Immunogenicity of an Inactivated Senecavirus A Vaccine with a Contemporary Brazilian Strain in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Viral Amplification and Sequencing
2.2. Selection of the Candidate Vaccine Strain
2.3. Vaccine Formulation
2.4. Animals and Vaccine Protocol
2.5. RNA Extraction and RT-qPCR
2.6. Morphologic Assessment
2.7. ELISA IgG for SVA Antibodies
2.8. In Vitro Stimulation and Flow Cytometry
2.9. Statistical Analysis
3. Results
3.1. Selection of a Vaccine Candidate for SVA
3.2. Vaccine Safety
3.3. Viral Load in Feces and Tissues
3.4. Evaluation of Microscopic Lesions in Tissues
3.5. Immunogenic Potential of Vaccine
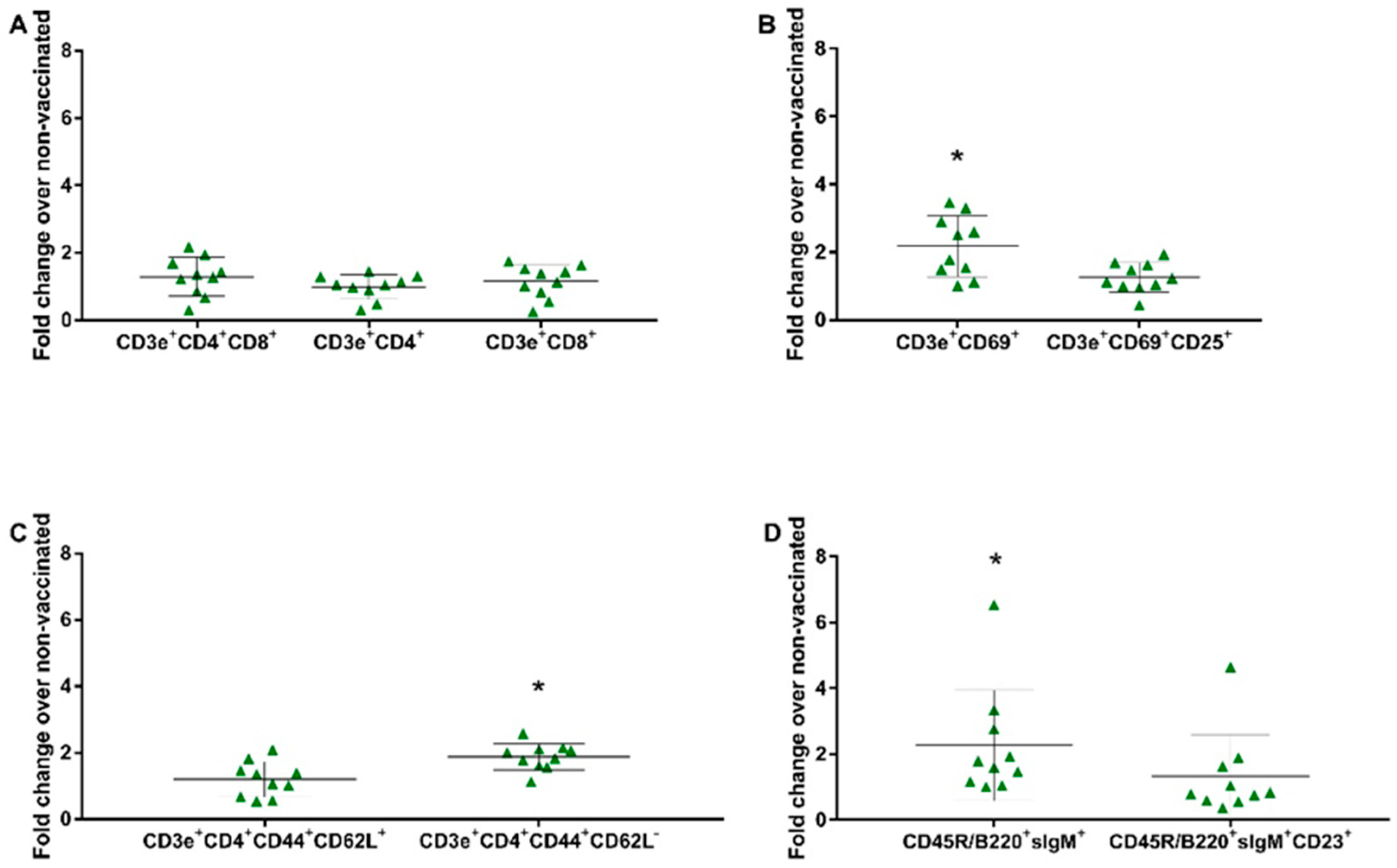
3.6. Evaluation of the Cellular Response against the SVA Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkataraman, S.; Reddy, S.P.; Loo, J.; Idamakanti, N.; Hallenbeck, P.L.; Reddy, V.S. Structure of Seneca Valley Virus-001: An Oncolytic Picornavirus Representing a New Genus. Structure 2008, 16, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Hales, L.M.; Knowles, N.J.; Reddy, P.S.; Xu, L.; Hay, C.; Hallenbeck, P.L. Complete Genome Sequence Analysis of Seneca Valley Virus-001, a Novel Oncolytic Picornavirus. J. Gen. Virol. 2008, 89, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Knowles, N.J.; Hales, L.M.; Jones, B.H.; Landgraf, J.G.; House, J.A.; Skele, K.L.; Burroughs, K.D.; Hallenbeck, P.L. Epidemiology of Seneca Valley Virus: Identification and Characterization of Isolates from Pigs in the United States. XIV Meet. Eur. Study Gr. Mol. Biol. Picornaviruses 2006, Abstract G2. Available online: https://www.researchgate.net/publication/267416696_Epidemiology_of_Seneca_Valley_Virus_Identification_and_Characterization_of_Isolates_from_Pigs_in_the_United_States (accessed on 1 March 2024).
- Pasma, T.; Davidson, S.; Shaw, S.L. Idiopathic Vesicular Disease in Swine in Manitoba. Can. Vet. J. 2008, 49, 84–85. [Google Scholar] [PubMed]
- Houston, E.; Temeeyasen, G.; Piñeyro, P.E. Comprehensive Review on Immunopathogenesis, Diagnostic and Epidemiology of Senecavirus A. Virus Res. 2020, 286, 198038. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Yasumitsu, C.Y.; Dall Agnol, A.M.; Leme, R.A.; Alfieri, A.F.; Alfieri, A.A. The Third Wave of Seneca Valley Virus Outbreaks in Pig Herds in Southern Brazil. Braz. J. Microbiol. 2022, 53, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.R.; Fernandes, M.H.V.; Clement, T.; Lawson, S.; Pillatzki, A.; Resende, T.P.; Vannucci, F.A.; Kutish, G.F.; Nelson, E.A.; Diel, D.G. Pathogenesis of Senecavirus a Infection in Finishing Pigs. J. Gen. Virol. 2016, 97, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.R.; Mohr, K.A.; Gava, D.; Kutish, G.; Buysse, A.S.; Vannucci, F.A.; Piñeyro, P.E.; Crossley, B.M.; Schiltz, J.J.; Jenkins-Moore, M.; et al. Genetic Diversity and Evolution of the Emerging Picornavirus Senecavirus A. J. Gen. Virol. 2020, 101, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.H.V.; Maggioli, M.F.; Joshi, L.R.; Clement, T.; Faccin, T.C.; Rauh, R.; Bauermann, F.V.; Diel, D.G. Pathogenicity and Cross-Reactive Immune Responses of a Historical and a Contemporary Senecavirus a Strains in Pigs. Virology 2018, 522, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Yan, Q.; Du, P.; Yuan, Z.; Sun, Y.; Liu, X.; Zhang, L.; Liu, X.; Ding, H.; Yi, L.; et al. Evolutionary Dynamics and Adaptive Analysis of Seneca Valley Virus. Infect. Genet. Evol. 2023, 113, 105488. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Fernandes, M.H.V.; de Lima, M.; Joshi, L.R.; Lawson, S.; Diel, D.G. A Novel Live Attenuated Vaccine Candidate Protects Against Heterologous Senecavirus a Challenge. Front. Immunol. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Lager, K. Efficacy of an Inactivated Senecavirus a Vaccine in Weaned Pigs and Mature Sows. Vaccine 2022, 40, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liao, Y.; Sun, Y.; Ruan, Y.; Liu, C.; Zhang, M.; Li, F.; Li, X.; Fan, S.; et al. Preliminary Evaluation of Protective Efficacy of Inactivated Senecavirus a on Pigs. Life 2021, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhu, Z.; Cao, W.; Liu, H.; Zhang, K.; Tian, H.; Liu, X.; Zheng, H. Immunogenicity and Protective Efficacy of an Inactivated Cell Culture-Derived Seneca Valley Virus Vaccine in Pigs. Vaccine 2018, 36, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhu, Z.; Liu, H.; Cao, W.; Zhang, W.; Wei, T.; Zheng, M.; Zhang, K.; Tian, H.; Zeng, Q.; et al. Evaluation of Antibody Response in Sows after Vaccination with Senecavirus a Vaccine and the Effect of Maternal Antibody Transfer on Antibody Dynamics in Offspring. Vaccines 2021, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Sun, S.; Dong, H.; Bai, M.; Zhang, Y.; Teng, Z.; Ren, M.; Yin, S.; Guo, H. Potent Protective Immune Responses to Senecavirus Induced by Virus-like Particle Vaccine in Pigs. Vaccines 2020, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Li, Z.; Xie, Y.; Jin, N.; Han, J.; Zhang, H.; Lu, H. Construction and Immunogenicity of Senecavirus a Virus-Like Particle Vaccine with Adjuvant. Vet. Microbiol. 2023, 289, 109971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, F.; Lv, J.; Ding, Y.; Liu, X.; Zhang, L.; Ma, Z.; Zhou, P.; Wang, Y.; Guo, H.; et al. Identification of B-Cell Epitopes on Structural Proteins VP1 and VP2 of Senecavirus A and Development of a Multi-Epitope Recombinant Protein Vaccine. Virology 2023, 582, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qiao, Q.L.; Guo, H.F.; Wang, B.Y.; Huang, Q.; Wang, Z.; Li, Y.T.; Zhao, J. Evaluation of Immunogenicity and Protective Efficacy of a Novel Senecavirus A Strain-Based Inactivated Vaccine in Mice. Res. Vet. Sci. 2022, 142, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Zhu, L.; Xu, L.; Yang, Y.; Zhang, Y.; Liu, Z.; Xu, T.; Wen, J.; Deng, L.; Zhou, Y.; et al. The Construction and Immunogenicity Analyses of a Recombinant Pseudorabies Virus with Senecavirus A VP2 Protein Coexpression. Microbiol. Spectr. 2023, 11, e05229-22. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V.; van Drunen Little-van den Hurk, S.; Griebel, P.J.; Babiuk, L.A. Use of Animal Models in the Development of Human Vaccines. Future Microbiol. 2007, 2, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.R.; Mohr, K.A.; Clement, T.; Hain, K.S.; Myers, B.; Yaros, J.; Nelson, E.A.; Christopher-Hennings, J.; Gava, D.; Schaefer, R.; et al. Detection of the Emerging Picornavirus Senecavirus a in Pigs, Mice, and Houseflies. J. Clin. Microbiol. 2016, 54, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Abajian, C.; Green, P. Consed: A Graphical Tool for Sequence Finishing. Genome Res. 1998, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Markin, A.; Wagle, S.; Grover, S.; Vincent Baker, A.L.; Eulenstein, O.; Anderson, T.K. PARNAS: Objectively Selecting the Most Representative Taxa on a Phylogeny. Syst. Biol. 2023, 72, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R.; Teeling, E. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, H.G. Inactivation of Viral Antigens for Vaccine Preparation with Particular Reference to the Application of Binary Ethylenimine. Vaccine 1990, 8, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Di Canzio, J.; Zurakowski, D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 1998, 221, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.N.; Haach, V.; Bellaver, F.V.; Bombassaro, G.; Gava, D.; da Silva, L.P.; Baron, L.F.; Simonelly, M.; Carvalho, W.A.; Schaefer, R.; et al. Immunological Profile of Mice Immunized with a Polyvalent Virosome-Based Influenza Vaccine. Virol. J. 2023, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.S.; Garon, J.; Seib, K.; Orenstein, W.A. Polio Vaccination: Past, Present and Future. Future Microbiol. 2015, 10, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.L.; Grubman, M.J. Foot and Mouth Disease Virus Vaccines. Vaccine 2009, 27, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Nunnally, B.K.; Turula, V.E.; Sitrin, R.D. Vaccine Analysis: Strategies, Principles, and Control; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783662450246. [Google Scholar]
- Maggioli, M.F.; Lawson, S.; de Lima, M.; Joshi, L.R.; Faccin, T.C.; Bauermann, F.V.; Diel, D.G. Adaptive Immune Responses Following Senecavirus a Infection in Pigs. J. Virol. 2018, 92, e01717-17. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, E.E.B.; Waters, J.; Phillips, M.; Yamamoto, R.; Long, B.R.; Yang, Y.; Gerstein, R.; Stoddart, C.A.; Nakauchi, H.; Herzenberg, L.A. Fetal Hematopoietic Stem Cell Transplantation Fails to Fully Regenerate the B-Lymphocyte Compartment. Stem Cell Rep. 2016, 6, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, T.L.; Griffin, D.O.; Holodick, N.E.; Quach, T.D.; Kaku, H. Human B-1 Cells Take the Stage. Ann. N. Y. Acad. Sci. 2013, 1285, 97–114. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Q.; Huang, Y.; Wang, N.; Shan, H. A 5-Year Review of Senecavirus A in China since Its Emergence in 2015. Front. Vet. Sci. 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.D.; Thomas, M.N.; Anderson, T.K.; Zeller, M.A.; Gauger, P.C.; Vincent Baker, A.L. 2018–2019 human seasonal H3N2 influenza A virus spillovers into swine with demonstrated virus transmission in pigs were not sustained in the pig population. bioRxiv 2024. bioRxiv:2024.01.12.575431. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 2, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, X.; Zhang, H.; Hao, G.; Shang, X.; Wang, H.; Chen, H.; Qian, P. Evaluation of Immunoreactivity and Protection Efficacy of Seneca Valley Virus Inactivated Vaccine in Finishing Pigs Based on Screening of Inactivated Agents and Adjuvants. Vaccines 2022, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Charerntantanakul, W. Adjuvants for Swine Vaccines: Mechanisms of Actions and Adjuvant Effects. Vaccine 2020, 38, 6659–6681. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.E.S.; Gamal, W.M.; Hassan, A.I.; Mahdy, S.E.D.; Hegazy, A.Z.; Abdel-Atty, M.M. Comparative Study on the Immunopotentiator Effect of ISA 201, ISA 61, ISA 50, ISA 206 Used in Trivalent Foot and Mouth Disease Vaccine. Vet. World 2015, 8, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
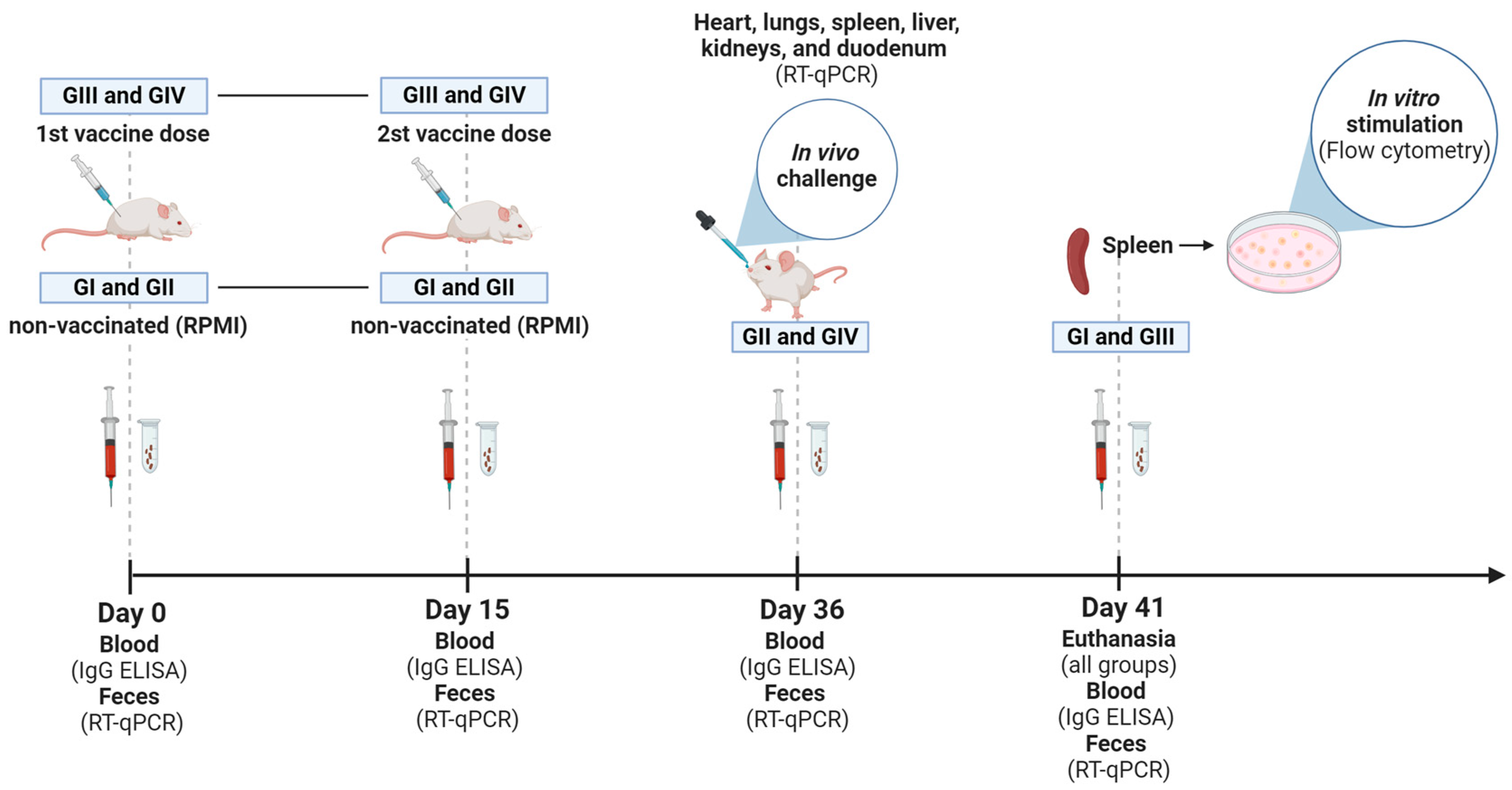


| Experimental Groups | Treatments | |
|---|---|---|
| G I | Non-vaccinated (RPMI, IM *) | in vitro stimulation a |
| G II | Non-vaccinated (RPMI, IM *) | challenge b |
| G III | Vaccinated (107 TCID50/100 µL + ISA 201 VG, IM *) | in vitro stimulation a |
| G IV | Vaccinated (107 TCID50/100 µL + ISA 201 VG, IM *) | challenge b |
| Panel | Antibody | Fluorochrome |
|---|---|---|
| Mouse Naïve/Memory T cell (cod 561609, BD Becton Dickinson) | anti-CD44 anti-CD4 anti-CD62L anti-CD3e | PE PerCP-Cy™5.5 APC APC-Cy™7 |
| Mouse T Lymphocyte Activation Antibody Cocktail (cod 557908, BD Becton Dickinson) | anti-CD25 anti-CD69 anti-CD3e | PE-Cy™7 PE APC |
| Mouse T Lymphocyte Subset Antibody Cocktail (cod 558431, BD Becton Dickinson) | anti-CD3e anti-CD4 anti-CD8α | PE-Cy™7 PE APC |
| Mouse B Lymphocyte Subset Antibody Cocktail (cod 558332, BD Becton Dickinson) | anti-CD45R/B220 anti-CD23 (FcεRII) anti-sIgM | PE-Cy™7 PE APC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, A.d.O.; Gava, D.; Tochetto, C.; Ribeiro, L.C.; Bastos, A.P.A.; Morés, M.A.Z.; Schaefer, R.; de Lima, M. Immunogenicity of an Inactivated Senecavirus A Vaccine with a Contemporary Brazilian Strain in Mice. Vaccines 2024, 12, 845. https://doi.org/10.3390/vaccines12080845
Barbosa AdO, Gava D, Tochetto C, Ribeiro LC, Bastos APA, Morés MAZ, Schaefer R, de Lima M. Immunogenicity of an Inactivated Senecavirus A Vaccine with a Contemporary Brazilian Strain in Mice. Vaccines. 2024; 12(8):845. https://doi.org/10.3390/vaccines12080845
Chicago/Turabian StyleBarbosa, Amanda de Oliveira, Danielle Gava, Caroline Tochetto, Leonardo Clasen Ribeiro, Ana Paula Almeida Bastos, Marcos Antônio Zanella Morés, Rejane Schaefer, and Marcelo de Lima. 2024. "Immunogenicity of an Inactivated Senecavirus A Vaccine with a Contemporary Brazilian Strain in Mice" Vaccines 12, no. 8: 845. https://doi.org/10.3390/vaccines12080845
APA StyleBarbosa, A. d. O., Gava, D., Tochetto, C., Ribeiro, L. C., Bastos, A. P. A., Morés, M. A. Z., Schaefer, R., & de Lima, M. (2024). Immunogenicity of an Inactivated Senecavirus A Vaccine with a Contemporary Brazilian Strain in Mice. Vaccines, 12(8), 845. https://doi.org/10.3390/vaccines12080845











