Comparison of Post-Vaccination Response (Humoral and Cellular) to BNT162b2 in Clinical Cases, Kidney and Pancreas Transplant Recipient with Immunocompetent Subjects over Almost Two Years of Parallel Monitoring
Abstract
1. Introduction
2. Materials and Methods
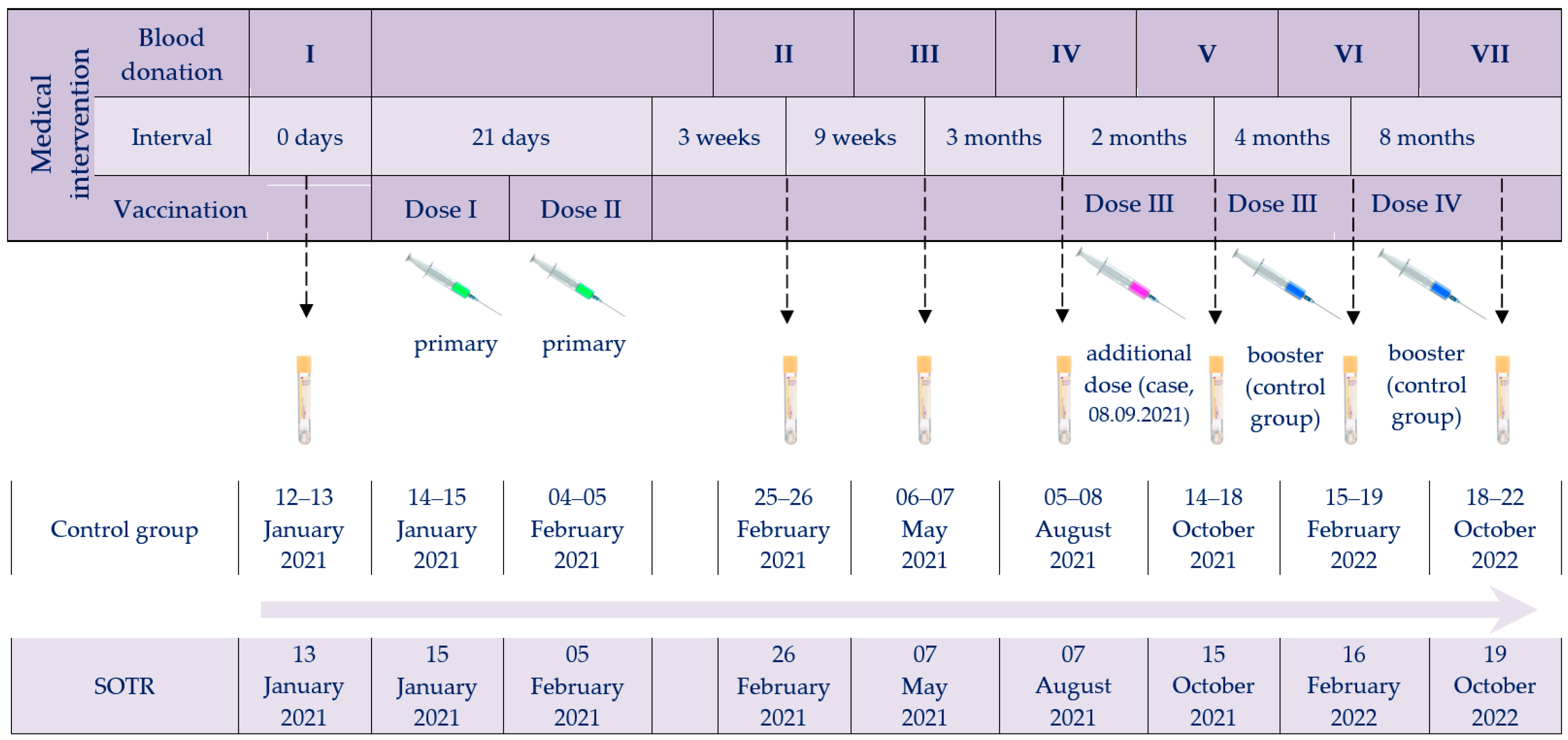
2.1. Study Design, Data Collection, and Participant Characteristics
2.2. SARS-CoV-2 Testing of SOTR Case
2.3. Anti-SARS-CoV-2 Immune Response Tests
3. Results
3.1. Participant Characteristics
3.2. SARS-CoV-2 Detection and Variant Identification
3.3. Anti-SARS-CoV-2 Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 7 May 2024).
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Hasan, I.; Rashid, T.; Suliman, S.; Amer, H.; Chirila, R.M.; Mai, M.L.; Jarmi, T.; Khouzam, S.; Franco, P.M.; Heilig, C.W.; et al. Predictors of disease severity and outcome of hospitalized renal transplant recipients with COVID-19 infection: A systematic review of a globally representative sample. Rom. J. Intern. Med. 2021, 59, 10–42. [Google Scholar] [CrossRef]
- Asderakis, A.; Khalid, U.; Koimtzis, G.; Ponsford, M.J.; Szabo, L.; Chalklin, C.; Bramhall, K.; Grant, L.; Moat, S.J.; Humphreys, I.R.; et al. An Analysis of Serological Response and Infection Outcomes Following Oxford-AstraZeneca (AZD1222) and Pfizer-BioNTech (mRNA BNT162b2) SARS-CoV-2 Vaccines in Kidney and Kidney-pancreas Transplants. Transplantation 2022, 1, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Wadei, H.M.; Gonwa, T.A.; Leoni, J.C.; Shah, S.Z.; Aslam, N.; Speicher, L.L. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am. J. Transplant. 2021, 21, 3496–3499. [Google Scholar] [CrossRef]
- Subramanian, V. Susceptibility to SARS-CoV-2 Infection and Immune Responses to COVID-19 Vaccination Among Recipients of Solid Organ Transplants. J. Infect. Dis. 2023, 4, S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Walory, J.; Ksiazek, I.; Karynski, M.; Baraniak, A. Twenty-Month Monitoring of Humoral Immune Response to BNT162b2 Vaccine: Antibody Kinetics, Breakthrough Infections, and Adverse Effects. Vaccines 2023, 10, 1578. [Google Scholar] [CrossRef]
- Komiazyk, M.; Walory, J.; Kozinska, A.; Wasko, I.; Baraniak, A. Impact of the Nucleic Acid Extraction Method and the RT-qPCR Assay on SARS-CoV-2 Detection in Low-Viral Samples. Diagnostics 2021, 11, 2247. [Google Scholar] [CrossRef]
- Komiazyk, M.; Walory, J.; Gawor, J.; Ksiazek, I.; Gromadka, R.; Baraniak, A. Case Report of COVID-19 after full vaccination: Viral loads and anti-SARS-CoV-2 antibodies. Diagnostics 2021, 11, 1815. [Google Scholar] [CrossRef]
- Wegrzynska, K.; Komiazyk, M.; Walory, J.; Kozinska, A.; Wasko, I.; Baraniak, A. Differentiation of SARS-CoV-2 Variants Using RT-qPCRs by Targeting Recurrent Mutation Sites: A Diagnostic Laboratory Experience from Multi-Center Regional Study, August 2020–December 2021, Poland. Int. J. Mol. Sci. 2022, 23, 9416. [Google Scholar] [CrossRef]
- Caillard, S.; Thaunat, O. COVID-19 vaccination in kidney transplant recipients. Nat. Rev. Nephrol. 2021, 17, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, A.; Todesco, E.; Drouin, S.; Hazan, F.; Marot, S.; Thabut, D.; Varnous, S.; Soulié, C.; Barrou, B.; Marcelin, A.G.; et al. Poor Antibody Response After Two Doses of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine in Transplant Recipients. Clin. Infect. Dis. 2022, 74, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, D.; Mei, B.; Du, J.; Liu, X.; Xie, H.; Lin, L.; Song, S.; Gang, M. Immunogenicity of COVID-19 vaccines in solid organ transplant recipients: A systematic review and meta-analysis. Clin. Microbiol. Infection. 2023, 29, 441–456. [Google Scholar] [CrossRef]
- Alotaibi, A.S.; Shalabi, H.A.; Alhifany, A.A.; Alotaibi, N.E.; Alnuhait, M.A.; Altheaby, A.R.; Alhazmi, A.Y. Humoral and Cellular Immunity following Five Doses of COVID-19 Vaccines in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 1166. [Google Scholar] [CrossRef] [PubMed]
- Thomson, T.; Prendecki, M.; Gleeson, S.; Martin, P.; Spensley, K.; De Aguiar, R.C.; Sandhu, B.; Seneschall, C.; Gan, J.; Clarke, C.L.; et al. Immune responses following 3rd and 4th doses of heterologous and homologous COVID-19 vaccines in kidney transplant recipients. EClinicalMedicine 2022, 53, 101642. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Esposito, L.; Hebral, A.L.; Médrano, C.; Guitard, J.; Lavayssière, L.; Cointault, O.; Nogier, M.B.; et al. Anti-SARS-CoV-2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2 vaccine in a large cohort of solid organ transplant patients. Am. J. Transplant. 2022, 22, 1467–1474. [Google Scholar] [CrossRef]
- Tsoutsoura, P.; Xagas, E.; Roussos, S.; Hatzakis, A.; Gourzi, P.; Boletis, I.N.; Marinaki, S. Assessment of mRNA Vaccine Immunogenicity in Solid Organ Transplant Recipients. Medicina 2023, 59, 1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Xie, M.; Rao, W. Clinical application of COVID-19 vaccine in liver transplant recipients. Hepatobiliary Pancreat. Dis. Int. 2024, 23, 339–343. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O‘Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Saharia, K.K.; Husson, J.S.; Niederhaus, S.V.; Iraguha, T.; Avila, S.V.; Yoo, Y.J.; Hardy, N.M.; Fan, X.; Omili, D.; Crane, A.; et al. Humoral immunity against SARS-CoV-2 variants including omicron in solid organ transplant recipients after three doses of a COVID-19 mRNA vaccine. Clin. Transl. Immunol. 2022, 11, e1391. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Hu, Q.; Samson, R.; Ferreira, V.H.; Hall, V.G.; Ierullo, M.; Majchrzak-Kita, B.; Hardy, W.; Gingras, A.C.; Humar, A. Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine. Am. J. Transplant. 2022, 22, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Al Jurdi, A.; Gassen, R.B.; Borges, T.J.; Lape, I.T.; Morena, L.; Efe, O.; Solhjou, Z.; El Fekih, R.; Deban, C.; Bohan, B.; et al. Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int. 2022, 101, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control, ECDC. Technical Report: Overview of the Implementation of COVID-19 Vaccination Strategies and Deployment Plans in the EU/EEA, 3 March 2023. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Public-health-considerations-to-support-decisions-on-implementing-a-second-mRNA-COVID-19-vaccine-booster-dose.pdf (accessed on 10 July 2024).

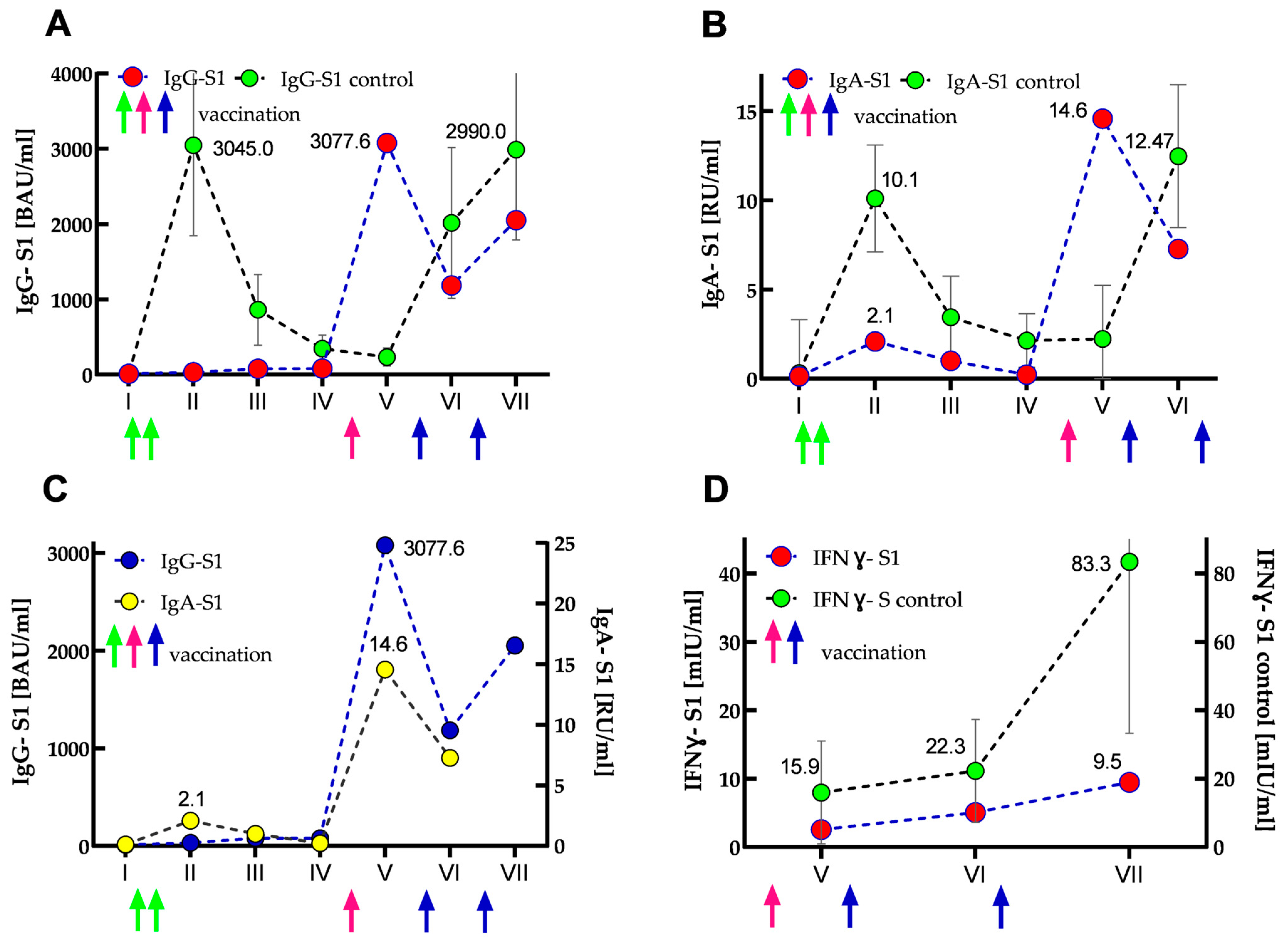
| Variable | IgG-S1 (BAU/mL) | IgA-S1 (RU/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | I | II | III | IV | V | VI | ||
| Control | N | 88 | 88 | 88 | 87 | 84 | 83 | 74 | 88 | 88 | 88 | 87 | 84 | 83 |
| Median | 11.90 | 3045 | 861 | 345 | 233 | 2015 | 2990 | 0.32 | 10.1 | 3.44 | 2.14 | 2.23 | 12.47 | |
| IQR | 9.40 | 2490 | 942 | 370 | 252 | 2056 | 3481 | 6.12 | 6.48 | 4.70 | 3.23 | 6.19 | 18.53 | |
| Percentage | 0.39 | 100 | 30.61 | 11.58 | 8.27 | 50.68 | 83.31 | 3.16 | 100 | 38.36 | 24.46 | 23.71 | 104.31 | |
| SOTR | Value Percentage | 11.78 0.38 | 32.0 1.05 | 77.81 2.55 | 79.21 2.60 | 3077.6 101.07 | 1185.6 38.93 | 2052.9 67.41 | 0.13 1.28 | 2.09 20.69 | 1.0 9.90 | 0.22 2.17 | 14.57 144.25 | 7.27 71.98 |
| Variable | IFNγ-S1 (mIU/mL) | Non-Specific IFNγ (mIU/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| V | VI | VII | V | VI | VII | |||
| Raw data | Control | N | 78 | 78 | 76 | 78 | 78 | 76 |
| Median | 1303.2 | 1590.4 | 4250.5 | 7283.2 | 7127.1 | 4927.9 | ||
| IQR | 1362.4 | 1577.7 | 5002.1 | 8533.6 | 6209.6 | 4022.4 | ||
| Percentage | 100 | 122.1 | 326.1 | 100 | 97.6 | 67.6 | ||
| SOTR | Value Percentage | 15.8 1.2 | 39.7 3.1 | 48.9 3.75 | 619.6 8.5 | 786.6 10.8 | 516.9 7.1 | |
| Normalized data * | Control | N | 78 | 78 | 76 | - | - | - |
| Median | 15.9 | 22.3 | 83.3 | |||||
| IQR | 33.0 | 29.7 | 159.6 | |||||
| Percentage | 100 | 140.3 | 1003.7 | |||||
| SOTR | Value Percentage | 2.6 16.4 | 5.1 32.1 | 9.5 59.7 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walory, J.; Ksiazek, I.; Wegrzynska, K.; Baraniak, A. Comparison of Post-Vaccination Response (Humoral and Cellular) to BNT162b2 in Clinical Cases, Kidney and Pancreas Transplant Recipient with Immunocompetent Subjects over Almost Two Years of Parallel Monitoring. Vaccines 2024, 12, 844. https://doi.org/10.3390/vaccines12080844
Walory J, Ksiazek I, Wegrzynska K, Baraniak A. Comparison of Post-Vaccination Response (Humoral and Cellular) to BNT162b2 in Clinical Cases, Kidney and Pancreas Transplant Recipient with Immunocompetent Subjects over Almost Two Years of Parallel Monitoring. Vaccines. 2024; 12(8):844. https://doi.org/10.3390/vaccines12080844
Chicago/Turabian StyleWalory, Jaroslaw, Iza Ksiazek, Karolina Wegrzynska, and Anna Baraniak. 2024. "Comparison of Post-Vaccination Response (Humoral and Cellular) to BNT162b2 in Clinical Cases, Kidney and Pancreas Transplant Recipient with Immunocompetent Subjects over Almost Two Years of Parallel Monitoring" Vaccines 12, no. 8: 844. https://doi.org/10.3390/vaccines12080844
APA StyleWalory, J., Ksiazek, I., Wegrzynska, K., & Baraniak, A. (2024). Comparison of Post-Vaccination Response (Humoral and Cellular) to BNT162b2 in Clinical Cases, Kidney and Pancreas Transplant Recipient with Immunocompetent Subjects over Almost Two Years of Parallel Monitoring. Vaccines, 12(8), 844. https://doi.org/10.3390/vaccines12080844








