Neisseria Gonorrhoeae Iga Protease. Nature 1987, 325, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Islam, E.A.; Jarvis, G.A.; Gray-Owen, S.D.; Russell, M.W. Neisseria Gonorrhoeae Selectively Suppresses the Development of Th1 and Th2 Cells, and Enhances Th17 Cell Responses, through TGF-β-Dependent Mechanisms. Mucosal Immunol. 2012, 5, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.; Criss, A.K. Gonococcal Defenses against Antimicrobial Activities of Neutrophils. Trends Microbiol. 2018, 26, 1022–1034. [Google Scholar] [CrossRef]
- Liu, Y.; Russell, M.W. Diversion of the Immune Response to Neisseria Gonorrhoeae from Th17 to Th1/Th2 by Treatment with Anti-Transforming Growth Factor β Antibody Generates Immunological Memory and Protective Immunity. mBio 2011, 2, e00095-11. [Google Scholar] [CrossRef]
- Girgis, M.M.; Christodoulides, M. Vertebrate and Invertebrate Animal and New In Vitro Models for Studying Neisseria Biology. Pathogens 2023, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.A.; Shafer, W.M.; Ram, S.; Jerse, A.E. Neisseria Gonorrhoeae: Drug Resistance, Mouse Models, and Vaccine Development. Annu. Rev. Microbiol. 2017, 71, 665–686. [Google Scholar] [CrossRef]
- Jerse, A.E. Experimental Gonococcal Genital Tract Infection and Opacity Protein Expression in Estradiol-Treated Mice. Infect. Immun. 1999, 67, 5699–5708. [Google Scholar] [CrossRef] [PubMed]
- Waltmann, A.; Duncan, J.A.; Pier, G.B.; Cywes-Bentley, C.; Cohen, M.S.; Hobbs, M.M. Experimental Urethral Infection with Neisseria Gonorrhoeae. Curr. Top. Microbiol. Immunol. 2022. [Google Scholar] [CrossRef]
- Mancini, F.; Micoli, F.; Necchi, F.; Pizza, M.; Berlanda Scorza, F.; Rossi, O. GMMA-Based Vaccines: The Known and the Unknown. Front. Immunol. 2021, 12, 715393. [Google Scholar] [CrossRef]
- Ruiz García, Y.; Sohn, W.Y.; Seib, K.L.; Taha, M.K.; Vázquez, J.A.; de Lemos, A.P.S.; Vadivelu, K.; Pizza, M.; Rappuoli, R.; Bekkat-Berkani, R. Looking Beyond Meningococcal B with the 4CMenB Vaccine: The Neisseria Effect. NPJ Vaccines 2021, 6, 130. [Google Scholar] [CrossRef]
- Russell, M.W.; Jerse, A.E.; Gray-Owen, S.D. Progress toward a Gonococcal Vaccine: The Way Forward. Front. Immunol. 2019, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hammer, L.A.; Liu, W.; Hobbs, M.M.; Zielke, R.A.; Sikora, A.E.; Jerse, A.E.; Egilmez, N.K.; Russell, M.W. Experimental Vaccine Induces Th1-Driven Immune Responses and Resistance to Neisseria Gonorrhoeae Infection in a Murine Model. Mucosal Immunol. 2017, 10, 1594–1608. [Google Scholar] [CrossRef] [PubMed]
- Addetia, A.; Tantalo, L.C.; Lin, M.J.; Xie, H.; Huang, M.-L.; Marra, C.M.; Greninger, A.L. Comparative Genomics and Full-Length Tprk Profiling of Treponema Pallidum Subsp. Pallidum Reinfection. PLOS Neglected Trop. Dis. 2020, 14, e0007921. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Nieto, C.; Pedreño-López, N.; Mitjà, O.; Clotet, B.; Blanco, J.; Carrillo, J. Syphilis Vaccine: Challenges, Controversies and Opportunities. Front. Immunol. 2023, 14, 1126170. [Google Scholar] [CrossRef]
- Baker-Zander, S.A.; Lukehart, S.A. Macrophage-Mediated Killing of Opsonized Treponema Pallidum. J. Infect. Dis. 1992, 165, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Hawley, K.L.; Cruz, A.R.; Benjamin, S.J.; La Vake, C.J.; Cervantes, J.L.; LeDoyt, M.; Ramirez, L.G.; Mandich, D.; Fiel-Gan, M.; Caimano, M.J.; et al. IFNγ Enhances Cd64-Potentiated Phagocytosis of Treponema Pallidum Opsonized with Human Syphilitic Serum by Human Macrophages. Front. Immunol. 2017, 8, 1227. [Google Scholar] [CrossRef] [PubMed]
- Baker-Zander, S.; Sell, S. A Histopathologic and Immunologic Study of the Course of Syphilis in the Experimentally Infected Rabbit. Demonstration of Long-Lasting Cellular Immunity. Am. J. Pathol. 1980, 101, 387–414. [Google Scholar]
- Edmondson, D.G.; Hu, B.; Norris, S.J. Long-Term In Vitro Culture of the Syphilis Spirochete Treponema Pallidum Subsp. Pallidum. mBio 2018, 9, e01153-18. [Google Scholar] [CrossRef]
- Lu, S.; Zheng, K.; Wang, J.; Xu, M.; Xie, Y.; Yuan, S.; Wang, C.; Wu, Y. Characterization of Treponema Pallidum Dissemination in C57bl/6 Mice. Front. Immunol. 2020, 11, 577129. [Google Scholar] [CrossRef]
- Lithgow, K.V.; Cameron, C.E. Vaccine Development for Syphilis. Expert. Rev. Vaccines 2017, 16, 37–44. [Google Scholar] [CrossRef]
- O’Connell, C.M.; Ferone, M.E. Chlamydia Trachomatis Genital Infections. Microb. Cell 2016, 3, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Sexually Transmitted Infections Surveillance: National Overview of Stis. 2022. Available online: https://www.cdc.gov/std/statistics/2022/overview.htm (accessed on 26 July 2024).
- Thompson, E.L.; Griner, S.B.; Galvin, A.M.; Lowery, A.D.; Lewis, M.A. Correlates of Sti Testing among Us Young Adults: Opportunities for Prevention. Prev. Sci. 2021, 22, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [PubMed]
- Molano, M.; Meijer, C.J.; Weiderpass, E.; Arslan, A.; Posso, H.; Franceschi, S.; Ronderos, M.; Muñoz, N.; van den Brule, A.J. The Natural Course of Chlamydia Trachomatis Infection in Asymptomatic Colombian Women: A 5-Year Follow-Up Study. J. Infect. Dis. 2005, 191, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Criss, A.K. Resistance of Neisseria Gonorrhoeae to Neutrophils. Front. Microbiol. 2011, 2, 77. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.A.S.; Butcher, S.A.; Saunders, N.J. Comparative Whole-Genome Analyses Reveal over 100 Putative Phase-Variable Genes in the Pathogenic Neisseria spp. Microbiology 2001, 147, 2321–2332. [Google Scholar] [CrossRef] [PubMed]
- Belcher, T.; Rollier, C.S.; Dold, C.; Ross, J.D.C.; MacLennan, C.A. Immune Responses to Neisseria Gonorrhoeae and Implications for Vaccine Development. Front. Immunol. 2023, 14, 1248613. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Seifert, H.S.; Hook, E.W., 3rd; Hawkes, S.; Ndowa, F.; Dillon, J.R. Gonorrhoea. Nat. Rev. Dis. Primers 2019, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.-S.; Radolf, J.D.; Benzaken, A.S. Syphilis. Nat. Rev. Dis. Primers 2017, 3, 17073. [Google Scholar] [CrossRef]
- Brown, D.L.; Frank, J.E. Diagnosis and Management of Syphilis. Am. Fam. Physician 2003, 68, 283–290. [Google Scholar]
- Whiting, C.; Schwartzman, G.; Khachemoune, A. Syphilis in Dermatology: Recognition and Management. Am. J. Clin. Dermatol. 2023, 24, 287–297. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.; O’Callaghan, K.; Torrone, E.; Barbee, L.; Grey, J.; Jackson, D.; Woodworth, K.; Olsen, E.; Ludovic, J.; Mayes, N.; et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Thean, L.; Moore, A.; Nourse, C. New Trends in Congenital Syphilis: Epidemiology, Testing in Pregnancy, and Management. Curr. Opin. Infect. Dis. 2022, 35, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Slifka, M.K. Mechanisms That Determine Plasma Cell Lifespan and the Duration of Humoral Immunity. Immunol. Rev. 2010, 236, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, H.D.; Perry, L.J. Neutralization of Chlamydia Trachomatis Infectivity with Antibodies to the Major Outer Membrane Protein. Infect. Immun. 1982, 38, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Hybiske, K.; Stephens, R.S. Mechanisms of Chlamydia Trachomatis Entry into Nonphagocytic Cells. Infect. Immun. 2007, 75, 3925–3934. [Google Scholar] [CrossRef]
- Bastidas, R.J.; Valdivia, R.H. The Emerging Complexity of Chlamydia Trachomatis Interactions with Host Cells as Revealed by Molecular Genetic Approaches. Curr. Opin. Microbiol. 2023, 74, 102330. [Google Scholar] [CrossRef]
- Su, H.; Parnell, M.; Caldwell, H.D. Protective Efficacy of a Parenterally Administered Momp-Derived Synthetic Oligopeptide Vaccine in a Murine Model of Chlamydia Trachomatis Genital Tract Infection: Serum Neutralizing Igg Antibodies Do Not Protect against Chlamydial Genital Tract Infection. Vaccine 1995, 13, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.W.; Meng, Q.; Shen, Z.L.; Zhang, Y.X.; Su, H.; Caldwell, H.D. Protective Efficacy of Major Outer Membrane Protein-Specific Immunoglobulin a (Iga) and Igg Monoclonal Antibodies in a Murine Model of Chlamydia Trachomatis Genital Tract Infection. Infect. Immun. 1995, 63, 4704–4714. [Google Scholar] [CrossRef]
- Morrison, S.G.; Su, H.; Caldwell, H.D.; Morrison, R.P. Immunity to Murine Chlamydia Trachomatis Genital Tract Reinfection Involves B Cells and Cd4(+) T Cells but Not Cd8(+) T Cells. Infect. Immun. 2000, 68, 6979–6987. [Google Scholar] [CrossRef]
- Morrison, S.G.; Morrison, R.P. Resolution of Secondary Chlamydia Trachomatis Genital Tract Infection in Immune Mice with Depletion of Both Cd4+ and Cd8+ T Cells. Infect. Immun. 2001, 69, 2643–2649. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.G.; Morrison, R.P. A Predominant Role for Antibody in Acquired Immunity to Chlamydial Genital Tract Reinfection. J. Immunol. 2005, 175, 7536–7542. [Google Scholar] [CrossRef]
- Naglak, E.K.; Morrison, S.G.; Morrison, R.P. IFNγ Is Required for Optimal Antibody-Mediated Immunity against Genital Chlamydia Infection. Infect. Immun. 2016, 84, 3232–3242. [Google Scholar] [CrossRef]
- Schiff, D.E.; Rae, J.; Martin, T.R.; Davis, B.H.; Curnutte, J.T. Increased Phagocyte Fc Gammari Expression and Improved Fc Gamma-Receptor-Mediated Phagocytosis after In Vivo Recombinant Human Interferon-Gamma Treatment of Normal Human Subjects. Blood 1997, 90, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Naglak, E.K.; Morrison, S.G.; Morrison, R.P. Neutrophils Are Central to Antibody-Mediated Protection against Genital Chlamydia. Infect. Immun. 2017, 85, e00409-17. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.T.; Rank, R.G. Role of NK Cells in Early Host Response to Chlamydial Genital Infection. Infect. Immun. 1998, 66, 5867–5875. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.; Ananaba, G.A.; Bolier, J.; Bowers, S.; Belay, T.; Eko, F.O.; Igietseme, J.U. Fc Receptor Regulation of Protective Immunity against Chlamydia Trachomatis. Immunology 2002, 105, 213–221. [Google Scholar] [CrossRef]
- Trifonova, R.T.; Lieberman, J.; van Baarle, D. Distribution of Immune Cells in the Human Cervix and Implications for Hiv Transmission. Am. J. Reprod. Immunol. 2014, 71, 252–264. [Google Scholar] [CrossRef]
- Johansson, E.L.; Rudin, A.; Wassén, L.; Holmgren, J. Distribution of Lymphocytes and Adhesion Molecules in Human Cervix and Vagina. Immunology 1999, 96, 272–277. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The Neonatal Fc Receptor Comes of Age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Knee, R.A.; Hickey, D.K.; Beagley, K.W.; Jones, R.C. Transport of Igg across the Blood-Luminal Barrier of the Male Reproductive Tract of the Rat and the Effect of Estradiol Administration on Reabsorption of Fluid and Igg by the Epididymal Ducts. Biol. Reprod. 2005, 73, 688–694. [Google Scholar] [CrossRef]
- Li, Z.; Palaniyandi, S.; Zeng, R.; Tuo, W.; Roopenian, D.C.; Zhu, X. Transfer of Igg in the Female Genital Tract by Mhc Class I-Related Neonatal Fc Receptor (Fcrn) Confers Protective Immunity to Vaginal Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 4388–4393. [Google Scholar] [CrossRef] [PubMed]
- Armitage, C.W.; O’Meara, C.P.; Harvie, M.C.; Timms, P.; Blumberg, R.S.; Beagley, K.W. Divergent Outcomes Following Transcytosis of Igg Targeting Intracellular and Extracellular Chlamydial Antigens. Immunol. Cell Biol. 2014, 92, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Hackstadt, T.; Scidmore-Carlson, M.A.; Shaw, E.I.; Fischer, E.R. The Chlamydia Trachomatis Inca Protein Is Required for Homotypic Vesicle Fusion. Cell Microbiol. 1999, 1, 119–130. [Google Scholar] [CrossRef]
- Rzomp, K.A.; Moorhead, A.R.; Scidmore, M.A. The Gtpase Rab4 Interacts with Chlamydia Trachomatis Inclusion Membrane Protein Ct229. Infect. Immun. 2006, 74, 5362–5373. [Google Scholar] [CrossRef]
- Kutteh, W.H.; Prince, S.J.; Hammond, K.R.; Kutteh, C.C.; Mestecky, J. Variations in Immunoglobulins and Iga Subclasses of Human Uterine Cervical Secretions around the Time of Ovulation. Clin. Exp. Immunol. 1996, 104, 538–542. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Mucosal Immunity in the Female Genital Tract. J. Reprod. Immunol. 1997, 36, 23–50. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.-P.; Raffatellu, M. Mucosal Immunity to Pathogenic Intestinal Bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.G.; Morrison, R.P. The Protective Effect of Antibody in Immunity to Murine Chlamydial Genital Tract Reinfection Is Independent of Immunoglobulin A. Infect. Immun. 2005, 73, 6183–6186. [Google Scholar] [CrossRef][Green Version]
- Woodhall, S.C.; Gorwitz, R.J.; Migchelsen, S.J.; Gottlieb, S.L.; Horner, P.J.; Geisler, W.M.; Winstanley, C.; Hufnagel, K.; Waterboer, T.; Martin, D.L.; et al. Advancing the Public Health Applications of Chlamydia Trachomatis Serology. Lancet Infect. Dis. 2018, 18, e399–e407. [Google Scholar]
- Albritton, H.L.; Kozlowski, P.A.; Lillis, R.A.; McGowin, C.L.; Siren, J.D.; Taylor, S.N.; Ibana, J.A.; Buckner, L.R.; Shen, L.; Quayle, A.J. A Novel Whole-Bacterial Enzyme Linked-Immunosorbant Assay to Quantify Chlamydia Trachomatis Specific Antibodies Reveals Distinct Differences between Systemic and Genital Compartments. PLoS ONE 2017, 12, e0183101. [Google Scholar] [CrossRef] [PubMed]
- Batteiger, B.E.; Fraiz, J.; Newhall, W.J.; Katz, B.P.; Jones, R.B. Association of Recurrent Chlamydial Infection with Gonorrhea. J. Infect. Dis. 1989, 159, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Batteiger, B.E.; Tu, W.; Ofner, S.; Van Der Pol, B.; Stothard, D.R.; Orr, D.P.; Katz, B.P.; Fortenberry, J.D. Repeated Chlamydia Trachomatis Genital Infections in Adolescent Women. J. Infect. Dis. 2010, 201, 42–51. [Google Scholar] [CrossRef]
- Blythe, M.J.; Katz, B.P.; Batteiger, B.E.; Ganser, J.A.; Jones, R.B. Recurrent Genitourinary Chlamydial Infections in Sexually Active Female Adolescents. J. Pediatr. 1992, 121, 487–493. [Google Scholar] [CrossRef]
- Brunham, R.C.; Kimani, J.; Bwayo, J.; Maitha, G.; Maclean, I.; Yang, C.; Shen, C.; Roman, S.; Nagelkerke, N.J.; Cheang, M.; et al. The Epidemiology of Chlamydia Trachomatis within a Sexually Transmitted Diseases Core Group. J. Infect. Dis. 1996, 173, 950–956. [Google Scholar] [CrossRef]
- Russell, A.N.; Zheng, X.; O‘Connell, C.M.; Wiesenfeld, H.C.; Hillier, S.L.; Taylor, B.D.; Picard, M.D.; Flechtner, J.B.; Zhong, W.; Frazer, L.C.; et al. Identification of Chlamydia trachomatis Antigens Recognized by T Cells From Highly Exposed Women Who Limit or Resist Genital Tract Infection. J Infect Dis. 2016, 214, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B.; Soper, D.E.; Richter, H.E.; Randall, H.; Peipert, J.F.; Nelson, D.B.; Schubeck, D.; McNeeley, S.G.; Trout, W.; Bass, D.C.; et al. Chlamydia Antibodies, Chlamydia Heat Shock Protein, and Adverse Sequelae after Pelvic Inflammatory Disease: The PID Evaluation and Clinical Health (Peach) Study. Sex. Transm. Dis. 2008, 35, 129–135. [Google Scholar] [CrossRef]
- Tiitinen, A.; Surcel, H.M.; Halttunen, M.; Birkelund, S.; Bloigu, A.; Christiansen, G.; Koskela, P.; Morrison, S.G.; Morrison, R.P.; Paavonen, J. Chlamydia Trachomatis and Chlamydial Heat Shock Protein 60-Specific Antibody and Cell-Mediated Responses Predict Tubal Factor Infertility. Hum. Reprod. 2006, 21, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- El Hakim, E.A.; Gordon, U.D.; Akande, V.A. The Relationship between Serum Chlamydia Antibody Levels and Severity of Disease in Infertile Women with Tubal Damage. Arch. Gynecol. Obs. 2010, 281, 727–733. [Google Scholar] [CrossRef]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking Mucosal Antibody Responses: Igm, Igg and Igd Join Iga. Nat. Rev. Immunol. 2020, 20, 427–441. [Google Scholar] [CrossRef]
- Hedges, S.R.; Mayo, M.S.; Mestecky, J.; Hook, E.W., 3rd; Russell, M.W. Limited Local and Systemic Antibody Responses to Neisseria Gonorrhoeae during Uncomplicated Genital Infections. Infect. Immun. 1999, 67, 3937–3946. [Google Scholar] [CrossRef] [PubMed]
- Hedges, S.R.; Sibley, D.A.; Mayo, M.S.; Hook, E.W., 3rd; Russell, M.W. Cytokine and Antibody Responses in Women Infected with Neisseria Gonorrhoeae: Effects of Concomitant Infections. J. Infect. Dis. 1998, 178, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Lukehart, S.A.; Baker-Zander, S.A.; Sell, S. Characterization of the Humoral Immune Response of the Rabbit to Antigens of Treponema Pallidum after Experimental Infection and Therapy. Sex. Transm. Dis. 1986, 13, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Leader, B.T.; Hevner, K.; Molini, B.J.; Barrett, L.K.; Van Voorhis, W.C.; Lukehart, S.A. Antibody Responses Elicited against the Treponema Pallidum Repeat Proteins Differ during Infection with Different Isolates of Treponema Pallidum subsp. Pallidum. Infect. Immun. 2003, 71, 6054–6057. [Google Scholar] [CrossRef] [PubMed]
- Baker-Zander, S.A.; Hook, E.W., 3rd; Bonin, P.; Handsfield, H.H.; Lukehart, S.A. Antigens of Treponema Pallidum Recognized by Igg and Igm Antibodies during Syphilis in Humans. J. Infect. Dis. 1985, 151, 264–272. [Google Scholar] [CrossRef]
- Bishop, N.H.; Miller, J.N. Humoral Immunity in Experimental Syphilis. I. The Demonstration of Resistance Conferred by Passive Immunization. J. Immunol. 1976, 117, 191–196. [Google Scholar] [CrossRef]
- Schell, R.F.; Chan, J.K.; Le Frock, J.L. Endemic Syphilis: Passive transfer of Resistance with Serum and Cells in Hamsters. J. Infect. Dis. 1979, 140, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.R.; Ramirez, L.G.; Zuluaga, A.V.; Pillay, A.; Abreu, C.; Valencia, C.A.; La Vake, C.; Cervantes, J.L.; Dunham-Ems, S.; Cartun, R.; et al. Immune Evasion and Recognition of the Syphilis Spirochete in Blood and Skin of Secondary Syphilis Patients: Two Immunologically Distinct Compartments. PLoS Negl. Trop. Dis. 2012, 6, e1717. [Google Scholar] [CrossRef]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Amezcua Vesely, M.C.; Pallis, P.; Bielecki, P.; Low, J.S.; Zhao, J.; Harman, C.C.D.; Kroehling, L.; Jackson, R.; Bailis, W.; Licona-Limon, P.; et al. Effector T(H)17 Cells Give Rise to Long-Lived T(Rm) Cells That Are Essential for an Immediate Response against Bacterial Infection. Cell 2019, 178, 1176–1188.e15. [Google Scholar] [CrossRef]
- Yang, X.; Hayglass, K.T.; Brunham, R.C. Different Roles Are Played by Alpha Beta and Gamma Delta T Cells in Acquired Immunity to Chlamydia Trachomatis Pulmonary Infection. Immunology 1998, 94, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.P.; Feilzer, K.; Tumas, D.B. Gene Knockout Mice Establish a Primary Protective Role for Major Histocompatibility Complex Class Ii-Restricted Responses in Chlamydia Trachomatis Genital Tract Infection. Infect. Immun. 1995, 63, 4661–4668. [Google Scholar] [CrossRef]
- Magee, D.M.; Igietseme, J.U.; Smith, J.G.; Bleicker, C.A.; Grubbs, B.G.; Schachter, J.; Rank, R.G.; Williams, D.M. Chlamydia Trachomatis Pneumonia in the Severe Combined Immunodeficiency (SCID) Mouse. Reg. Immunol. 1993, 5, 305–311. [Google Scholar] [PubMed]
- Su, H.; Feilzer, K.; Caldwell, H.D.; Morrison, R.P. Chlamydia Trachomatis Genital Tract Infection of Antibody-Deficient Gene Knockout Mice. Infect. Immun. 1997, 65, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.L.; Feilzer, K.; Caldwell, H.D. Immunity to Chlamydia Trachomatis Is Mediated by T Helper 1 Cells through Ifn-Gamma-Dependent and -Independent Pathways. J. Immunol. 1997, 158, 3344–3352. [Google Scholar] [CrossRef]
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.; et al. A Mucosal Vaccine against Chlamydia Trachomatis Generates Two Waves of Protective Memory T Cells. Science 2015, 348, aaa8205. [Google Scholar] [CrossRef] [PubMed]
- Rixon, J.A.; Depew, C.E.; McSorley, S.J. Th1 Cells Are Dispensable for Primary Clearance of Chlamydia from the Female Reproductive Tract of Mice. PLoS Pathog. 2022, 18, e1010333. [Google Scholar] [CrossRef] [PubMed]
- Lampe, M.F.; Wilson, C.B.; Bevan, M.J.; Starnbach, M.N. Gamma Interferon Production by Cytotoxic T Lymphocytes Is Required for Resolution of Chlamydia Trachomatis Infection. Infect. Immun. 1998, 66, 5457–5461. [Google Scholar] [CrossRef]
- Nelson, D.E.; Virok, D.P.; Wood, H.; Roshick, C.; Johnson, R.M.; Whitmire, W.M.; Crane, D.D.; Steele-Mortimer, O.; Kari, L.; McClarty, G.; et al. Chlamydial IFN-γ Immune Evasion Is Linked to Host Infection Tropism. Proc. Natl. Acad. Sci. USA 2005, 102, 10658–10663. [Google Scholar] [CrossRef]
- Hawkins, R.A.; Rank, R.G.; Kelly, K.A. A Chlamydia Trachomatis-Specific Th2 Clone Does Not Provide Protection against a Genital Infection and Displays Reduced Trafficking to the Infected Genital Mucosa. Infect. Immun. 2002, 70, 5132–5139. [Google Scholar] [CrossRef]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 Cells at the Crossroads of Innate and Adaptive Immunity against Infectious Diseases at the Mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef]
- Paroli, M.; Caccavale, R.; Fiorillo, M.T.; Spadea, L.; Gumina, S.; Candela, V.; Paroli, M.P. The Double Game Played by Th17 Cells in Infection: Host Defense and Immunopathology. Pathogens 2022, 11, 1547. [Google Scholar] [CrossRef]
- Vicetti Miguel, R.D.; Harvey, S.A.K.; LaFramboise, W.A.; Reighard, S.D.; Matthews, D.B.; Cherpes, T.L. Human Female Genital Tract Infection by the Obligate Intracellular Bacterium Chlamydia Trachomatis Elicits Robust Type 2 Immunity. PLoS ONE 2013, 8, e58565. [Google Scholar] [CrossRef]
- Scurlock, A.M.; Frazer, L.C.; Andrews, C.W., Jr.; O’Connell, C.M.; Foote, I.P.; Bailey, S.L.; Chandra-Kuntal, K.; Kolls, J.K.; Darville, T. Interleukin-17 Contributes to Generation of Th1 Immunity and Neutrophil Recruitment during Chlamydia Muridarum Genital Tract Infection But Is Not Required for Macrophage Influx or Normal Resolution of Infection. Infect. Immun. 2011, 79, 1349–1362. [Google Scholar] [CrossRef]
- Barral, R.; Desai, R.; Zheng, X.; Frazer, L.C.; Sucato, G.S.; Haggerty, C.L.; O’Connell, C.M.; Zurenski, M.A.; Darville, T. Frequency of Chlamydia Trachomatis-Specific T Cell Interferon-Gamma and Interleukin-17 Responses in Cd4-Enriched Peripheral Blood Mononuclear Cells of Sexually Active Adolescent Females. J. Reprod. Immunol. 2014, 103, 29–37. [Google Scholar] [CrossRef]
- Feinen, B.; Jerse, A.E.; Gaffen, S.L.; Russell, M.W. Critical Role of Th17 Responses in a Murine Model of Neisseria Gonorrhoeae Genital Infection. Mucosal Immunol. 2010, 3, 312–321. [Google Scholar] [CrossRef]
- Feinen, B.; Russell, M.W. Contrasting Roles of IL-22 and IL-17 in Murine Genital Tract Infection by Neisseria Gonorrhoeae. Front. Immunol. 2012, 3, 11. [Google Scholar] [CrossRef]
- Gagliardi, M.C.; Starnino, S.; Teloni, R.; Mariotti, S.; Dal Conte, I.; Di Carlo, A.; Stefanelli, P. Circulating Levels of Interleukin-17a and Interleukin-23 Are Increased in Patients with Gonococcal Infection. FEMS Immunol. Med. Microbiol. 2011, 61, 129–132. [Google Scholar] [CrossRef][Green Version]
- Leader, B.T.; Godornes, C.; VanVoorhis, W.C.; Lukehart, S.A. Cd4+ Lymphocytes and Gamma Interferon Predominate in Local Immune Responses in Early Experimental Syphilis. Infect. Immun. 2007, 75, 3021–3026. [Google Scholar] [CrossRef]
- Lukehart, S.A.; Baker-Zander, S.A.; Lloyd, R.M.; Sell, S. Characterization of Lymphocyte Responsiveness in Early Experimental Syphilis. Ii. Nature of Cellular Infiltration and Treponema Pallidum Distribution in Testicular Lesions. J. Immunol. 1980, 124, 461–467. [Google Scholar] [CrossRef]
- Van Voorhis, W.C.; Barrett, L.K.; Koelle, D.M.; Nasio, J.M.; Plummer, F.A.; Lukehart, S.A. Primary and Secondary Syphilis Lesions Contain Mrna for Th1 Cytokines. J. Infect. Dis. 1996, 173, 491–495. [Google Scholar] [CrossRef]
- van Voorhis, W.C.; Barrett, L.K.; Nasio, J.M.; Plummer, F.A.; Lukehart, S.A. Lesions of Primary and Secondary Syphilis Contain Activated Cytolytic T Cells. Infect. Immun. 1996, 64, 1048–1050. [Google Scholar] [CrossRef]
- Salazar, J.C.; Cruz, A.R.; Pope, C.D.; Valderrama, L.; Trujillo, R.; Saravia, N.G.; Radolf, J.D. Treponema Pallidum Elicits Innate and Adaptive Cellular Immune Responses in Skin and Blood during Secondary Syphilis: A Flow-Cytometric Analysis. J. Infect. Dis. 2007, 195, 879–887. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two Subsets of Memory T Lymphocytes with Distinct Homing Potentials and Effector Functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Lange, J.; Rivera-Ballesteros, O.; Buggert, M. Human Mucosal Tissue-Resident Memory T Cells in Health and Disease. Mucosal Immunol. 2022, 15, 389–397. [Google Scholar] [CrossRef]
- Morris, S.E.; Farber, D.L.; Yates, A.J. Tissue-Resident Memory T Cells in Mice and Humans: Towards a Quantitative Ecology. J. Immunol. 2019, 203, 2561–2569. [Google Scholar] [CrossRef]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte Egress from Thymus and Peripheral Lymphoid Organs Is Dependent on S1P Receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef]
- Shiow, L.R.; Rosen, D.B.; Brdicková, N.; Xu, Y.; An, J.; Lanier, L.L.; Cyster, J.G.; Matloubian, M. Cd69 Acts Downstream of Interferon-Alpha/Beta to Inhibit S1p1 and Lymphocyte Egress from Lymphoid Organs. Nature 2006, 440, 540–544. [Google Scholar] [CrossRef]
- Mackay, L.K.; Braun, A.; Macleod, B.L.; Collins, N.; Tebartz, C.; Bedoui, S.; Carbone, F.R.; Gebhardt, T. Cutting Edge: Cd69 Interference with Sphingosine-1-Phosphate Receptor Function Regulates Peripheral T Cell Retention. J. Immunol. 2015, 194, 2059–2063. [Google Scholar] [CrossRef]
- Woodward Davis, A.S.; Vick, S.C.; Pattacini, L.; Voillet, V.; Hughes, S.M.; Lentz, G.M.; Kirby, A.C.; Fialkow, M.F.; Gottardo, R.; Hladik, F.; et al. The Human Memory T Cell Compartment Changes across Tissues of the Female Reproductive Tract. Mucosal Immunol. 2021, 14, 862–872. [Google Scholar] [CrossRef]
- Pattacini, L.; Woodward Davis, A.; Czartoski, J.; Mair, F.; Presnell, S.; Hughes, S.M.; Hyrien, O.; Lentz, G.M.; Kirby, A.C.; Fialkow, M.F.; et al. A Pro-Inflammatory Cd8+ T-Cell Subset Patrols the Cervicovaginal Tract. Mucosal Immunol. 2019, 12, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.-h.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Woodward Davis, A.S.; Roozen, H.N.; Dufort, M.J.; DeBerg, H.A.; Delaney, M.A.; Mair, F.; Erickson, J.R.; Slichter, C.K.; Berkson, J.D.; Klock, A.M.; et al. The Human Tissue-Resident CCR5+ T Cell Compartment Maintains Protective and Functional Properties during Inflammation. Sci. Transl. Med. 2019, 11, eaaw8718. [Google Scholar] [CrossRef]
- Hladik, F.; Lentz, G.; Delpit, E.; McElroy, A.; McElrath, M.J. Coexpression of Ccr5 and Il-2 in Human Genital but Not Blood T Cells: Implications for the Ontogeny of the Ccr5+ Th1 Phenotype. J. Immunol. 1999, 163, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.M.L.; Caron, D.P.; Wang, Z.; Wells, S.B.; Chen, D.; Meng, W.; Szabo, P.A.; Lam, N.; Kubota, M.; Matsumoto, R.; et al. Tissue Adaptation and Clonal Segregation of Human Memory T Cells in Barrier Sites. Nat. Immunol. 2023, 24, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Iwasaki, A. A Local Macrophage Chemokine Network Sustains Protective Tissue-Resident Memory Cd4 T Cells. Science 2014, 346, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Iwasaki, A. A Vaccine Strategy Protects against Genital Herpes by Establishing Local Memory T Cells. Nature 2012, 491, 463–467. [Google Scholar] [CrossRef]
- Shin, H.; Kumamoto, Y.; Gopinath, S.; Iwasaki, A. Cd301b+ Dendritic Cells Stimulate Tissue-Resident Memory Cd8+ T Cells to Protect against Genital Hsv-2. Nat. Commun. 2016, 7, 13346. [Google Scholar] [CrossRef]
- Koelle, D.M.; Dong, L.; Jing, L.; Laing, K.J.; Zhu, J.; Jin, L.; Selke, S.; Wald, A.; Varon, D.; Huang, M.L.; et al. Hsv-2-Specific Human Female Reproductive Tract Tissue Resident Memory T Cells Recognize Diverse Hsv Antigens. Front. Immunol. 2022, 13, 867962. [Google Scholar] [CrossRef]
- Posavad, C.M.; Zhao, L.; Dong, L.; Jin, L.; Stevens, C.E.; Magaret, A.S.; Johnston, C.; Wald, A.; Zhu, J.; Corey, L.; et al. Enrichment of Herpes Simplex Virus Type 2 (Hsv-2) Reactive Mucosal T Cells in the Human Female Genital Tract. Mucosal Immunol. 2017, 10, 1259–1269. [Google Scholar] [CrossRef]
- Peng, T.; Phasouk, K.; Bossard, E.; Klock, A.; Jin, L.; Laing, K.J.; Johnston, C.; Williams, N.A.; Czartoski, J.L.; Varon, D.; et al. Distinct Populations of Antigen-Specific Tissue-Resident Cd8+ T Cells in Human Cervix Mucosa. JCI Insight 2021, 6, e149950. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, P.; Swan, D.A.; Duke, E.; Corey, L.; Zhu, J.; Davé, V.; Spuhler, L.R.; Lund, J.M.; Prlic, M.; Schiffer, J.T. Tissue-Resident T Cell-Derived Cytokines Eliminate Herpes Simplex Virus-2-Infected Cells. J. Clin. Investig. 2020, 130, 2903–2919. [Google Scholar] [CrossRef] [PubMed]
- Posavad, C.M.; Zhao, L.; Mueller, D.E.; Stevens, C.E.; Huang, M.-L.W.; Wald, A.; Corey, L. Persistence of Mucosal T Cell Responses to Herpes Simplex Virus Type 2 (Hsv-2) in the Female Genital Tract. Mucosal Immunol. 2014, 8, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.K.; Stock, A.T.; Ma, J.Z.; Jones, C.M.; Kent, S.J.; Mueller, S.N.; Heath, W.R.; Carbone, F.R.; Gebhardt, T. Long-Lived Epithelial Immunity by Tissue-Resident Memory T (Trm) Cells in the Absence of Persisting Local Antigen Presentation. Proc. Natl. Acad. Sci. USA 2012, 109, 7037–7042. [Google Scholar] [CrossRef] [PubMed]
- Beura, L.K.; Mitchell, J.S.; Thompson, E.A.; Schenkel, J.M.; Mohammed, J.; Wijeyesinghe, S.; Fonseca, R.; Burbach, B.J.; Hickman, H.D.; Vezys, V.; et al. Intravital Mucosal Imaging of Cd8+ Resident Memory T Cells Shows Tissue-Autonomous Recall Responses That Amplify Secondary Memory. Nat. Immunol. 2018, 19, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Fraser, K.A.; Vezys, V.; Masopust, D. Sensing and Alarm Function of Resident Memory Cd8⁺ T Cells. Nat. Immunol. 2013, 14, 509–513. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Fraser, K.A.; Beura, L.K.; Pauken, K.E.; Vezys, V.; Masopust, D. Resident Memory Cd8 T Cells Trigger Protective Innate and Adaptive Immune Responses. Science 2014, 346, 101–198. [Google Scholar] [CrossRef] [PubMed]
- Buggert, M.; Nguyen, S.; Salgado-Montes de Oca, G.; Bengsch, B.; Darko, S.; Ransier, A.; Roberts, E.R.; del Alcazar, D.; Brody, I.B.; Vella, L.A.; et al. Identification and Characterization of Hiv-Specific Resident Memory Cd8+ T Cells in Human Lymphoid Tissue. Sci. Immunol. 2018, 3, eaar4526. [Google Scholar] [CrossRef]
- Cantero-Pérez, J.; Grau-Expósito, J.; Serra-Peinado, C.; Rosero, D.A.; Luque-Ballesteros, L.; Astorga-Gamaza, A.; Castellví, J.; Sanhueza, T.; Tapia, G.; Lloveras, B.; et al. Resident Memory T Cells Are a Cellular Reservoir for Hiv in the Cervical Mucosa. Nat. Commun. 2019, 10, 4739. [Google Scholar] [CrossRef]
- Gibbs, A.; Buggert, M.; Edfeldt, G.; Ranefall, P.; Introini, A.; Cheuk, S.; Martini, E.; Eidsmo, L.; Ball, T.B.; Kimani, J.; et al. Human Immunodeficiency Virus-Infected Women Have High Numbers of Cd103-Cd8+ T Cells Residing Close to the Basal Membrane of the Ectocervical Epithelium. J. Infect. Dis. 2018, 218, 453–465. [Google Scholar] [CrossRef]
- Gupta, P.; Collins, K.B.; Ratner, D.; Watkins, S.C.; Naus, G.J.; Landers, D.V.; Patterson, B.K. Memory Cd4+ T Cells Are the Earliest Detectable Human Immunodeficiency Virus Type 1 (Hiv-1)-Infected Cells in the Female Genital Mucosal Tissue during Hiv-1 Transmission in an Organ Culture System. J. Virol. 2002, 76, 9868–9876. [Google Scholar] [CrossRef] [PubMed]
- Moylan, D.; Goepfert, P.A.; Kempf, M.-C.; Saag, M.S.; Richter, H.E.; Mestecky, J.F.; Sabbaj, S. Diminished Cd103 (αEβ7) Expression on Resident T Cells from the Female Genital Tract of Hiv-Positive Women. Pathog. Immun. 2017, 1, 371–389. [Google Scholar] [CrossRef]
- Petitdemange, C.; Kasturi, S.P.; Kozlowski, P.A.; Nabi, R.; Quarnstrom, C.F.; Reddy, P.B.J.; Derdeyn, C.A.; Spicer, L.M.; Patel, P.J.; Legere, T.; et al. Vaccine Induction of Antibodies and Tissue-Resident Cd8+ T Cells Enhances Protection against Mucosal Shiv-Infection in Young Macaques. JCI Insight 2019, 4, e126047. [Google Scholar] [CrossRef] [PubMed]
- Shacklett, B.L. Mucosal Immunity in Hiv/Siv Infection: T Cells, B Cells and Beyond. Curr. Immunol. Rev. 2019, 15, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-X.; Wheatley, A.K.; Esterbauer, R.; Jegaskanda, S.; Glass, J.J.; Masopust, D.; Rose, R.D.; Rose, R.D.; Kent, S.J.; Kent, S.J. Induction of Vaginal-Resident Hiv-Specific Cd8 T Cells with Mucosal Prime–Boost Immunization. Mucosal Immunol. 2017, 11, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A. Antiviral Immune Responses in the Genital Tract: Clues for Vaccines. Nat. Rev. Immunol. 2010, 10, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.G.; Morrison, R.P. In Situ Analysis of the Evolution of the Primary Immune Response in Murine Chlamydia Trachomatis Genital Tract Infection. Infect. Immun. 2000, 68, 2870–2879. [Google Scholar] [CrossRef] [PubMed]
- Van Voorhis, W.C.; Barrett, L.K.; Sweeney, Y.T.; Kuo, C.C.; Patton, D.L. Repeated Chlamydia Trachomatis Infection of Macaca Nemestrina Fallopian Tubes Produces a Th1-Like Cytokine Response Associated with Fibrosis and Scarring. Infect. Immun. 1997, 65, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Kiviat, N.B.; Paavonen, J.A.; Wølner-Hanssen, P.; Critchlow, C.W.; Stamm, W.E.; Douglas, J.; Eschenbach, D.A.; Corey, L.A.; Holmes, K.K. Histopathology of Endocervical Infection Caused by Chlamydia Trachomatis, Herpes Simplex Virus, Trichomonas Vaginalis, and Neisseria Gonorrhoeae. Hum. Pathol. 1990, 21, 831–837. [Google Scholar] [CrossRef]
- Kiviat, N.B.; Wolner-Hanssen, P.; Eschenbach, D.A.; Wasserheit, J.N.; Paavonen, J.A.; Bell, T.A.; Critchlow, C.W.; Stamm, W.E.; Moore, D.E.; Holmes, K.K. Endometrial Histopathology in Patients with Culture-Proved Upper Genital Tract Infection and Laparoscopically Diagnosed Acute Salpingitis. Am. J. Surg. Pathol. 1990, 14, 167–175. [Google Scholar] [CrossRef]
- Igietseme, J.U.; Uriri, I.M.; Hawkins, R.; Rank, R.G. Integrin-Mediated Epithelial-T Cell Interaction Enhances Nitric Oxide Production and Increased Intracellular Inhibition of Chlamydia. J. Leukoc. Biol. 1996, 59, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Igietseme, J.U. Molecular Mechanism of T-Cell Control of Chlamydia in Mice: Role of Nitric Oxide In Vivo. Immunology 1996, 88, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Rank, R.G. Identification of Homing Receptors That Mediate the Recruitment of Cd4 T Cells to the Genital Tract Following Intravaginal Infection with Chlamydia Trachomatis. Infect. Immun. 1997, 65, 5198–5208. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Rank, R.G.; Kelly, K.A. Expression of Mucosal Homing Receptor Alpha4beta7 Is Associated with Enhanced Migration to the Chlamydia-Infected Murine Genital Mucosa In Vivo. Infect. Immun. 2000, 68, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Olsen, A.W.; Lorenzen, E.; Andersen, P.; Hvid, M.; Follmann, F.; Dietrich, J. Parenteral Vaccination Protects against Transcervical Infection with Chlamydia Trachomatis and Generate Tissue-Resident T Cells Post-Challenge. NPJ Vaccines 2020, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Guleed, S.; Olsen, A.W.; Follmann, F.; Christensen, J.P.; Dietrich, J. Th1/Th17 T Cell Tissue-Resident Immunity Increases Protection, but Is Not Required in a Vaccine Strategy Against Genital Infection with Chlamydia trachomatis. Front. Immunol. 2021, 12, 790463. [Google Scholar] [CrossRef]
- Jiao, L.; Gao, X.; Joyee, A.G.; Zhao, L.; Qiu, H.; Yang, M.; Fan, Y.; Wang, S.; Yang, X. NK Cells Promote Type 1 T Cell Immunity through Modulating the Function of Dendritic Cells during Intracellular Bacterial Infection. J. Immunol. 2011, 187, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Dong, X.; Zhou, X.; Zhao, L.; Wang, X.; Rashu, R.; Zhao, W.; Yang, X. NK Cells Contribute to Protective Memory T Cell Mediated Immunity to Chlamydia Muridarum Infection. Front. Cell Infect. Microbiol. 2020, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Colonna, M. Innate Lymphoid Cells in Mucosal Immunity. Front. Immunol. 2019, 10, 861. [Google Scholar] [CrossRef]
- Doisne, J.M.; Balmas, E.; Boulenouar, S.; Gaynor, L.M.; Kieckbusch, J.; Gardner, L.; Hawkes, D.A.; Barbara, C.F.; Sharkey, A.M.; Brady, H.J.; et al. Composition, Development, and Function of Uterine Innate Lymphoid Cells. J. Immunol. 2015, 195, 3937–3945. [Google Scholar] [CrossRef]
- Miller, D.; Motomura, K.; Garcia-Flores, V.; Romero, R.; Gomez-Lopez, N. Innate Lymphoid Cells in the Maternal and Fetal Compartments. Front. Immunol. 2018, 9, 2396. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.H.; Howard, J.K.; Lord, G.M. Transcription Factor-Driven Regulation of Ilc1 and Ilc3. Trends Immunol. 2022, 43, 564–579. [Google Scholar] [CrossRef]
- Barth, S.; Kirschnek, S.; Ortmann, N.; Tanriver, Y.; Häcker, G. The Reaction of Innate Lymphoid Cells in the Mouse Female Genital Tract to Chlamydial Infection. Infect. Immun. 2021, 89, e0080020. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, H.; Song, C.; Koprivsek, J.J.; Arulanandam, B.; Yang, H.; Tao, L.; Zhong, G. Adoptive Transfer of Group 3-Like Innate Lymphoid Cells Restores Mouse Colon Resistance to Colonization of a Gamma Interferon-Susceptible Chlamydia muridarum Mutant. Infect. Immun. 2021, 89, e00533-20. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Koay, H.F.; McCluskey, J.; Gherardin, N.A. The Biology and Functional Importance of Mait Cells. Nat. Immunol. 2019, 20, 1110–1128. [Google Scholar] [CrossRef]
- Le Bourhis, L.; Martin, E.; Péguillet, I.; Guihot, A.; Froux, N.; Coré, M.; Lévy, E.; Dusseaux, M.; Meyssonnier, V.; Premel, V.; et al. Antimicrobial Activity of Mucosal-Associated Invariant T Cells. Nat. Immunol. 2010, 11, 701–708. [Google Scholar] [CrossRef]
- Gibbs, A.; Leeansyah, E.; Introini, A.; Paquin-Proulx, D.; Hasselrot, K.; Andersson, E.; Broliden, K.; Sandberg, J.K.; Tjernlund, A. MAIT Cells Reside in the Female Genital Mucosa and Are Biased towards IL-17 and IL-22 Production in Response to Bacterial Stimulation. Mucosal Immunol. 2016, 10, 35–45. [Google Scholar] [CrossRef]
- Bister, J.; Crona Guterstam, Y.; Strunz, B.; Dumitrescu, B.; Haij Bhattarai, K.; Özenci, V.; Brännström, M.; Ivarsson, M.A.; Gidlöf, S.; Björkström, N.K. Human Endometrial Mait Cells Are Transiently Tissue Resident and Respond to Neisseria Gonorrhoeae. Mucosal Immunol. 2021, 14, 357–365. [Google Scholar] [CrossRef]
- Hosenfeld, C.B.; Workowski, K.A.; Berman, S.; Zaidi, A.; Dyson, J.; Mosure, D.; Bolan, G.; Bauer, H.M. Repeat Infection with Chlamydia and Gonorrhea among Females: A Systematic Review of the Literature. Sex. Transm. Dis. 2009, 36, 478–489. [Google Scholar] [CrossRef]
- Cha, S.; Newman, D.R.; Rahman, M.; Peterman, T.A. High Rates of Repeat Chlamydial Infections among Young Women-Louisiana, 2000–2015. Sex. Transm. Dis. 2019, 46, 52–57. [Google Scholar] [CrossRef]
- Newman, D.R.; Matthias, J.; Rahman, M.M.; Brantley, A.; Peterman, T.A. Repeat Syphilis Among Hiv-Infected Men in Florida and Louisiana 2000–2018: Implications for Screening Recommendations. AIDS Patient Care STDS 2021, 35, 435–440. [Google Scholar] [CrossRef]
- Hsu, K.K.; Molotnikov, L.E.; Roosevelt, K.A.; Elder, H.R.; Klevens, R.M.; DeMaria, A., Jr.; Aral, S.O. Characteristics of Cases With Repeated Sexually Transmitted Infections, Massachusetts, 2014–2016. Clin. Infect. Dis. 2018, 67, 99–104. [Google Scholar] [CrossRef]
- Geisler, W.M.; Lensing, S.Y.; Press, C.G.; Hook, E.W., 3rd. Spontaneous Resolution of Genital Chlamydia Trachomatis Infection in Women and Protection from Reinfection. J. Infect. Dis. 2013, 207, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Arno, J.N.; Katz, B.P.; McBride, R.; Carty, G.A.; Batteiger, B.E.; Caine, V.A.; Jones, R.B. Age and Clinical Immunity to Infections with Chlamydia Trachomatis. Sex. Transm. Dis. 1994, 21, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Tsoumanis, A.; Osbak, K.; Van Esbroeck, M.; Florence, E.; Crucitti, T.; Kestens, L. Repeat Syphilis Has a Different Immune Response Compared with Initial Syphilis: An Analysis of Biomarker Kinetics in Two Cohorts. Sex. Transm. Infect. 2018, 94, 180–186. [Google Scholar] [CrossRef]
- Kenyon, C.; Osbak, K.K.; Apers, L. Repeat Syphilis Is More Likely to Be Asymptomatic in Hiv-Infected Individuals: A Retrospective Cohort Analysis with Important Implications for Screening. Open Forum Infect. Dis. 2018, 5, ofy096. [Google Scholar] [CrossRef]
- Darville, T.; Albritton, H.L.; Zhong, W.; Dong, L.; O’Connell, C.M.; Poston, T.B.; Quayle, A.J.; Goonetilleke, N.; Wiesenfeld, H.C.; Hillier, S.L.; et al. Anti-Chlamydia Igg and Iga Are Insufficient to Prevent Endometrial Chlamydia Infection in Women, and Increased Anti-Chlamydia Igg Is Associated with Enhanced Risk for Incident Infection. Am. J. Reprod. Immunol. 2019, 81, e13103. [Google Scholar] [CrossRef]
- Nichols, R.L.; Bell, S.D., Jr.; Murray, E.S.; Haddad, N.A.; Bobb, A.A. Studies on Trachoma. V. Clinical Observations in a Field Trial of Bivalent Trachoma Vaccine at Three Dosage Levels in Saudi Arabia. Am. J. Trop. Med. Hyg. 1966, 15, 639–647. [Google Scholar] [CrossRef]
- Nichols, R.L.; Bell, S.D., Jr.; Haddad, N.A.; Bobb, A.A. Studies on Trachoma. Vi. Microbiological Observations in a Field Trial in Saudi Arabia of Bivalent Rachoma Vaccine at Three Dosage Levels. Am. J. Trop. Med. Hyg. 1969, 18, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Grayston, J.T.; Woolridge, R.L.; Wang, S.P.; Yen, C.H.; Yang, C.Y.; Cheng, K.H.; Chang, I.H. Field Studies of Protection from Infection by Experimental Trachoma Virus Vaccine in Preschool-Aged Children on Taiwan. Proc. Soc. Exp. Biol. Med. 1963, 112, 589–595. [Google Scholar] [CrossRef]
- Woolridge, R.L.; Grayston, J.T.; Chang, I.H.; Cheng, K.H.; Yang, C.Y.; Neave, C. Field Trial of a Monovalent and of a Bivalent Mineral Oil Adjuvant Trachoma Vaccine in Taiwan School Children. Am. J. Ophthalmol. 1967, 63, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Sowa, S.; Sowa, J.; Collier, L.H.; Blyth, W.A. Trachoma Vaccine Field Trials in the Gambia. J. Hyg. 1969, 67, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Dhir, S.P.; Agarwal, L.P.; Detels, R.; Wang, S.P.; Grayston, J.T. Field Trial of Two Bivalent Trachoma Vaccines in Children of Punjab Indian Villages. Am. J. Ophthalmol. 1967, 63, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.C.; Hu, V.; Bailey, R.L.; Burton, M.J.; Holland, M.J. Towards a Safe and Effective Chlamydial Vaccine: Lessons from the Eye. Vaccine 2014, 32, 1572–1578. [Google Scholar] [CrossRef]
- O’Connell, C.M.; Ingalls, R.R.; Andrews, C.W., Jr.; Scurlock, A.M.; Darville, T. Plasmid-Deficient Chlamydia Muridarum Fail to Induce Immune Pathology and Protect against Oviduct Disease. J. Immunol. 2007, 179, 4027–4034. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.M.; Zurenski, M.A.; Frazer, L.C.; O’Connell, C.M.; Andrews, C.W., Jr.; Mintus, M.; Darville, T. The Recall Response Induced by Genital Challenge with Chlamydia Muridarum Protects the Oviduct from Pathology But Not from Reinfection. Infect. Immun. 2012, 80, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, H.D.; Kromhout, J.; Schachter, J. Purification and Partial Characterization of the Major Outer Membrane Protein of Chlamydia Trachomatis. Infect. Immun. 1981, 31, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Proctor, J.; Stadler, M.; Cortes, L.M.; Brodsky, D.; Poisson, L.; Gerdts, V.; Smirnov, A.I.; Smirnova, T.I.; Barua, S.; Leahy, D.; et al. A TriAdj-Adjuvanted Chlamydia trachomatis Cpaf Protein Vaccine Is Highly Immunogenic in Pigs. Vaccines 2024, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.K.; Li, W.; Guentzel, M.N.; Zhong, G.; Arulanandam, B.P. Vaccination with the Defined Chlamydial Secreted Protein Cpaf Induces Robust Protection against Female Infertility Following Repeated Genital Chlamydial Challenge. Vaccine 2011, 29, 2519–2522. [Google Scholar] [CrossRef]
- Murphey, C.; Murthy, A.K.; Meier, P.A.; Neal Guentzel, M.; Zhong, G.; Arulanandam, B.P. The Protective Efficacy of Chlamydial Protease-Like Activity Factor Vaccination Is Dependent Upon Cd4+ T Cells. Cell Immunol. 2006, 242, 110–117. [Google Scholar] [CrossRef]
- Cong, Y.; Jupelli, M.; Guentzel, M.N.; Zhong, G.; Murthy, A.K.; Arulanandam, B.P. Intranasal Immunization with Chlamydial Protease-Like Activity Factor and Cpg Deoxynucleotides Enhances Protective Immunity against Genital Chlamydia Muridarum Infection. Vaccine 2007, 25, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Favaroni, A.; Tifrea, D.F.; Hanisch, P.T.; Luczak, S.E.T.; Hegemann, J.H.; de la Maza, L.M. Comparison of the Nine Polymorphic Membrane Proteins of Chlamydia Trachomatis for Their Ability to Induce Protective Immune Responses in Mice against a C. Muridarum Challenge. Vaccine 2017, 35, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Becker, E.; Stallmann, S.; Waldhuber, A.; Römmler-Dreher, F.; Albrecht, S.; Mohr, F.; Hegemann, J.H.; Miethke, T. Vaccination with the Polymorphic Membrane Protein a Reduces Chlamydia Muridarum Induced Genital Tract Pathology. Vaccine 2017, 35, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Lanfermann, C.; Wintgens, S.; Ebensen, T.; Kohn, M.; Laudeley, R.; Schulze, K.; Rheinheimer, C.; Hegemann, J.H.; Guzmán, C.A.; Klos, A. Prophylactic Multi-Subunit Vaccine against Chlamydia trachomatis: In Vivo Evaluation in Mice. Vaccines 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Karunakaran, K.P.; Jiang, X.; Shen, C.; Andersen, P.; Brunham, R.C. Chlamydia Muridarum T Cell Antigens and Adjuvants That Induce Protective Immunity in Mice. Infect. Immun. 2012, 80, 1510–1518. [Google Scholar] [CrossRef]
- Poston, T.B.; Girardi, J.; Polson, A.G.; Bhardwaj, A.; Yount, K.S.; Jaras Salas, I.; Trim, L.K.; Li, Y.; O’Connell, C.M.; Leahy, D.; et al. Viral-Vectored Boosting of Omcb- or Cpaf-Specific T-Cell Responses Fail to Enhance Protection from Chlamydia Muridarum in Infection-Immune Mice and Elicits a Non-Protective Cd8-Dominant Response in Naïve Mice. Mucosal Immunol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Muenzner, P.; Hauck, C.R. Neisseria Gonorrhoeae Blocks Epithelial Exfoliation by Nitric-Oxide-Mediated Metabolic Cross Talk to Promote Colonization in Mice. Cell Host Microbe 2020, 27, 793–808.e795. [Google Scholar] [CrossRef] [PubMed]
- Muenzner, P.; Bachmann, V.; Zimmermann, W.; Hentschel, J.; Hauck, C.R. Human-Restricted Bacterial Pathogens Block Shedding of Epithelial Cells by Stimulating Integrin Activation. Science 2010, 329, 1197–1201. [Google Scholar] [CrossRef]
- Ladhani, S.N.; White, P.J.; Campbell, H.; Mandal, S.; Borrow, R.; Andrews, N.; Bhopal, S.; Saunders, J.; Mohammed, H.; Drisdale-Gordon, L.; et al. Use of a Meningococcal Group B Vaccine (4cmenb) in Populations at High Risk of Gonorrhoea in the UK. Lancet Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Marjuki, H.; Topaz, N.; Joseph, S.J.; Gernert, K.M.; Kersh, E.N.; Wang, X. Genetic Similarity of Gonococcal Homologs to Meningococcal Outer Membrane Proteins of Serogroup B Vaccine. mBio 2019, 10, e01668-19. [Google Scholar] [CrossRef]
- Semchenko, E.A.; Tan, A.; Borrow, R.; Seib, K.L. The Serogroup B Meningococcal Vaccine Bexsero Elicits Antibodies to Neisseria Gonorrhoeae. Clin. Infect. Dis. 2019, 69, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Longtin, J.; Dion, R.; Simard, M.; Betala Belinga, J.-F.; Longtin, Y.; Lefebvre, B.; Labbé, A.-C.; Deceuninck, G.; De Wals, P. Possible Impact of Wide-scale Vaccination Against Serogroup B Neisseria Meningitidis on Gonorrhea Incidence Rates in One Region of Quebec, Canada. Open Forum Infect. Dis. 2017, 4, S734–S735. [Google Scholar] [CrossRef]
- Abara, W.E.; Bernstein, K.T.; Lewis, F.M.T.; Schillinger, J.A.; Feemster, K.; Pathela, P.; Hariri, S.; Islam, A.; Eberhart, M.; Cheng, I.; et al. Effectiveness of a Serogroup B Outer Membrane Vesicle Meningococcal Vaccine against Gonorrhoea: A Retrospective Observational Study. Lancet Infect. Dis. 2022, 22, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Giles, L.; Andraweera, P.; McMillan, M.; Almond, S.; Beazley, R.; Mitchell, J.; Ahoure, M.; Denehy, E.; Flood, L.; et al. 4cmenb Sustained Vaccine Effectiveness against Invasive Meningococcal B Disease and Gonorrhoea at Three Years Post Programme Implementation. J. Infect. 2023, 87, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.M.; Castro, C.D.; Kuller, L.; Dukes, A.C.; Centurion-Lara, A.; Morton, W.R.; Lukehart, S.A. Mechanisms of Clearance of Treponema Pallidum from the Csf in a Nonhuman Primate Model. Neurology 1998, 51, 957–961. [Google Scholar] [CrossRef]
- Schell, R.F.; LeFrock, J.L.; Chan, J.K.; Bagasra, O. LSH Hamster Model of Syphilitic Infection. Infect. Immun. 1980, 28, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.; Michalska, E.; Podwińska, J.; Smogór, W. Immunogenic Properties of the Protein Component of Treponema Pallidum. Br. J. Vener. Dis. 1969, 45, 299–304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, K.; Xu, M.; Xiao, Y.; Luo, H.; Xie, Y.; Yu, J.; Tan, M.; Li, Y.; Zhao, F.; Zeng, T.; et al. Immunogenicity and Protective Efficacy against Treponema Pallidum in New Zealand Rabbits Immunized with Plasmid DNA Encoding Flagellin. Emerg. Microbes Infect. 2018, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, X.; Liu, S.; Zeng, T.; Yu, J.; Gu, W.; Zhang, Y.; Chen, X.; Wu, Y. Assessment of the Immune Responses to Treponema Pallidum Gpd DNA Vaccine Adjuvanted with Il-2 and Chitosan Nanoparticles before and after Treponema Pallidum Challenge in Rabbits. Sci. China Life Sci. 2013, 56, 174–180. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, Y.; Zhang, X.; Yu, J.; Gu, W.; Liu, S.; Zeng, T.; Zhang, Y.; Wang, S. Enhanced Immune Response and Protective Efficacy of a Treponema Pallidum Tp92 DNA Vaccine Vectored by Chitosan Nanoparticles and Adjuvanted with IL-2. Hum. Vaccines 2011, 7, 1083–1089. [Google Scholar] [CrossRef][Green Version]
- Xiong, S.; Liu, Z.; Zhang, X.; Huang, S.; Ding, X.; Zhou, J.; Yao, J.; Li, W.; Liu, S.; Zhao, F. Resurgence of Syphilis: Focusing on Emerging Clinical Strategies and Preclinical Models. J. Transl. Med. 2023, 21, 917. [Google Scholar] [CrossRef] [PubMed]
- Darville, T. Pelvic Inflammatory Disease Due to Neisseria Gonorrhoeae and Chlamydia Trachomatis: Immune Evasion Mechanisms and Pathogenic Disease Pathways. J. Infect. Dis. 2021, 224, S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, H.; Fang, C.; Li, Z. Insights into Innate Immune Cell Evasion by Chlamydia Trachomatis. Front. Immunol. 2024, 15, 1289644. [Google Scholar] [CrossRef]
- Scurtu, L.G.; Jinga, V.; Simionescu, O. Fascinating Molecular and Immune Escape Mechanisms in the Treatment of Stis (Syphilis, Gonorrhea, Chlamydia, and Herpes Simplex). Int. J. Mol. Sci. 2022, 23, 3550. [Google Scholar] [CrossRef]
| Disease [Pathogen] | Symptoms of Female Genital Tract Infection | Immune Evasion Mechanisms | Protective Immune Responses | Experimental Models | Vaccine Development |
|---|---|---|---|---|---|
| Chlamydia [Chlamydia trachomatis] | Lower genital tract discharge, dysuria, dyspareunia, urethritis, menstrual irregularities, PID; long term complications include chronic pelvic pain, infertility, ectopic pregnancy, adverse pregnancy outcomes Often asymptomatic Mother to child transmission during birth can cause neonatal conjunctivitis and pneumonia | Evades recognition in intracellular niche [4] Can persist within inclusion in a low metabolic state [4] Multiple molecular mechanisms of epithelial cell invasion [4] Inhibition of neutrophil functions [5] Responses to natural infection are skewed toward Th2 in humans, supporting antibody production which is not associated with long-term protective immunity [6,7,8] | IFNγ-mediated tryptophan depletion in humans restricts chlamydial growth and replication [9] CD4+ Th1 and Th17 responses associated with reduced reinfection in humans [6,8] | Mice, guinea pigs, non-human primates, koalas [10,11] | Recombinant MOMP vaccine adjuvanted with CAF01 in Phase 1 human clinical trials (NCT02787109, NCT03926728) [12,13,14] Several preclinical vaccine candidates using MOMP, CPAF, PmpG recombinant proteins with an emphasis on Th1-inducing adjuvants [15,16,17] |
| Gonorrhea [Neisseria gonorrhoeae] | Lower genital tract discharge, dysuria, dyspareunia, urethritis, menstrual irregularities, PID; long term complications include chronic pelvic pain, infertility, ectopic pregnancy, adverse pregnancy outcomes Often asymptomatic Disseminated infections can lead to sepsis, skin rash, arthritis, endocarditis, meningitis Mother to child transmission during birth can cause ocular or disseminated infection | Antigenic and phase variation [18] Induces IL-10 and regulatory T cells that suppresses antibody responses [19] Inhibits complement deposition and blocks activation of complement [20,21] Epitope masking by IgA1 protease [22] Natural infection in mice drives Th17 and neutrophil responses, which N. gonorrhoeae resists, while suppressing protective Th1 responses [23,24] | CD4+ Th1 responses associated with protection in mice [25] | Antibiotic-treated mice, humanized mice [26,27,28] Controlled human infection model (CHIM) [29] | Phase 2 and Phase 4 human clinical trials evaluating efficacy of currently licensed meningococcal vaccine 4CMenB (Bexsero, GSK) against N. gonnorrhoea infections (NCT04722003, NCT04094883) [30,31] OMV-based vaccine in Phase1/2 human clinical trials (NCT05630859) Pre-clinical studies evaluating OMV-based vaccines in mice and determining candidate antigens for recombinant protein vaccines [32,33] |
| Syphilis [Treponema pallidum] | Primary stage: chancre at infection site Secondary stage: skin lesions, fever, fatigue, rash, muscle aches, weight loss, headaches, hair loss and swollen lymph nodes Tertiary stage: neurological and cardiovascular complications, seizures, deafness, and vision problems including blindness Congenital syphilis: severe outcomes in newborns including prematurity, low birth weight, pneumonia, rash, neurologic disease, and bone abnormalities | Ability to remain dormant for long periods Antigenic variation [34] | CD4+ Th1 IFNγ-mediated recruitment of macrophages [35] Macrophage mediated opsonophagocytosis and killing [36,37] | Rabbits are most widely used [38] Recent development of mouse and in vitro models [39,40] | Pre-clinical studies identifying potential target antigens for recombinant protein vaccines [35,41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yount, K.S.; Darville, T. Immunity to Sexually Transmitted Bacterial Infections of the Female Genital Tract: Toward Effective Vaccines. Vaccines 2024, 12, 863. https://doi.org/10.3390/vaccines12080863
Yount KS, Darville T. Immunity to Sexually Transmitted Bacterial Infections of the Female Genital Tract: Toward Effective Vaccines. Vaccines. 2024; 12(8):863. https://doi.org/10.3390/vaccines12080863
Chicago/Turabian StyleYount, Kacy S., and Toni Darville. 2024. "Immunity to Sexually Transmitted Bacterial Infections of the Female Genital Tract: Toward Effective Vaccines" Vaccines 12, no. 8: 863. https://doi.org/10.3390/vaccines12080863
APA StyleYount, K. S., & Darville, T. (2024). Immunity to Sexually Transmitted Bacterial Infections of the Female Genital Tract: Toward Effective Vaccines. Vaccines, 12(8), 863. https://doi.org/10.3390/vaccines12080863





