An Overview of Nanoparticle-Based Delivery Platforms for mRNA Vaccines for Treating Cancer
Abstract
1. Introduction
2. Cancer Vaccines
2.1. Traditional Tumor Vaccine Types
2.2. mRNA Vaccines
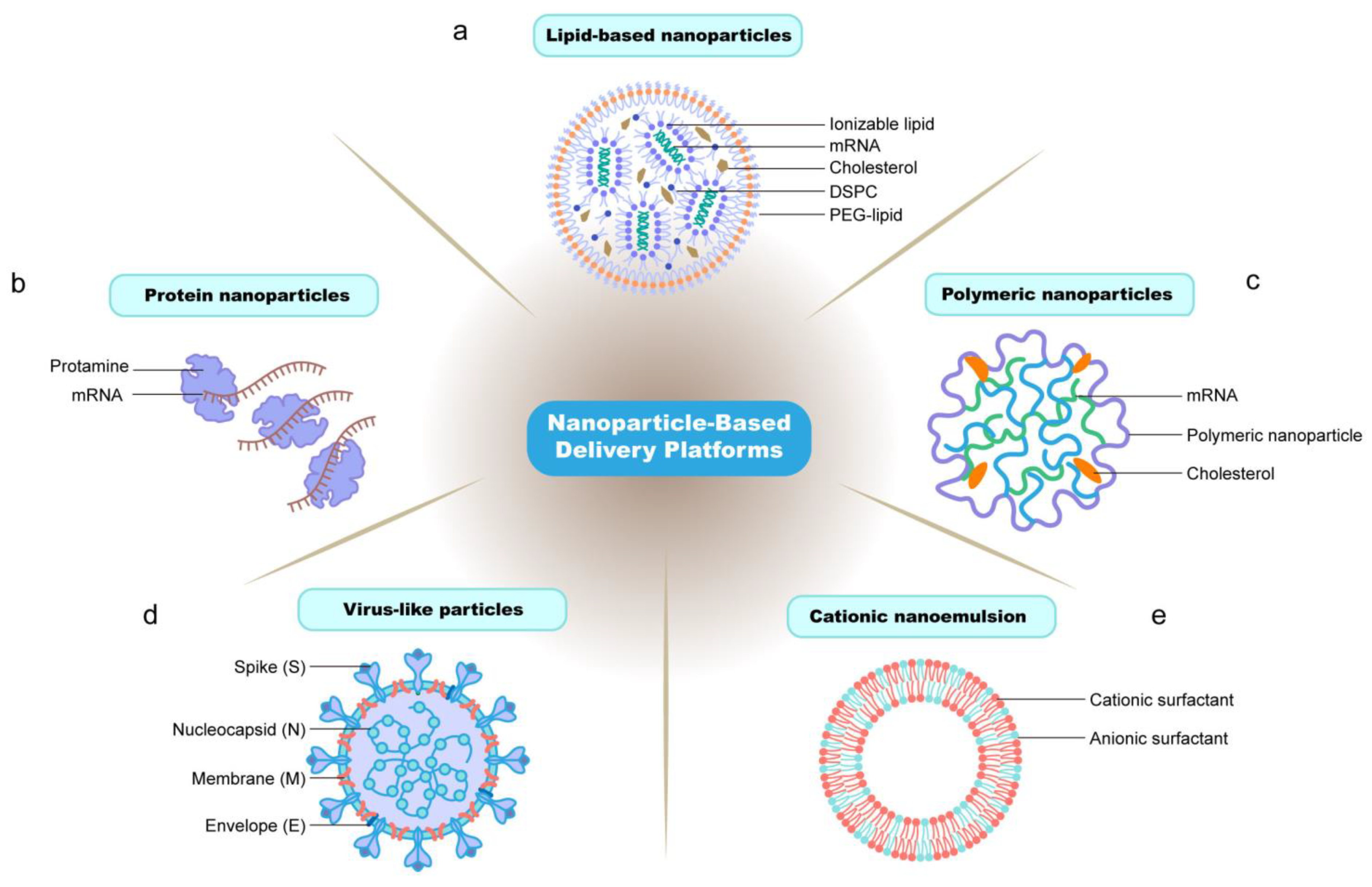
3. Nanotechnology in mRNA Vaccine Platforms
3.1. Lipid-Based Nanoparticle Delivery Systems
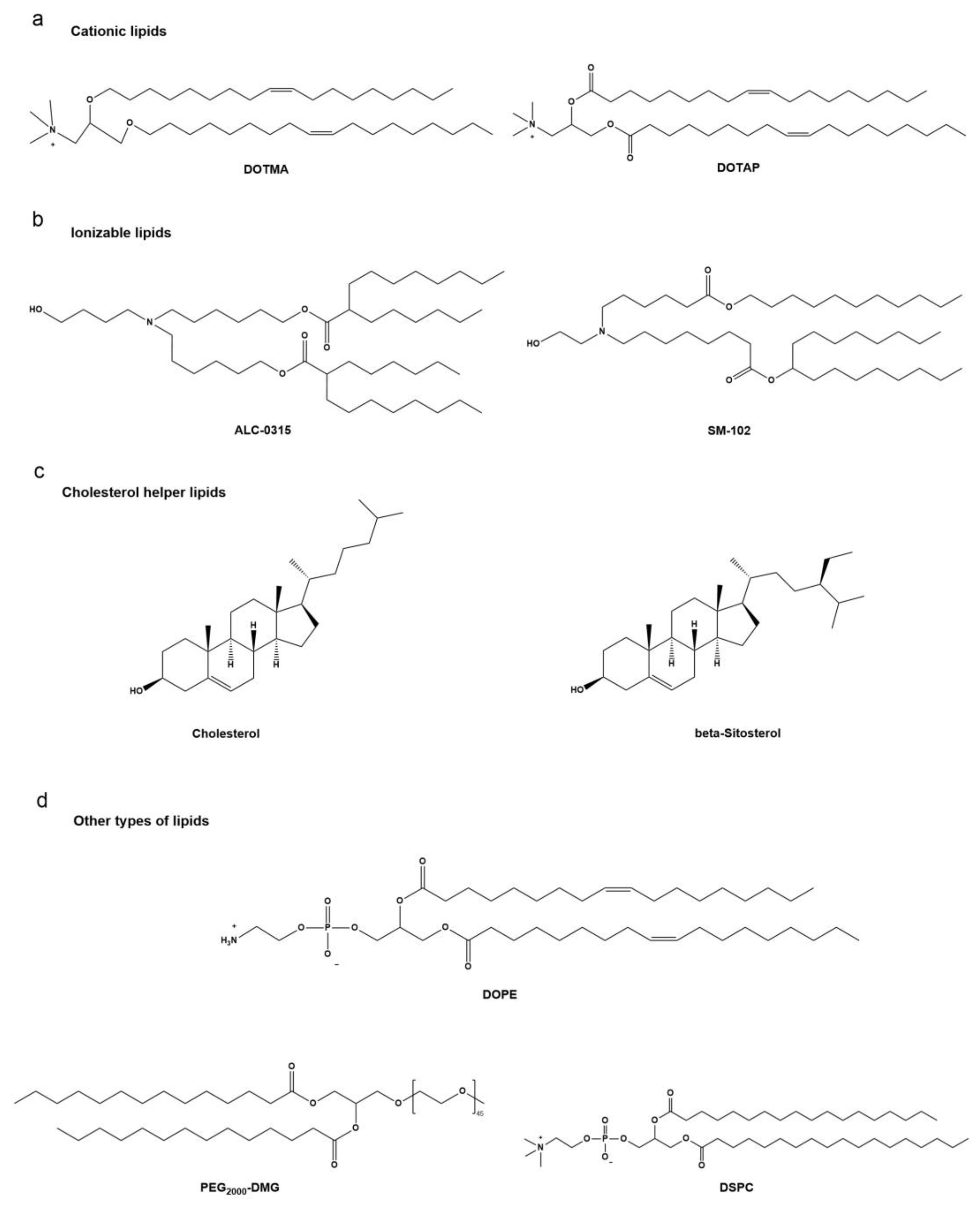
3.1.1. Composition of Lipid-Based Nanoparticles
3.1.2. Stability and Modification of Lipid-Based Nanoparticle Delivery Platforms
3.1.3. Application of Lipid-Based Nanoparticle Delivery Platforms
3.1.4. Disadvantages and Optimization of Lipid-Based Nanoparticle Delivery Platforms
3.1.5. Self-Adjuvants and Adjuvants in Lipid-Based Nanoparticle Delivery Platforms
3.2. Polymer-Based Nanoparticle Delivery Systems
3.3. Protein-Based mRNA Delivery Systems
3.4. Other Formulations Used in mRNA Delivery
3.4.1. Virus-like Particles (VLPs)
3.4.2. Inorganic-Based Nanoparticles
3.4.3. Cationic Nanoemulsions
4. Nanovaccines and Anti-Tumor Immunity
5. Conclusion and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guan, G.; Wu, M.; Han, M.-Y. Stimuli-Responsive Hybridized Nanostructures. Adv. Funct. Mater. 2020, 30, 1903439. [Google Scholar] [CrossRef]
- Gabizon, A.; Isacson, R.; Rosengarten, O.; Tzemach, D.; Shmeeda, H.; Sapir, R. An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemother. Pharmacol. 2008, 61, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Aulic, S.; Marson, D.; Laurini, E.; Fermeglia, M.; Pricl, S. 16–Breast cancer nanomedicine market update and other industrial perspectives of nanomedicine. In Nanomedicines for Breast Cancer Theranostics; Thorat, N.D., Bauer, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–404. [Google Scholar] [CrossRef]
- von Roemeling, C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y.S. Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Guo, M.; Duan, X.; Peng, X.; Jin, Z.; Huang, H.; Xiao, W.; Zheng, Q.; Deng, Y.; Fan, N.; Chen, K.; et al. A lipid-based LMP2-mRNA vaccine to treat nasopharyngeal carcinoma. Nano Res. 2023, 16, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, C.L.; Haanen, J.B.; Met, O.; Svane, I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.K.; Moynihan, K.D.; Irvine, D.J. Engineering New Approaches to Cancer Vaccines. Cancer Immunol. Res. 2015, 3, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Coulie, P.G.; Van den Eynde, B.J.; Agostinis, P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017, 38, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-T.; Liu, X.-H.; An, J.-X.; Liang, J.-L.; Li, C.-X.; Jin, X.-K.; Ji, P.; Zhang, X.-Z. Dendritic Cell-Based In Situ Nanovaccine for Reprogramming Lipid Metabolism to Boost Tumor Immunotherapy. ACS Nano 2023, 17, 24947–24960. [Google Scholar] [CrossRef] [PubMed]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [PubMed]
- Drew, L. Cancer-vaccine trials give reasons for optimism. Nature 2024, 627, S33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Hazama, S.; Shindo, Y.; Nagano, H. Combination treatment of advanced pancreatic cancer using novel vaccine and traditional therapies. Expert. Rev. Anticancer. Ther. 2018, 18, 1205–1217. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Fiedler, K.; Lazzaro, S.; Lutz, J.; Rauch, S.; Heidenreich, R. mRNA Cancer Vaccines. In Current Strategies in Cancer Gene Therapy; Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2016; Volume 209, pp. 61–85. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, A.Y.; Raji, I.; Atyeo, C.; Raimondo, T.M.; Gordon, A.G.R.; Rhym, L.H.; Samad, T.; MacIsaac, C.; Witten, J.; et al. Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA. Nat. Biomed. Eng. 2023, 6, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, Y.; He, Y.; Boucetta, H.; Wu, J.; Chen, Z.; He, W. Lipid carriers for mRNA delivery. Acta Pharm. Sin. B 2023, 13, 4105–4126. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Dobrowolski, C.; Paunovska, K.; Hatit, M.Z.C.; Lokugamage, M.P.; Dahlman, J.A.-O.X. Therapeutic RNA Delivery for COVID and Other Diseases. Adv. Healthc. Mater. 2021, 10, e2002022. [Google Scholar] [CrossRef]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef] [PubMed]
- Dirisala, A.; Uchida, S.; Tockary, T.A.; Yoshinaga, N.; Li, J.; Osawa, S.; Gorantla, L.; Fukushima, S.; Osada, K.; Kataoka, K. Precise tuning of disulphide crosslinking in mRNA polyplex micelles for optimising extracellular and intracellular nuclease tolerability. J. Drug Target. 2019, 27, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.; Mithu, M.D.S.H.; Douroumis, D. Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D. Controlling the Growth of Charged-Nanoparticle Chains through Interparticle Electrostatic Repulsion. Angew. Chem. Int. Ed. 2008, 47, 3984–3987. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective. Bioconjugate Chem. 2023, 34, 941–960. [Google Scholar] [CrossRef]
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug. Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Qiang, C.; Tuo, W. Surface engineering of lipid nanoparticles: Targeted nucleic acid delivery and beyond. Biophys. Rep. 2023, 9, 255–278. [Google Scholar] [CrossRef]
- Patel, P.; Ibrahim, N.M.; Cheng, K. The Importance of Apparent pKa in the Development of Nanoparticles Encapsulating siRNA and mRNA. Trends Pharmacol. Sci. 2021, 42, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Dyett, B.; Kirby, N.; Cai, X.; Mohamad, M.E.; Bozinovski, S.; Drummond, C.J.; Zhai, J. pH-Dependent Lyotropic Liquid Crystalline Mesophase and Ionization Behavior of Phytantriol-Based Ionizable Lipid Nanoparticles. Small 2024, 20, e2309200. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeong, M.; Hur, S.; Cho, Y.; Park, J.; Jung, H.; Seo, Y.; Woo, H.A.; Nam, K.T.; Lee, K.; et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021, 7, eabf4398. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Y.; Han, X. Ionizable Lipid Nanoparticles for mRNA Delivery. Adv. NanoBiomed Res. 2023, 3, 2300006. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2022, 55, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Ramos da Silva, J.; Bitencourt Rodrigues, K.; Formoso Pelegrin, G.; Silva Sales, N.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.F.M.M.; Moreno, A.C.R.; Aps, L.R.M.M.; Venceslau-Carvalho, A.A.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl. Acad. Sci. USA 2022, 119, e2207841119. [Google Scholar] [CrossRef]
- Appidi, T.; Sivasankaran, R.P.; Chinchulkar, S.A.; Patra, P.; Murugaiyan, K.; Veeresh, B.; Rengan, A.K. A lipo-polymeric hybrid nanosystem with metal enhanced fluorescence for targeted imaging of metastatic breast cancer. Nanotheranostics 2024, 8, 239–246. [Google Scholar] [CrossRef]
- Zhang, H.; You, X.; Wang, X.; Cui, L.; Wang, Z.; Xu, F.; Li, M.; Yang, Z.; Liu, J.; Huang, P.; et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2005191118. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Kitte, R.; Rabel, M.; Geczy, R.; Park, S.; Fricke, S.; Koehl, U.; Tretbar, U.S. Lipid nanoparticles outperform electroporation in mRNA-based CAR T cell engineering. Mol. Ther. Methods Clin. Dev. 2023, 31, 101139. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, C.J.; Vargas, J.R.; Blake, T.R.; Hardy, J.W.; Kanada, M.; Contag, C.H.; Wender, P.A.; Waymouth, R.M. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc. Natl. Acad. Sci. USA 2017, 114, E448–E456. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Amaya, L.; Pi, R.; Wang, S.K.; Ranjan, A.; Waymouth, R.M.; Blish, C.A.; Chang, H.Y.; Wender, P.A. Charge-altering releasable transporters enhance mRNA delivery in vitro and exhibit in vivo tropism. Nat. Commun. 2023, 14, 6983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Zhou, Z. Lipid Nanoparticle-Based Delivery System-A Competing Place for mRNA Vaccines. ACS Omega 2024, 9, 6219–6234. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Boettler, T.; Csernalabics, B.; Salié, H.; Luxenburger, H.; Wischer, L.; Salimi Alizei, E.; Zoldan, K.; Krimmel, L.; Bronsert, P.; Schwabenland, M.; et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J. Hepatol. 2022, 77, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Storm, G.; Ljubimova, J.Y.; Castells, M.; Phillips, E.J.; Turjeman, K.; Barenholz, Y.; Crommelin, D.J.A.; Dobrovolskaia, M.A. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat. Nanotechnol. 2022, 17, 337–346. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, C.; Yi, X.; Han, J.; Wang, J.; Liu, F.; Ling, Q.; Li, H.; Gu, Z. Fluorinated Lipid Nanoparticles for Enhancing mRNA Delivery Efficiency. ACS Nano 2024, 18, 7825–7836. [Google Scholar] [CrossRef]
- Yang, K.; Bai, B.; Lei, J.; Yu, X.; Qi, S.; Wang, Y.; Huang, F.; Tong, Z.; Yu, G. Biodegradable Lipid-Modified Poly(Guanidine Thioctic Acid)s: A Fortifier of Lipid Nanoparticles to Promote the Efficacy and Safety of mRNA Cancer Vaccines. J. Am. Chem. Soc. 2024, 146, 11679–11693. [Google Scholar] [CrossRef]
- Lei, J.; Qi, S.; Yu, X.; Gao, X.; Yang, K.; Zhang, X.; Cheng, M.; Bai, B.; Feng, Y.; Lu, M.; et al. Development of Mannosylated Lipid Nanoparticles for mRNA Cancer Vaccine with High Antigen Presentation Efficiency and Immunomodulatory Capability. Angew. Chem. Int. Ed. 2024, 63, e202318515. [Google Scholar] [CrossRef]
- Agrawal, S.; Garg, A.; Varshney, V. Recent Updates on Applications of Lipid-Based Nanoparticles for Site-Specific Drug Delivery. Pharm. Nanotechnol. 2022, 10, 24–41. [Google Scholar] [CrossRef]
- Tombácz, I.; Laczkó, D.; Shahnawaz, H.; Muramatsu, H.; Natesan, A.; Yadegari, A.; Papp, T.E.; Alameh, M.-G.; Shuvaev, V.; Mui, B.L.; et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol. Ther. 2021, 29, 3293–3304. [Google Scholar] [CrossRef]
- Tilsed, C.M.; Sadiq, B.A.; Papp, T.E.; Areesawangkit, P.; Kimura, K.; Noguera-Ortega, E.; Scholler, J.; Cerda, N.; Aghajanian, H.; Bot, A.; et al. IL7 increases targeted lipid nanoparticle-mediated mRNA expression in T cells in vitro and in vivo by enhancing T cell protein translation. Proc. Natl. Acad. Sci. USA 2024, 121, e2319856121. [Google Scholar] [CrossRef] [PubMed]
- Swingle, K.L.; Safford, H.C.; Geisler, H.C.; Hamilton, A.G.; Thatte, A.S.; Billingsley, M.M.; Joseph, R.A.; Mrksich, K.; Padilla, M.S.; Ghalsasi, A.A.; et al. Ionizable Lipid Nanoparticles for In Vivo mRNA Delivery to the Placenta during Pregnancy. J. Am. Chem. Soc. 2023, 145, 4691–4706. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Heydarkhan-Hagvall, S.; Tangruksa, B.; González-King Garibotti, H.; Jing, Y.; Maugeri, M.; Kohl, F.; Hultin, L.; Reyahi, A.; Camponeschi, A.; et al. Lipid Nanoparticles Deliver the Therapeutic VEGFA mRNA In Vitro and In Vivo and Transform Extracellular Vesicles for Their Functional Extensions. Adv. Sci. 2023, 10, 2206187. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- Ahn, J.; Barber, G.N. STING signaling and host defense against microbial infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Han, X.; Alameh, M.-G.; Butowska, K.; Knox, J.J.; Lundgreen, K.; Ghattas, M.; Gong, N.; Xue, L.; Xu, Y.; Lavertu, M.; et al. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat. Nanotechnol. 2023, 18, 1105–1114. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Peppas, N.A. Robotic pills for gastrointestinal-tract-targeted oral mRNA delivery. Matter 2022, 5, 775–777. [Google Scholar] [CrossRef]
- Xiao, Y.; Tang, Z.; Huang, X.; Chen, W.; Zhou, J.; Liu, H.; Liu, C.; Kong, N.; Tao, W. Emerging mRNA technologies: Delivery strategies and biomedical applications. Chem. Soc. Rev. 2022, 51, 3828–3845. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Deng, H.; Zhou, Y.; Chen, X. The roles of polymers in mRNA delivery. Matter 2022, 5, 1670–1699. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.; Munson, M.J.; Sabirsh, A.; England, R.M.; Hemmerling, M.; Alexander, C.; Ashford, M.B. Precise and systematic end group chemistry modifications on PAMAM and poly(l-lysine) dendrimers to improve cytosolic delivery of mRNA. J. Control. Release 2023, 356, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.C.I.M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Guizze, F.; Serra, C.H.d.R.; Giarolla, J. PAMAM Dendrimers: A Review of Methodologies Employed in Biopharmaceutical Classification. J. Pharm. Sci. 2022, 111, 2662–2673. [Google Scholar] [CrossRef]
- Kharwade, R.; More, S.; Warokar, A.; Agrawal, P.; Mahajan, N. Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications. Arab. J. Chem. 2020, 13, 6009–6039. [Google Scholar] [CrossRef]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ta, W.; Hua, R.; Song, J.; Lu, W. A Review on Increasing the Targeting of PAMAM as Carriers in Glioma Therapy. Biomedicines 2022, 10, 2455. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yeudall, W.A.; Yang, H. Folic acid-decorated polyamidoamine dendrimer exhibits high tumor uptake and sustained highly localized retention in solid tumors: Its utility for local siRNA delivery. Acta Biomater. 2017, 57, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, D.; Zhou, Z.; Piao, Y.; Tang, J.; Shen, Y. Glutathione-Specific and Intracellularly Labile Polymeric Nanocarrier for Efficient and Safe Cancer Gene Delivery. ACS Appl. Mater. Interfaces 2020, 12, 14825–14838. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Cázares, H.; Tzeng, S.Y.; Young, N.P.; Abutaleb, A.O.; Quiñones-Hinojosa, A.; Green, J.J. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano 2014, 8, 5141–5153. [Google Scholar] [CrossRef] [PubMed]
- Dosta, P.; Cryer, A.M.; Dion, M.Z.; Shiraishi, T.; Langston, S.P.; Lok, D.; Wang, J.; Harrison, S.; Hatten, T.; Ganno, M.L.; et al. Investigation of the enhanced antitumour potency of STING agonist after conjugation to polymer nanoparticles. Nat. Nanotechnol. 2023, 18, 1351–1363. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, S.; Qi, Y.; Gao, Y.; Zhang, Y.; Li, M.; Chen, J.; Song, W.; Chen, X. A polyamino acid-based phosphatidyl polymer library for in vivo mRNA delivery with spleen targeting ability. Mater. Horiz. 2024, 11, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Suberi, A.; Grun, M.K.; Mao, T.; Israelow, B.; Reschke, M.; Grundler, J.; Akhtar, L.; Lee, T.; Shin, K.; Piotrowski-Daspit, A.S.; et al. Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination. Sci. Transl. Med. 2023, 15, eabq0603. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 1–24. [Google Scholar] [CrossRef]
- Jarzebska, N.T.; Mellett, M.; Frei, J.; Kündig, T.M.; Pascolo, S. Protamine-Based Strategies for RNA Transfection. Pharmaceutics 2021, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Q.; Zhu, F.; Zhao, M.; Yan, Y. mRNA delivery via non-viral carriers for biomedical applications. Int. J. Pharm. 2021, 607, 121020. [Google Scholar] [CrossRef] [PubMed]
- Bäumer, N.; Scheller, A.; Wittmann, L.; Faust, A.; Apel, M.; Nimmagadda, S.C.; Geyer, C.; Grunert, K.; Kellmann, N.; Peipp, M.; et al. Electrostatic anti-CD33-antibody-protamine nanocarriers as platform for a targeted treatment of acute myeloid leukemia. J. Hematol. Oncol. 2022, 15, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Antibody–oligonucleotide conjugates enter the clinic. Nat. Rev. Drug Discov. 2021, 21, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Gilabert, M.; Artero, R.; López-Castel, A. The myotonic dystrophy type 1 drug development pipeline: 2022 edition. Drug Discov. Today 2023, 28, 103489. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.T.; A Poliskey, J.; Baumhover, N.J.; Rice, K.G. Efficient expression of stabilized mRNA PEG-peptide polyplexes in liver. Gene Ther. 2015, 22, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Houser, K.V.; Chen, G.L.; Carter, C.; Crank, M.C.; Nguyen, T.A.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Gordon, I.J.; et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: A phase 1 trial. Nat. Med. 2022, 28, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Udhayakumar, V.K.; De Beuckelaer, A.; McCaffrey, J.; McCrudden, C.M.; Kirschman, J.L.; Vanover, D.; Van Hoecke, L.; Roose, K.; Deswarte, K.; De Geest, B.G.; et al. Arginine-Rich Peptide-Based mRNA Nanocomplexes Efficiently Instigate Cytotoxic T Cell Immunity Dependent on the Amphipathic Organization of the Peptide. Adv. Healthc. Mater. 2017, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.S.; Tandler, C.; Marconato, M.; Nelde, A.; Habibzada, T.; Rittig, S.M.; Tegeler, C.M.; Maringer, Y.; Jaeger, S.U.; Denk, M.; et al. Phase I/II trial of a peptide-based COVID-19 T-cell activator in patients with B-cell deficiency. Nat. Commun. 2023, 14, 5032. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, Y.; Ding, X.; Wang, C.; Hua, B.; Hong, S.; Huang, X.; Lin, J.; Zhang, P.; Chen, W. PEGylated pH-responsive peptide-mRNA nano self-assemblies enhance the pulmonary delivery efficiency and safety of aerosolized mRNA. Drug Deliv. 2023, 30, 2219870. [Google Scholar] [CrossRef]
- D’Haese, S.; Laeremans, T.; Roover, S.D.; Allard, S.D.; Vanham, G.; Aerts, J.L. Efficient Induction of Antigen-Specific CD8+ T-Cell Responses by Cationic Peptide-Based mRNA Nanoparticles. Pharmaceutics 2022, 14, 1387. [Google Scholar] [CrossRef] [PubMed]
- Frietze, K.M.; Peabody, D.S.; Chackerian, B. Engineering virus-like particles as vaccine platforms. Curr. Opin. Virol. 2016, 18, 44–49. [Google Scholar] [CrossRef]
- Unti, M.J.; Jaffrey, S.R. Highly efficient cellular expression of circular mRNA enables prolonged protein expression. Cell Chem. Biol. 2024, 31, 163–176.e165. [Google Scholar] [CrossRef]
- Ling, S.; Yang, S.; Hu, X.; Yin, D.; Dai, Y.; Qian, X.; Wang, D.; Pan, X.; Hong, J.; Sun, X.; et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 2021, 5, 144–156. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Chang, X.; Krenger, P.; Krueger, C.C.; Zha, L.; Han, J.; Yermanos, A.; Roongta, S.; Mohsen, M.O.; Oxenius, A.; Vogel, M.; et al. TLR7 Signaling Shapes and Maintains Antibody Diversity Upon Virus-Like Particle Immunization. Front. Immunol. 2022, 12, 827256. [Google Scholar] [CrossRef]
- Hoffmann, M.A.G.; Yang, Z.; Huey-Tubman, K.E.; Cohen, A.A.; Gnanapragasam, P.N.P.; Nakatomi, L.M.; Storm, K.N.; Moon, W.J.; Lin, P.J.C.; West, A.P., Jr.; et al. ESCRT recruitment to SARS-CoV-2 spike induces virus-like particles that improve mRNA vaccines. Cell 2023, 186, 2380–2391.e9. [Google Scholar] [CrossRef]
- Li, S.; Xu, X.; Xu, L.; Lin, H.; Kuang, H.; Xu, C. Emerging trends in chiral inorganic nanomaterials for enantioselective catalysis. Nat. Commun. 2024, 15, 3506. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- OuYang, X.; Xu, X.; Qin, Q.; Dai, C.; Wang, H.; Liu, S.; Hu, L.; Xiong, X.; Liu, H.; Zhou, D. Manganese-Based Nanoparticle Vaccine for Combating Fatal Bacterial Pneumonia. Adv. Mater. 2023, 35, e2304514. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.F.; Bruxel, F.; Fraga, M.; Schuh, R.S.; Zorzi, G.K.; Matte, U.; Fattal, E. Cationic nanoemulsions as nucleic acids delivery systems. Int. J. Pharm. 2017, 534, 356–367. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Liu, Y.; Liu, J.; Guo, X.; Li, J.; Gong, L.; Zhou, X.; Cheng, G.; Qiu, Y.; et al. Structural and biochemical characteristics of mRNA nanoparticles determine anti–SARS-CoV-2 humoral and cellular immune responses. Sci. Adv. 2022, 8, eabo1827. [Google Scholar] [CrossRef]
- Brito, L.A.; Chan, M.; Shaw, C.A.; Hekele, A.; Carsillo, T.; Schaefer, M.; Archer, J.; Seubert, A.; Otten, G.R.; Beard, C.W.; et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol. Ther. 2014, 22, 2118–2129. [Google Scholar] [CrossRef]
- Aucouturier, J.; Dupuis, L.; Deville, S.; Ascarateil, S.; Ganne, V. Montanide ISA 720 and 51: A new generation of water in oil emulsions as adjuvants for human vaccines. Expert. Rev. Vaccines 2002, 1, 111–118. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Mahalingam, G.; Rachamalla, H.K.; Arjunan, P.; Karuppusamy, K.V.; Periyasami, Y.; Mohan, A.; Subramaniyam, K.; Salma, M.; Rajendran, V.; Moorthy, M.; et al. SMART-lipid nanoparticles enabled mRNA vaccine elicits cross-reactive humoral responses against the omicron sub-variants. Mol. Ther. 2024, 32, 1284–1297. [Google Scholar] [CrossRef]
- Kon, E.; Ad-El, N.; Hazan-Halevy, I.; Stotsky-Oterin, L.; Peer, D. Targeting cancer with mRNA–lipid nanoparticles: Key considerations and future prospects. Nat. Rev. Clin. Oncol. 2023, 20, 739–754. [Google Scholar] [CrossRef]
- Pine, M.; Arora, G.; Hart, T.M.; Bettini, E.; Gaudette, B.T.; Muramatsu, H.; Tombácz, I.; Kambayashi, T.; Tam, Y.K.; Brisson, D.; et al. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Mol. Ther. 2023, 31, 2702–2714. [Google Scholar] [CrossRef]
- Willis, E.; Pardi, N.; Parkhouse, K.; Mui, B.L.; Tam, Y.K.; Weissman, D.; Hensley, S.E. Nucleoside-modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci. Transl. Med. 2020, 12, eaav5701. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Gong, L.; Liang, Z.; Zhu, C.; Ren, C.; Zheng, N.; Zhang, Q.; Liu, H.; Liu, W.; et al. Manganese nanodepot augments host immune response against coronavirus. Nano Res. 2021, 14, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Chelvanambi, M.; Fecek, R.J.; Taylor, J.L.; Storkus, W.J. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J. Immunother. Cancer 2021, 9, e001906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef]
- Chen, F.; Li, T.; Zhang, H.; Saeed, M.; Liu, X.; Huang, L.; Wang, X.; Gao, J.; Hou, B.; Lai, Y.; et al. Acid-Ionizable Iron Nanoadjuvant Augments STING Activation for Personalized Vaccination Immunotherapy of Cancer. Adv. Mater. 2023, 35, e2209910. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 1–23. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Dong, Y. Lipid Nanoparticle–mRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Chen, X.; Wang, K.; Liang, L.; Zhang, H. An Overview of Nanoparticle-Based Delivery Platforms for mRNA Vaccines for Treating Cancer. Vaccines 2024, 12, 727. https://doi.org/10.3390/vaccines12070727
Lin Y, Chen X, Wang K, Liang L, Zhang H. An Overview of Nanoparticle-Based Delivery Platforms for mRNA Vaccines for Treating Cancer. Vaccines. 2024; 12(7):727. https://doi.org/10.3390/vaccines12070727
Chicago/Turabian StyleLin, Yang, Xuehua Chen, Ke Wang, Li Liang, and Hongxia Zhang. 2024. "An Overview of Nanoparticle-Based Delivery Platforms for mRNA Vaccines for Treating Cancer" Vaccines 12, no. 7: 727. https://doi.org/10.3390/vaccines12070727
APA StyleLin, Y., Chen, X., Wang, K., Liang, L., & Zhang, H. (2024). An Overview of Nanoparticle-Based Delivery Platforms for mRNA Vaccines for Treating Cancer. Vaccines, 12(7), 727. https://doi.org/10.3390/vaccines12070727




