Immunogenic Comparison of Nucleic Acid-Based Vaccines Administered by Pyro-Drive Jet Injector
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Plasmid DNA and mRNA Vaccines
2.3. Preparation of mRNA-LNPs
2.4. Mouse Vaccination Protocols
2.5. Detection of OVA Expression by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. ELISA-Based Anti-OVA Antibody Titer Analysis
2.7. Detection of Luciferase Expression in Skin Tissue Samples
2.8. In Vivo Bioluminescence Analysis
2.9. IFN-γ ELISpot Assay
2.10. Quantitative Real-Time PCR Analysis
2.11. Statistical Analysis
3. Results
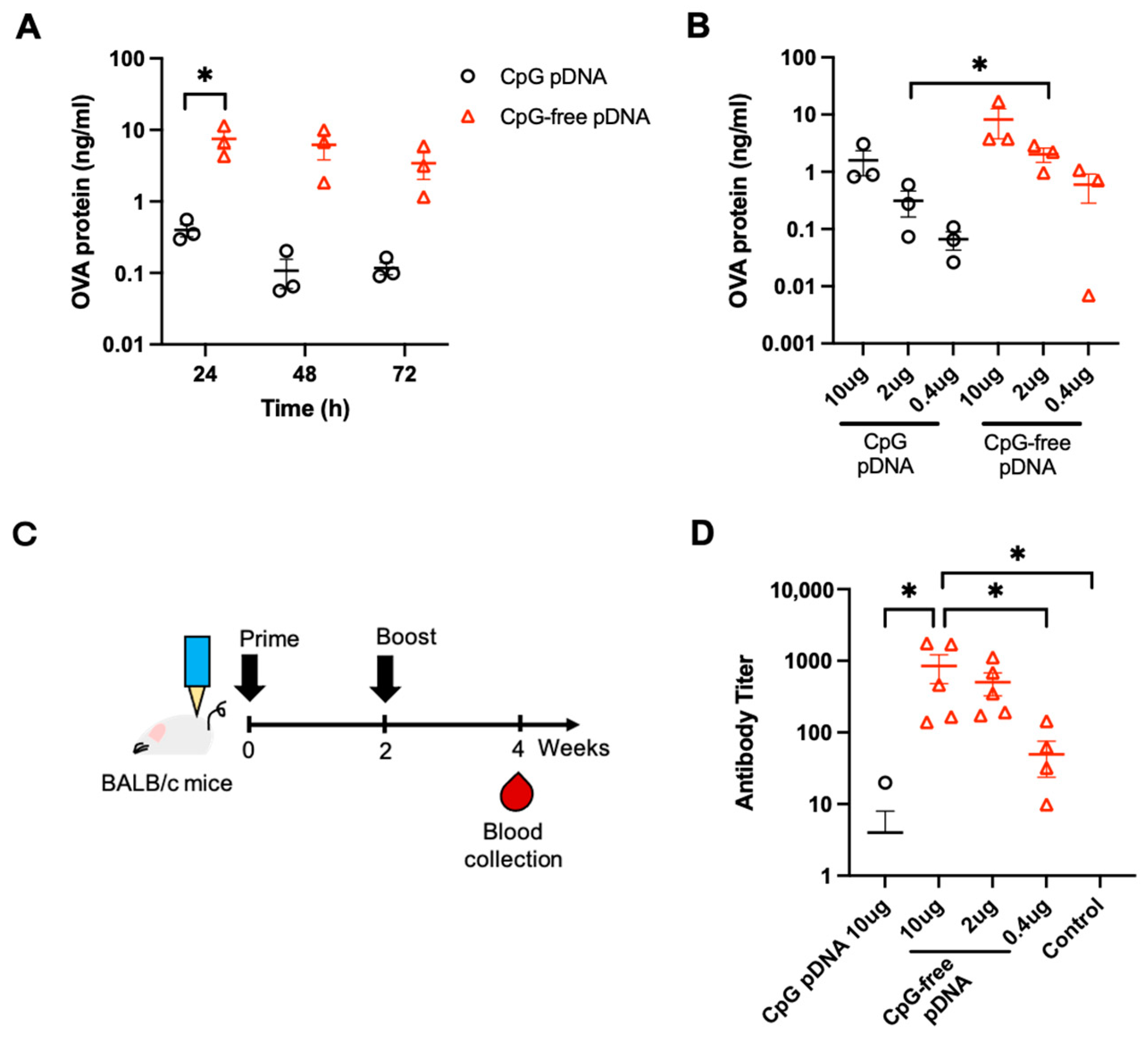
3.1. Effects of OVA pDNA Vaccine Delivered by PJI on Protein Expression and Antibody Response
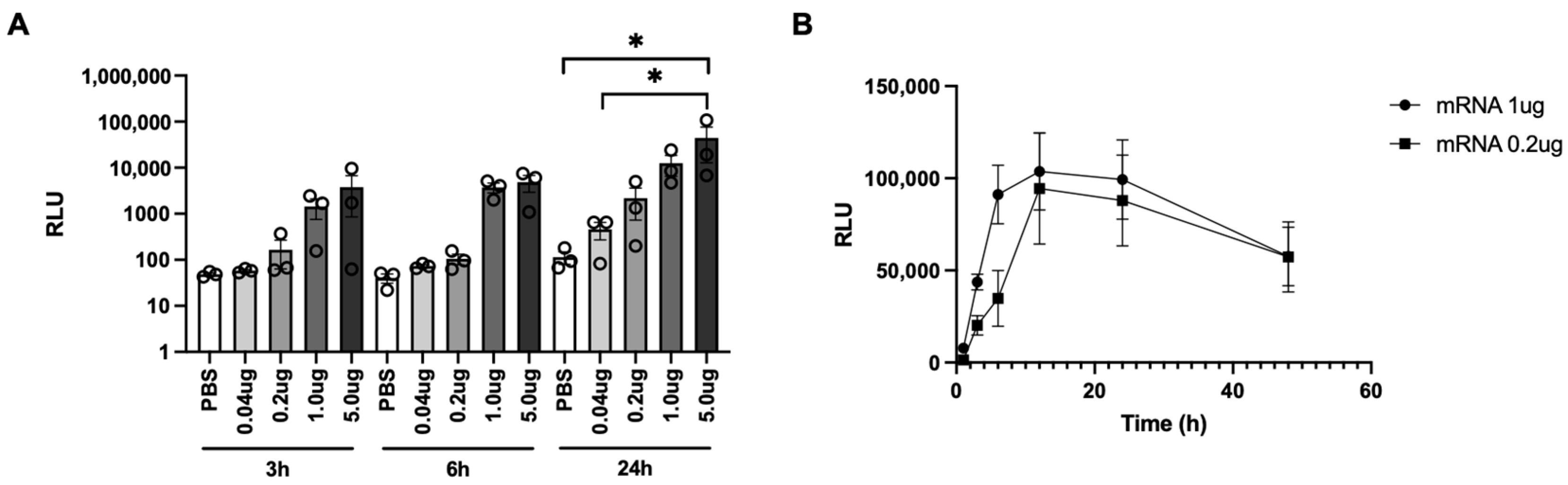
3.2. Evaluation of PJI-Delivered mRNA Vaccine on Gene Expression
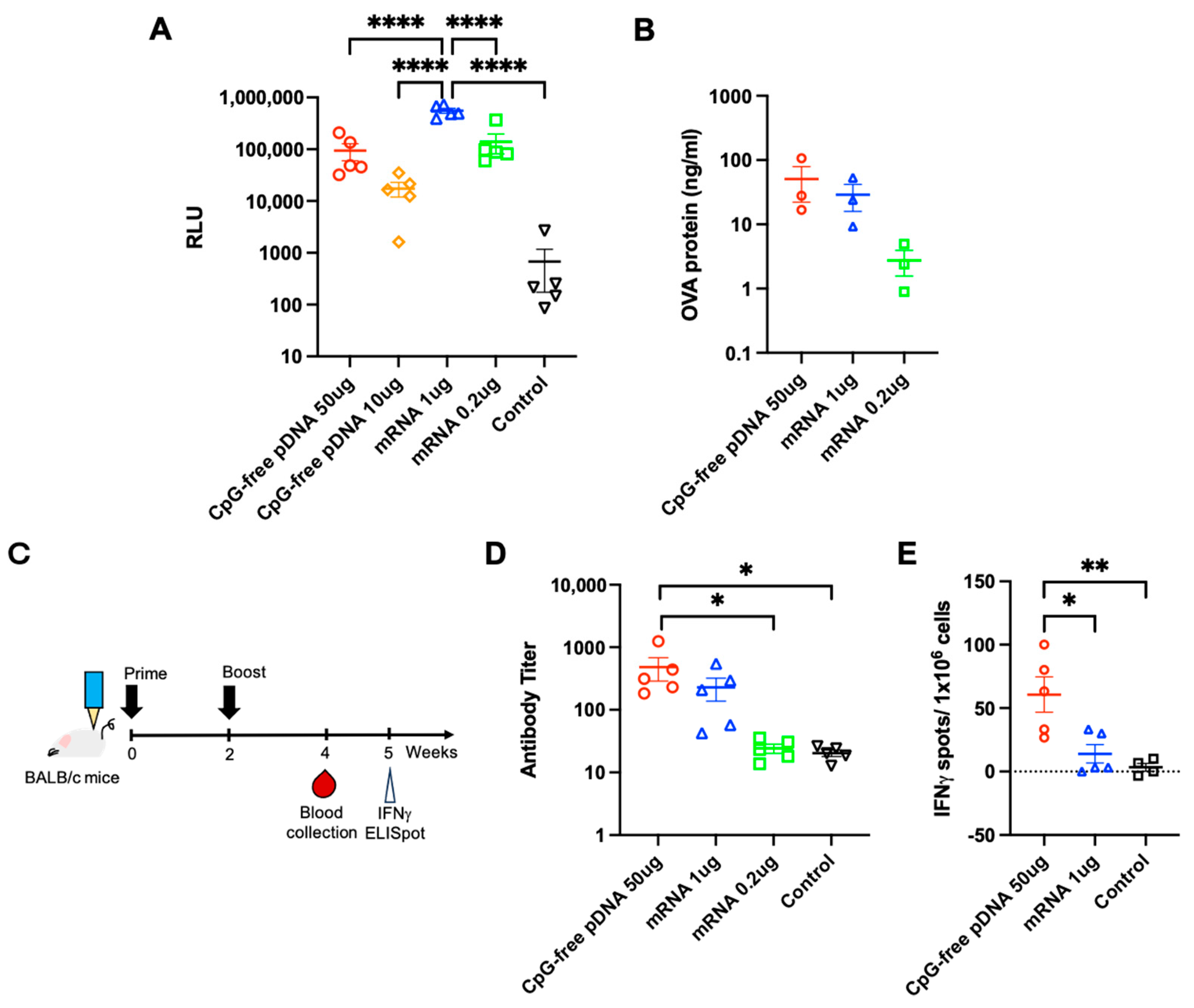
3.3. Comparison of PJI-Delivered pDNA and mRNA Vaccines on Gene Expression and Immune Response
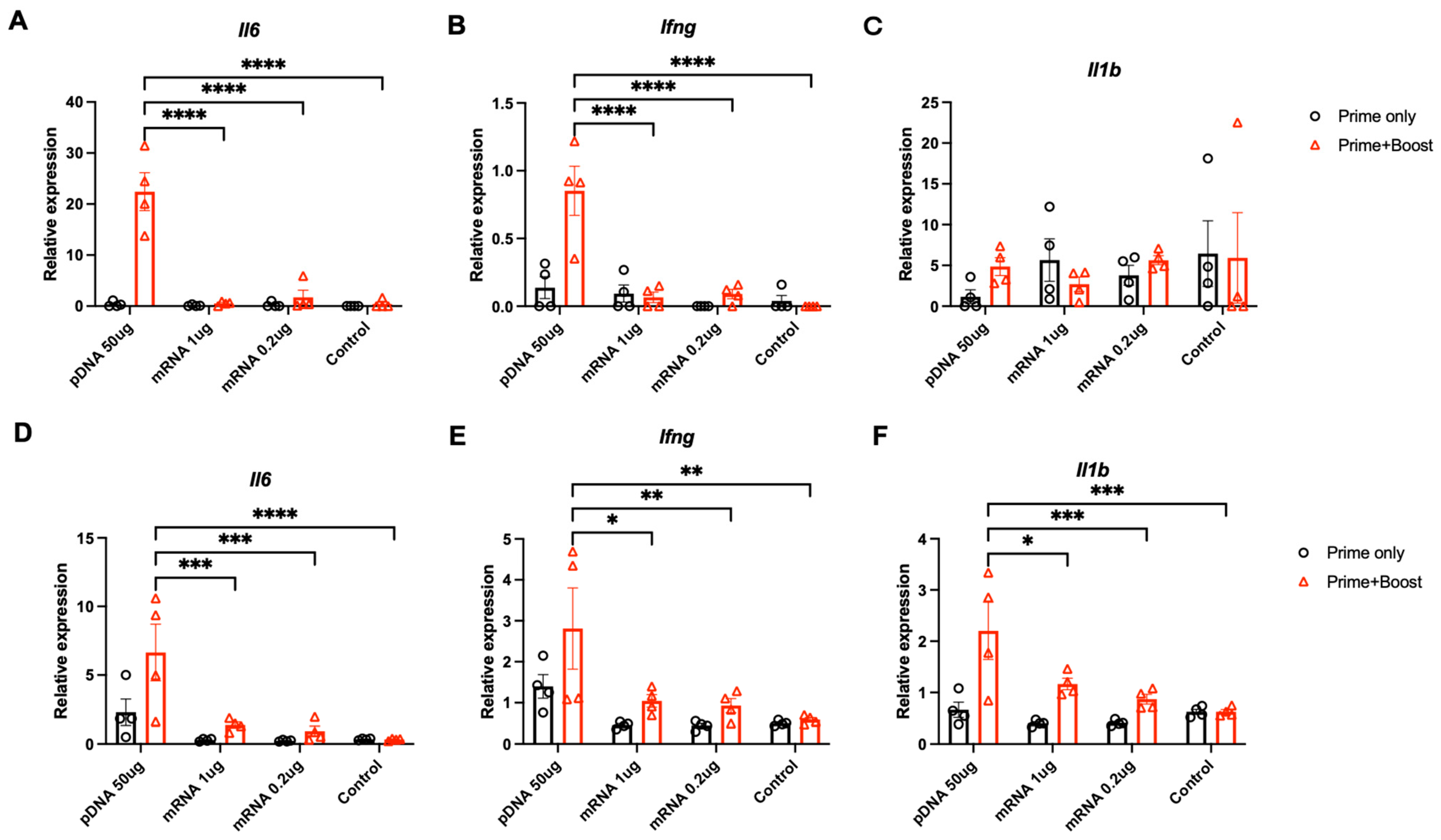
3.4. Pro-Inflammatory Factors at the Injection Site and Lymph Nodes
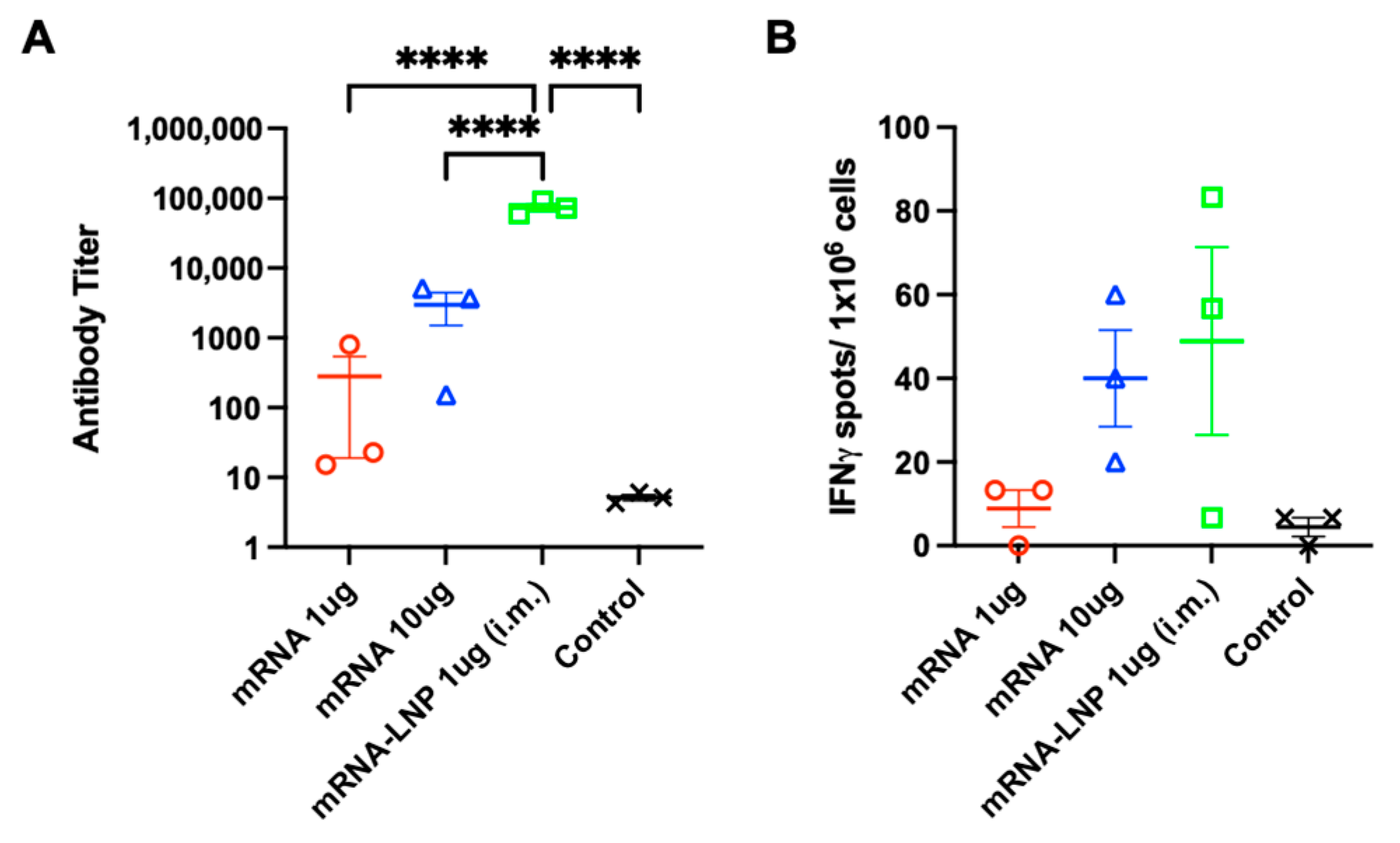
3.5. Comparison of mRNA Delivered by PJI and in Combination with LNP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Nakagami, H.; Hayashi, H.; Sun, J.; Yanagida, Y.; Otera, T.; Nakagami, F.; Hamaguchi, S.; Yoshida, H.; Okuno, H.; Yoshida, S.; et al. Phase I Study to Assess the Safety and Immunogenicity of an Intradermal COVID-19 DNA Vaccine Administered Using a Pyro-Drive Jet Injector in Healthy Adults. Vaccines 2022, 10, 1427. [Google Scholar] [CrossRef]
- Tebas, P.; Yang, S.; Boyer, J.D.; Reuschel, E.L.; Patel, A.; Christensen-Quick, A.; Andrade, V.M.; Morrow, M.P.; Kraynyak, K.; Agnes, J.; et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine 2021, 31, 100689. [Google Scholar] [CrossRef]
- Dauphin, G.; Zientara, S. West Nile virus: Recent trends in diagnosis and vaccine development. Vaccine 2007, 25, 5563–5576. [Google Scholar] [CrossRef]
- Atherton, M.J.; Morris, J.S.; McDermott, M.R.; Lichty, B.D. Cancer immunology and canine malignant melanoma: A comparative review. Vet. Immunol. Immunopathol. 2016, 169, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Suschak, J.J.; Williams, J.A.; Schmaljohn, C.S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum. Vaccin. Immunother. 2017, 13, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Enama, M.E.; Nason, M.C.; Gordon, I.J.; Peel, S.A.; Ledgerwood, J.E.; Plummer, S.A.; Mascola, J.R.; Bailer, R.T.; Roederer, M.; et al. DNA vaccine delivered by a needle-free injection device improves potency of priming for antibody and CD8+ T-cell responses after rAd5 boost in a randomized clinical trial. PLoS ONE 2013, 8, e59340. [Google Scholar] [CrossRef] [PubMed]
- Widera, G.; Austin, M.; Rabussay, D.; Goldbeck, C.; Barnett, S.W.; Chen, M.; Leung, L.; Otten, G.R.; Thudium, K.; Selby, M.J.; et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 2000, 164, 4635–4640. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Sun, J.; Hayashi, H.; Suzuki, A.; Sakaguchi, Y.; Miyazaki, H.; Nishikawa, T.; Nakagami, H.; Yamashita, K.; Kaneda, Y. Stable Immune Response Induced by Intradermal DNA Vaccination by a Novel Needleless Pyro-Drive Jet Injector. AAPS PharmSciTech 2019, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sun, J.; Yanagida, Y.; Otera, T.; Sasai, M.; Chang, C.Y.; Tai, J.A.; Nishikawa, T.; Yamashita, K.; Sakaguchi, N.; et al. Modified DNA vaccine confers improved humoral immune response and effective virus protection against SARS-CoV-2 delta variant. Sci. Rep. 2022, 12, 20923. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Chang, C.Y.; Tai, J.A.; Hayashi, H.; Sun, J.; Torii, S.; Ono, C.; Matsuura, Y.; Ide, R.; Mineno, J.; et al. Immune response induced in rodents by anti-CoVid19 plasmid DNA vaccine via pyro-drive jet injector inoculation. Immunol. Med. 2022, 45, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sun, J.; Yanagida, Y.; Otera, T.; Tai, J.A.; Nishikawa, T.; Yamashita, K.; Sakaguchi, N.; Yoshida, S.; Baba, S.; et al. Intradermal administration of DNA vaccine targeting Omicron SARS-CoV-2 via pyro-drive jet injector provides the prolonged neutralizing antibody production via germinal center reaction. Sci. Rep. 2023, 13, 13033. [Google Scholar] [CrossRef]
- Abbasi, S.; Matsui-Masai, M.; Yasui, F.; Hayashi, A.; Tockary, T.A.; Mochida, Y.; Akinaga, S.; Kohara, M.; Kataoka, K.; Uchida, S. Carrier-free mRNA vaccine induces robust immunity against SARS-CoV-2 in mice and non-human primates without systemic reactogenicity. Mol. Ther. 2024, 32, 1266–1283. [Google Scholar] [CrossRef]
- Diebold, S.S.; Cotten, M.; Koch, N.; Zenke, M. MHC class II presentation of endogenously expressed antigens by transfected dendritic cells. Gene Ther. 2001, 8, 487–493. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, Y. Preparation and Optimization of Lipid-Like Nanoparticles for mRNA Delivery. In RNA Nanostructures: Methods and Protocols; Bindewald, E., Shapiro, B.A., Eds.; Springer: New York, NY, USA, 2017; pp. 207–217. [Google Scholar]
- Jürgens, D.C.; Deßloch, L.; Porras-Gonzalez, D.; Winkeljann, J.; Zielinski, S.; Munschauer, M.; Hörner, A.L.; Burgstaller, G.; Winkeljann, B.; Merkel, O.M. Lab-scale siRNA and mRNA LNP manufacturing by various microfluidic mixing techniques—An evaluation of particle properties and efficiency. OpenNano 2023, 12, 100161. [Google Scholar] [CrossRef]
- Yew, N.S.; Zhao, H.; Przybylska, M.; Wu, I.H.; Tousignant, J.D.; Scheule, R.K.; Cheng, S.H. CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo. Mol. Ther. 2002, 5, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Yew, N.S.; Zhao, H.; Wu, I.H.; Song, A.; Tousignant, J.D.; Przybylska, M.; Cheng, S.H. Reduced inflammatory response to plasmid DNA vectors by elimination and inhibition of immunostimulatory CpG motifs. Mol. Ther. 2000, 1, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Desmet, C.J.; Ishii, K.J. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 2012, 12, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug. Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Wolff, J.A.; Budker, V. The mechanism of naked DNA uptake and expression. Adv. Genet. 2005, 54, 3–20. [Google Scholar] [CrossRef]
- Jorritsma, S.H.T.; Gowans, E.J.; Grubor-Bauk, B.; Wijesundara, D.K. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine 2016, 34, 5488–5494. [Google Scholar] [CrossRef] [PubMed]
- Peachman, K.K.; Rao, M.; Alving, C.R. Immunization with DNA through the skin. Methods 2003, 31, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Elnekave, M.; Furmanov, K.; Hovav, A.H. Intradermal naked plasmid DNA immunization: Mechanisms of action. Expert Rev. Vaccines 2011, 10, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, P.; Obermoser, G.; Nguyen, V.A.; Fritsch, P.; Sepp, N.; Romani, N. Distribution and maturation of skin dendritic cell subsets in two forms of cutaneous T-cell lymphoma: Mycosis fungoides and Sézary syndrome. Acta Derm. Venereol. 2012, 92, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Chozhavel Rajanathan, T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- AboulFotouh, K.; Cui, Z.; Williams, R.O., 3rd. Next-Generation COVID-19 Vaccines Should Take Efficiency of Distribution into Consideration. AAPS PharmSciTech 2021, 22, 126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, J.A.; Nishikawa, T.; Hayashi, H.; Kuan, Y.-D.; Yamashita, K.; Nakagami, H. Immunogenic Comparison of Nucleic Acid-Based Vaccines Administered by Pyro-Drive Jet Injector. Vaccines 2024, 12, 757. https://doi.org/10.3390/vaccines12070757
Tai JA, Nishikawa T, Hayashi H, Kuan Y-D, Yamashita K, Nakagami H. Immunogenic Comparison of Nucleic Acid-Based Vaccines Administered by Pyro-Drive Jet Injector. Vaccines. 2024; 12(7):757. https://doi.org/10.3390/vaccines12070757
Chicago/Turabian StyleTai, Jiayu A., Tomoyuki Nishikawa, Hiroki Hayashi, Yu-Diao Kuan, Kunihiko Yamashita, and Hironori Nakagami. 2024. "Immunogenic Comparison of Nucleic Acid-Based Vaccines Administered by Pyro-Drive Jet Injector" Vaccines 12, no. 7: 757. https://doi.org/10.3390/vaccines12070757
APA StyleTai, J. A., Nishikawa, T., Hayashi, H., Kuan, Y.-D., Yamashita, K., & Nakagami, H. (2024). Immunogenic Comparison of Nucleic Acid-Based Vaccines Administered by Pyro-Drive Jet Injector. Vaccines, 12(7), 757. https://doi.org/10.3390/vaccines12070757





