Cardiac and Neurological Complications Post COVID-19 Vaccination: A Systematic Review of Case Reports and Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Literature Search
2.3. Data Handling
2.4. Selection Criteria
2.5. PICO
2.6. Data Extraction
2.7. Data Analyses
3. Results
3.1. Search Results
3.2. Geographical Distribution of Included Studies
3.3. Characteristics of Patients
3.4. Complications and Outcomes Post COVID-19 Vaccination
4. Discussion
4.1. Cardiac Complications
4.2. Mechanisms Underlying Cardiac Complications following COVID-19 Vaccination
4.3. Neurological Complications
4.4. Neurological Complications in COVID-19-Vaccinated People Compared to COVID-19 Patients and/or Uninfected People
4.4.1. Guillain–Barré Syndrome (GBS)
4.4.2. Cerebrovascular Disorders
4.5. Mechanisms Underlying Neurological Complications following COVID-19 Vac-cination
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fragkou, P.C.; Dimopoulou, D. Serious complications of COVID-19 vaccines: A mini-review. Metab. Open 2021, 12, 100145. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Aurigemma, G.; Saucedo, J.; Gerson, D.S. Myocarditis following COVID-19 vaccination. Radiol. Case Rep. 2021, 16, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Bayas, A.; Hindi, F.; Rizvi, Z.; Espinosa, P.S. Neurological complications of COVID-19, Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus 2021, 13, e13426. [Google Scholar] [CrossRef] [PubMed]
- Goss, A.L.; Samudralwar, R.D.; Das, R.R.; Nath, A. ANA investigates: Neurological complications of COVID-19 vaccines. Ann. Neurol. 2021, 89, 856. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2022, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Gavin, J.; Madanchi, N.; Kim, C.; Shah, P.R.; Klein, K.; Boatman, J.; Roberts, C.; Patel, S.; Danielides, S. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int. J. Cardiol. 2021, 340, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K. Myocarditis after BNT162b2 and mRNA-1273 COVID-19 vaccination: A report of 7 cases. Ann. Med. Surg. 2022, 77, 103657. [Google Scholar] [CrossRef]
- Aiba, T.; Ishibashi, K.; Hattori, K.; Wada, M.; Ueda, N.; Miyazaki, Y.; Wakamiya, A.; Yamagata, K.; Inoue, Y.; Miyamoto, K.; et al. Frequent premature ventricular contraction and non-sustained ventricular tachycardia after the SARS-CoV-2 vaccination in patient with implantable cardioverter defibrillator due to acquired long-QT syndrome. Circ. J. 2021, 85, 2117. [Google Scholar] [CrossRef]
- Alizadeh, L.S.; Koch, V.; Yel, I.; Grünewald, L.D.; Mathies, D.; Martin, S.; Vogl, T.J.; Rauschning, D.; Booz, C. A case of Myocarditis after COVID-19 vaccination: Incidental or Consequential? Heliyon 2022, 8, e09537. [Google Scholar] [CrossRef]
- Allam, C.; Kounis, N.G.; Chlawit, R.; Saouma, M.; Badaoui, G. (Eds.) Kounis Syndrome following COVID-19 Vaccination; Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon, UK, 2022. [Google Scholar]
- Al-Rasbi, S.; Al-Mqbali, J.S.; Al-Farsi, R.; Al Shukaili, M.A.; Al-Riyami, M.H.; Al Falahi, Z.; Al Farhan, H.; Al Alawi, A.M. Myocarditis, pulmonary hemorrhage, and extensive myositis with rhabdomyolysis 12 days after first dose of Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine: A case report. Am. J. Case Rep. 2022, 23, e934399-1. [Google Scholar] [CrossRef] [PubMed]
- Ambati, S.; Colon, M.; Mihic, M.; Sanchez, J.; Bakar, A. Acute myopericarditis after COVID-19 vaccine in teenagers. Case Rep. Cardiol. 2021, 2021, 8268755. [Google Scholar] [CrossRef]
- Ameratunga, R.; Woon, S.T.; Sheppard, M.N.; Garland, J.; Ondruschka, B.; Wong, C.X.; Stewart, R.A.; Tatley, M.; Stables, S.R.; Tse, R.D. First identified case of fatal fulminant necrotizing eosinophilic myocarditis following the initial dose of the Pfizer-BioNTech mRNA COVID-19 vaccine (BNT162b2, Comirnaty): An extremely rare idiosyncratic hypersensitivity reaction. J. Clin. Immunol. 2022, 42, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ansari, U.; Britsch, S.; Rogowski, S.; Duerschmied, D.; Papavassiliu, T. Case Report: Transient Increase of CMR T1 Mapping Indices in a Patient With COVID-19 mRNA Vaccine Induced Acute Myocarditis. Front. Cardiovasc. Med. 2022, 9, 880717. [Google Scholar] [CrossRef]
- Ashaari, S.; Sohaib, H.A.; Bolger, K. A case report: Symptomatic pericarditis post-COVID-19 vaccination. Eur. Heart J.-Case Rep. 2021, 5, ytab375. [Google Scholar] [CrossRef]
- Azdaki, N.; Farzad, M. Long QT interval and syncope after a single dose of COVID-19 vaccination: A case report. Pan Afr. Med. J. 2021, 40. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.H.; Kim, M.; Lee, D.I.; Lee, J.H.; Kim, S.; Lee, S.Y.; Bae, J.W.; Hwang, K.K.; Kim, D.W.; Cho, M.C. Simultaneous Occurrence of Immune-Mediated Thrombocytopenia and Myocarditis After mRNA-1273 COVID-19 Vaccination: A Case Report. J. Korean Med. Sci. 2022, 37, e169. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, V.L.; Thomas, A.; Hur, D.J.; Malm, B. Myopericarditis with Significant Left Ventricular Dysfunction Following COVID-19 Vaccination: A Case Report. Am. J. Case Rep. 2021, 22, e934066-1. [Google Scholar] [CrossRef]
- Bengel, C.P.; Kacapor, R. A report of two cases of myocarditis following mRNA coronavirus disease 2019 vaccination. Eur. Heart J.-Case Rep. 2022, 6, ytac004. [Google Scholar] [CrossRef]
- Beshai, R.; Lee, J.J. Unusual Case of Takotsubo Cardiomyopathy Secondary to COVID-19 Vaccine: Case Report and Literature Review. Cureus 2022, 14, e25398. [Google Scholar] [CrossRef]
- Boivin, Z.; Martin, J. Untimely myocardial infarction or COVID-19 vaccine side effect. Cureus 2021, 13, e13651. [Google Scholar] [CrossRef] [PubMed]
- Berto, M.B.; Spano, G.; Wagner, B.; Bernhard, B.; Häner, J.; Huber, A.T.; Gräni, C. Takotsubo Cardiomyopathy After mRNA COVID-19 Vaccination. Heart Lung Circ. 2021, 30, e119–e120. [Google Scholar] [CrossRef] [PubMed]
- Cereda, A.; Conca, C.; Barbieri, L.; Ferrante, G.; Tumminello, G.; Lucreziotti, S.; Guazzi, M.; Mafrici, A. Acute myocarditis after the second dose of SARS-CoV-2 vaccine: Serendipity or atypical causal relationship? Anatol. J. Cardiol. 2021, 25, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, M.E.; Ünsalver, B.Ö.; Dönmez, A.; Yertutanol, F.D.K.; Evrensel, A.; Ceylan, H.Z. A case of myocarditis and isolated hypopotassemia after Biontech-Pfizer vaccine for COVID-19. Vaccine 2022, 40, 2897. [Google Scholar] [CrossRef]
- Chachar, T.S.; Yousuf, N.; Sulaibikh, L.; Abdulqader, F.; Alqahtani, M. First Report of Acute Myocarditis Post-Pfizer-BioNTech COVID-19 Vaccination in the Kingdom of Bahrain. Cureus 2021, 13, e20313. [Google Scholar] [CrossRef] [PubMed]
- Chamling, B.; Vehof, V.; Drakos, S.; Weil, M.; Stalling, P.; Vahlhaus, C.; Mueller, P.; Bietenbeck, M.; Reinecke, H.; Meier, C.; et al. Occurrence of acute infarct-like myocarditis following COVID-19 vaccination: Just an accidental co-incidence or rather vaccination-associated autoimmune myocarditis? Clin. Res. Cardiol. 2021, 110, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ojha, U.K.; Vardhan, B.; Tiwari, A. Myocardial infarction after COVID-19 vaccination-casual or causal? Diabetes Metab. Syndr. 2021, 15, 1055. [Google Scholar] [CrossRef] [PubMed]
- Chelala, L.; Jeudy, J.; Hossain, R.; Rosenthal, G.; Pietris, N.; White, C.S. Cardiac MRI findings of myocarditis after COVID-19 mRNA vaccination in adolescents. Am. J. Roentgenol. 2022, 218, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Chellapandian, S.B.; Turkmen, S.; Salim, I.; Chinnakaruppan, S.; Mohammad, J. Myocarditis following COVID-19 mRNA (mRNA-1273) vaccination. Clin. Case Rep. 2022, 10, e05741. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, S.; Seo, J.W.; Kim, M.J.; Jeon, Y.H.; Park, J.H.; Lee, J.K.; Yeo, N.S. Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings. J. Korean Med. Sci. 2021, 36, e286. [Google Scholar] [CrossRef]
- Crane, P.; Wong, C.; Mehta, N.; Barlis, P. Takotsubo (stress) cardiomyopathy after ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021, 14, e246580. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Cattafi, A.; Carerj, M.L.; Booz, C.; Ascenti, G.; Cicero, G.; Blandino, A.; Mazziotti, S. Myocarditis After SARS-CoV-2 Vaccination: A Vaccine-Induced Reaction? Can. J. Cardiol. 2021, 37, 1665–1667. [Google Scholar] [CrossRef] [PubMed]
- Dlewati, M.; Park, K.; Rawat, S.; Conte, J.; Bhadha, K. COVID-19 mRNA Vaccine-Associated Myocarditis Presenting as STEMI in a 48-Year-Old Male. Case Rep. Cardiol. 2022, 2022, 2284530. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.; Klingel, K.; Ohlmann-Knafo, S.; Hüttinger, S.; Sood, N.; Pickuth, D.; Kindermann, M. Biopsy-proven lymphocytic myocarditis following first mRNA COVID-19 vaccination in a 40-year-old male: Case report. Clin. Res. Cardiol. 2021, 110, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Elheet, A.A.; Farrag, M.H.; Elkeliei, M.M.; Alabdali, A.M. Acute myocardial infarction following COVID-19 vaccination: A cause or a coincidence? Cardiometry 2022, 22, 143–146. [Google Scholar] [CrossRef]
- Fearon, C.; Parwani, P.; Gow-Lee, B.; Abramov, D. Takotsubo syndrome after receiving the COVID-19 vaccine. J. Cardiol. Cases 2021, 24, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Federico, C.; Paolo, T.; Carlo, L.; Fulvio, C. A case of constrictive pericarditis after COVID-19 vaccine. J. Clin. Images Med. Case Rep. 2022, 3, 1827. [Google Scholar]
- Freise, N.F.; Kivel, M.; Grebe, O.; Meyer, C.; Wafaisade, B.; Peiper, M.; Zeus, T.; Schmidt, J.; Neuwahl, J.; Jazmati, D.; et al. Acute cardiac side effects after COVID-19 mRNA vaccination: A case series. Eur. J. Med. Res. 2022, 27, 80. [Google Scholar] [CrossRef]
- Gautam, N.; Saluja, P.; Fudim, M.; Jambhekar, K.; Pandey, T.; Al’Aref, S. A late presentation of COVID-19 vaccine-induced myocarditis. Cureus 2021, 13, e17890. [Google Scholar] [CrossRef]
- Gill, J.; Mallari, A.J.P.; Zahra, F. Transient Myopericarditis Following Vaccination for COVID-19. J. Med. Cases 2022, 13, 80. [Google Scholar] [CrossRef]
- Giublin, I.T.; Hartmann, C.; Mangili, O.C.; Shiozaki, A.A.; Moura, L.A.Z. Symptomatic Acute Myopericarditis after Pfizer Vaccine against COVID-19. ABC Heart Fail. Cardiomyopathy 2022, 2, 120–122. [Google Scholar] [CrossRef]
- Habedank, D.; Lagast, A.; Novoa-Usme, M.; Atmowihardjo, I. A case of myocarditis in a 60-year-old man 48 h after mRNA vaccination against SARS-CoV-2. Clin. Res. Cardiol. 2021, 111, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.B.; Hamamyh, T.; Elyas, A.; Altermanini, M.; Elhassan, M. Acute myocarditis following administration of BNT162b2 vaccine. IDCases 2021, 25, e01197. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, S.; Sadeghi, S.; Mirdamadi, A.; Nematollahi, A. Myocarditis following AstraZeneca (an adenovirus vector vaccine) COVID-19 vaccination: A case report. Clin. Case Rep. 2022, 10, e05744. [Google Scholar] [CrossRef] [PubMed]
- Hinton, J.; Briosa e Gala, A.; Corbett, S. mRNA COVID-19 Vaccine–Related Anaphylactoid Reaction and Coronary Thrombosis. Mayo Clin. Proc. 2021, 96, 3182–3183. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicki, A.T.; Tolia, V.M.; Nene, R.V. Cardiac Tamponade following COVID-19 Vaccination. J. Emerg. Med. 2022, 62, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Liu, Y.-H.; Lin, H.H.; Hsu, J.-C.; Tu, C.-M.; Wu, Y.-W. Acute Myocardial Infarction within 5 Days after COVID-19 Vaccination: Three Case Reports from a Regional Tertiary Center. Acta Cardiol. Sin. 2022, 38, 409. [Google Scholar] [PubMed]
- Hung, Y.-P.; Sun, K.-S. A case of myopericarditis with pleuritis following AstraZeneca COVID-19 vaccination. QJM Int. J. Med. 2021, 114, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Imran, A.; Park, W.; Sood, M. Partially Resolving Myocardial Fibrosis Five Months following the mRNA COVID-19 Vaccine: An MRI Based Case Report. Int. J. Clin. Cardiol. 2022, 9, 253. [Google Scholar]
- Iqbal, S.; Adnan, G.; Farhad, A.; Ahmed, I.; Rahman, M.N. Acute Myocardial Infarction after Coronavirus Vaccine: A Rare Adverse Effect. Cureus 2022, 14, e21544. [Google Scholar] [CrossRef] [PubMed]
- Isaak, A.; Feisst, A.; Luetkens, J.A. Myocarditis following COVID-19 vaccination. Radiology 2021, 301, E378–E379. [Google Scholar] [CrossRef] [PubMed]
- Iwamuro, A.; Sasa, T.; Kawai, T.; Taguchi, M.; Izuhara, M.; Uegaito, T.; Shioji, K. A 17-year-old male with acute myocarditis following mRNA-1273 vaccination in Japan. J. Cardiol. Cases 2022, 26, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Jani, C.; Leavitt, J.; Al Omari, O.; Dimaso, A.; Pond, K.; Gannon, S.; Chandran, A.K.; Dennis, C.; Colgrove, R. COVID-19 Vaccine–Associated Takotsubo Cardiomyopathy. Am. J. Ther. 2021, 28, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Medina, Y.; Tian, J.; Alvi, M.J.; Sahra, S.; Rojas-Marte, G. An Unusual Case of Hemorrhagic Pleuropericarditis after COVID-19 Vaccination. Cureus 2022, 14, e24828. [Google Scholar] [CrossRef] [PubMed]
- Kadwalwala, M.; Chadha, B.; Ortoleva, J.; Joyce, M. Multimodality imaging and histopathology in a young man presenting with fulminant lymphocytic myocarditis and cardiogenic shock after mRNA-1273 vaccination. BMJ Case Rep. CP 2021, 14, e246059. [Google Scholar] [CrossRef] [PubMed]
- Kaneta, K.; Yokoi, K.; Jojima, K.; Kotooka, N.; Node, K. Young male with myocarditis following mRNA-1273 vaccination against Coronavirus disease-2019 (COVID-19). Circ. J. 2022, 86, 721. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Na, J.Y.; Yang, J.H.; Moon, S.H.; Kim, S.H.; Jung, J.J.; Cha, H.J.; Ahn, J.H.; Park, Y.W.; Cho, S.Y.; et al. Fulminant Giant Cell Myocarditis following Heterologous Vaccination of ChAdOx1 nCoV-19 and Pfizer-BioNTech COVID-19. Medicina 2022, 58, 449. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Sreenivasan, J.; Goel, A.; Malik, A.; Bandyopadhyay, D.; Jin, C.; Sharma, M.; Levine, A.; Pan, S.; Fuisz, A.; et al. Myocarditis following COVID-19 vaccination. Int. J. Cardiol. Heart Vasc. 2021, 36, 1–4. [Google Scholar] [CrossRef]
- Kawakami, T.; Yahagi, K.; Sekiguchi, M.; Ishizawa, T.; Nonaka, H.; Setoguchi, N.; Watanabe, Y.; Nakase, M.; Horiuchi, Y.; Asami, M.; et al. Acute Myocarditis in a Patient Following mRNA-1273 SARS-CoV-2 Vaccination. Intern. Med. 2022, 61, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Motokawa, T.; Kurohama, H.; Okano, S.; Akashi, R.; Yonekura, T.; Ikeda, S.; Izumikawa, K.; Maemura, K. Fulminant Myocarditis 24 Days after Coronavirus Disease Messenger Ribonucleic Acid Vaccination: A Case Report. Intern. Med. 2022, 61, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Kazama, S.; Okumura, T.; Kimura, Y.; Ito, R.; Araki, T.; Mizutani, T.; Oishi, H.; Kuwayama, T.; Hiraiwa, H.; Kondo, T.; et al. Biopsy-proven fulminant myocarditis requiring mechanical circulatory support following COVID-19 mRNA vaccination. CJC Open 2022, 4, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Khogali, F.; Abdelrahman, R. Unusual Presentation of Acute Perimyocarditis following SARS-CoV-2 mRNA-1237 Moderna Vaccination. Cureus 2021, 13, e16590. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Choi, J.H.; Jang, J.Y.; So, O.; Cho, E.; Choi, H.; Hong, K.S.; Park, K.T. A case report for myopericarditis after BNT162b2 COVID-19 mRNA vaccination in a Korean young male. J. Korean Med. Sci. 2021, 36, e277. [Google Scholar] [CrossRef]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients with acute myocarditis following mrna COVID-19 vaccination. JAMA Cardiol. 2021, 6, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-C.; Kim, H.; Lee, H.J.; Kim, J.Y.; Kim, J.-Y. Cardiac imaging of acute myocarditis following COVID-19 mRNA vaccination. J. Korean Med. Sci. 2021, 36, e229. [Google Scholar] [CrossRef]
- Kimball, E.; Buchwalder, K.; Upchurch, C.; Kea, B. Intermittent complete heart block with ventricular standstill after Pfizer COVID-19 booster vaccination: A case report. J. Am. Coll. Emerg. Physicians Open 2022, 3, e12723. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Hashimoto, T.; Noda, E.; Ishikawa, Y.; Ishikita, A.; Fujino, T.; Matsushima, S.; Ide, T.; Kinugawa, S.; Nagaoka, K.; et al. Fulminant necrotizing eosinophilic myocarditis after COVID-19 vaccination survived with mechanical circulatory support. ESC Heart Failure 2022, 9, 2732–2737. [Google Scholar] [CrossRef]
- King, W.W.; Petersen, M.R.; Matar, R.M.; Budweg, J.B.; Pardo, L.C.; Petersen, J.W. Myocarditis following mRNA vaccination against SARS-CoV-2, a case series. Am. Heart J. Plus Cardiol. Res. Pract. 2021, 8, 100042. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Awaya, T.; Yoshioka, K.; Kitano, S.; Hayama, H.; Amemiya, K.; Enomoto, Y.; Yazaki, Y.; Moroi, M.; Nakamura, M. Myocarditis after COVID-19 mRNA vaccines. QJM Int. J. Med. 2021, 114, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Tada, H.; Okada, H.; Yoshida, S.; Sakata, K.; Usui, S.; Ikeda, H.; Okajima, M.; Kawashiri, M.A.; Takamura, M. Myocarditis associated with COVID-19 mRNA Vaccination following Myocarditis associated with Campylobacter Jejuni. Front. Cardiovasc. Med. 2022, 9, 837759. [Google Scholar] [CrossRef]
- Korzeniowska, K.; Cieślewicz, A.; Flotyńska, A.; Jabłecka, A. Polyserositis (pericarditis and bilateral pleural effusion) after COVID-19 mRNA vaccine. Hematol. Clin. Pract. 2022, 13, 15–22. [Google Scholar] [CrossRef]
- Kumar, B.; Sabbarwal, V.; Nigam, A.; Gore, P.; Chauhan, G.; Darbari, A. Two case reports of acute ST-elevation myocardial infarction after COVID-19 vaccination: Co-incidence or causal-association. J. Health Soc. Sci. 2021, 6, 293–298. [Google Scholar]
- Larson, K.F.; Ammirati, E.; Adler, E.D.; Cooper Jr, L.T.; Hong, K.N.; Saponara, G.; Couri, D.; Cereda, A.; Procopio, A.; Cavalotti, C.; et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation 2021, 144, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Lazaros, G.; Anastassopoulou, C.; Hatziantoniou, S.; Kalos, T.; Soulaidopoulos, S.; Lazarou, E.; Vlachopoulos, C.; Vassilopoulos, D.; Tsakris, A.; Tsioufis, C. A case series of acute pericarditis following COVID-19 vaccination in the context of recent reports from Europe and the United States. Vaccine 2021, 39, 6585–6590. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Chew, N.W.S.; Ng, P.; Yeo, T.J. A spectrum of cardiac manifestations post Pfizer-BioNTech COVID-19 vaccination. QJM Int. J. Med. 2021, 114, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.; Shimon, G.; Fadlon-Derai, M.; Gershovitz, L.; Shovali, A.; Sebbag, A.; Bader, S.; Fink, N.; Gordon, B. Myocarditis following COVID-19 vaccination–a case series. Vaccine 2021, 39, 6195–6200. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, B.; Ji, T.; Xu, S.G.; Jiang, W. A postoperative man with marfan syndrome with palpitations and chest pain after receiving the SARS-CoV-2 vaccine. Infect. Drug Resist. 2021, 14, 2953–2956. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Kim, M.C.; Kim, K.H.; Jeong, I.S.; Cho, Y.S.; Choi, Y.D.; Lee, J.E. Case report: Acute fulminant myocarditis and cardiogenic shock after messenger RNA coronavirus disease 2019 vaccination requiring extracorporeal cardiopulmonary resuscitation. Front. Cardiovasc. Med. 2021, 8, 758996. [Google Scholar] [CrossRef] [PubMed]
- Loch, A.; Siew, K.S.W.; Tan, K.L. Transient severe myocarditis and intraventricular thrombus associated with SARS-CoV-2 vaccination. Singap. Med. J. 2022, 1, 15. [Google Scholar]
- Mansour, J.; Short, R.G.; Bhalla, S.; Woodard, P.K.; Verma, A.; Robinson, X.; Raptis, D.A. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: A report of two cases. Clin. Imaging 2021, 78, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.; Ferguson, I.D.; Lewis, P.; Jaggi, P.; Gagliardo, C.; Collins, J.S.; Shaughnessy, R.; Caron, R.; Fuss, C.; Corbin, K.J.E.; et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination. Pediatrics 2021, 148, e2021052478. [Google Scholar] [CrossRef] [PubMed]
- Marsukjai, A.; Theerasuwipakorn, N.; Tumkosit, M.; Chattranukulchai, P.; Srichomkwun, P.; Prechawat, S. Concomitant myocarditis and painless thyroiditis after AstraZeneca coronavirus disease 2019 vaccination: A case report. J. Med. Case Rep. 2022, 16, 212. [Google Scholar] [CrossRef]
- Matta, A.; Kallamadi, R.; Matta, D.; Bande, D. Post-mRNA COVID-19 vaccination myocarditis. Eur. J. Case Rep. Intern. Med. 2021, 8, 1–3. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.; Johnson, T.J. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: A case report. Acad. Emerg. Med. 2021, 28, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, B.; Asenov, A.G.; Hirsh-Raccah, B.; Amir, O.; Pappo, O.; Asleh, R. Severe acute myocarditis after the third (booster) dose of mRNA COVID-19 vaccination. Vaccines 2022, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, H.; Bahouh, C.; Baddi, M.; Berichi, S.; Bkiyar, H.; Housni, B. Cardiogenic shock revealing myocarditis after mRNA vaccination against COVID-19, Case report and brief review for the first case in Morocco. Ann. Med. Surg. 2022, 74, 103210. [Google Scholar] [CrossRef] [PubMed]
- Minocha, P.K.; Better, D.; Singh, R.K.; Hoque, T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID-19) vaccine in a male adolescent. J. Pediatr. 2021, 238, 321–323. [Google Scholar] [CrossRef]
- Miqdad, M.A.; Nasser, H.; Alshehri, A.; Mourad, A.R. Acute myocarditis following the administration of the second BNT162b2 COVID-19 vaccine dose. Cureus 2021, 13, e18880. [Google Scholar] [CrossRef]
- Mishra, A.; Komut, O.; Kumar, A.; Ete, T.; Megeji, R.D. Acute Myocardial Infarction after COVID-19 Vaccination: A Case Report. Cureus 2022, 14, e25536. [Google Scholar] [CrossRef] [PubMed]
- Misumi, I.; Ogata, A.; Fukuda, K.; Sato, K.; Nagano, M.; Usuku, H.; Tsujita, K. Constrictive pericarditis following mRNA COVID-19 vaccination in a patient with systemic sclerosis. J. Cardiol. Cases 2022, 26, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, T.; Yokoi, M.; Shintani, Y.; Yamamoto, J.; Mori, K.; Fujita, H.; Ito, T.; Sugiura, T.; Seo, Y. A case of an elderly female who developed subacute pleuropericarditis following BNT162b2 mRNA COVID-19 vaccination. J. Cardiol. Cases 2022, 26, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Mouch, S.A.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Yanay, N.B. Myocarditis following COVID-19 mRNA vaccination. Vaccine 2021, 39, 3790–3793. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Shinohara, M.; Oka, Y.; Wada, R.; Noike, R.; Ohara, H.; Fujino, T.; Ikeda, T. Myocarditis following a COVID-19 messenger RNA vaccination: A Japanese case series. Intern. Med. 2022, 61, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, A.; Narasimhan, M.; Li, Q.Z.; Mahimainathan, L.; Hitto, I.; Fuda, F.; Batra, K.; Jiang, X.; Zhu, C.; Schoggins, J.; et al. In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation 2021, 144, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, T.; Koitabashi, N.; Ishibashi, Y.; Aihara, K.; Takama, N.; Ohyama, Y.; Yokoyama, T.; Kaneko, Y. Acute myocarditis associated with COVID-19 vaccination: A case report. J. Cardiol. Cases 2022, 25, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Naghashzadeh, F.; Shafaghi, S.; Dorudinia, A.; Naji, S.A.; Marjani, M.; Amin, A.; Mohamadifar, A.; Noorali, S.; Kashani, B.S. Myocarditis following rAd26 and rAd5 vector-based COVID-19 vaccine: Case report. ESC Heart Failure. 2022, 9, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Honda, S.; Yamano, M.; Kawasaki, T.; Yoshioka, K. Constrictive pericarditis after SARS-CoV-2 vaccination: A case report. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2022, 116, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Nso, N.; Gonzalez, C.; Lakhdar, S.; Alshamam, M.; Elshafey, M.; Abdalazeem, Y.; Nyein, A.; Punzalan, B.; Durrance, R.J.; et al. COVID-19 vaccine-induced myocarditis: Case report with literature review. Diabetes Metab. Syndr. 2021, 15, 102205. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Tanaka, Y.; Nishida, S.; Sugimoto, T. Case report of acute myocarditis after administration of coronavirus disease 2019 vaccine in Japan. Eur. Heart J.-Case Rep. 2022, 6, ytab534. [Google Scholar] [CrossRef] [PubMed]
- Oka, A.; Sudo, Y.; Miyoshi, T.; Ozaki, M.; Kimura, Y.; Takagi, W.; Ugawa, S.; Okada, T.; Nosaka, K.; Doi, M. Fulminant myocarditis after the second dose of COVID-19 mRNA vaccination. Clin. Case Rep. 2022, 10, e05378. [Google Scholar] [CrossRef] [PubMed]
- Olagunju, A.; Moradi, A.; Johnson, B.; Lebaron, Z.; Johnson, R.; Mehdizadeh, A. Acute Myocarditis Following Vaccination With the First Dose of the mRNA-1273 Vaccine. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221092291. [Google Scholar] [CrossRef] [PubMed]
- Olmos, C.V.; Trahan, S.; Rochon, A.; Ducharme, A. Severe myocarditis after SARS-CoV-2 vaccination in a 49-year-old woman. CMAJ 2022, 194, E581–E584. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, İ.H.; Özlek, B.; Özen, M.B.; Gündüz, R.; Bayturan, Ö. Type 1 Kounis Syndrome Induced by Inactivated SARS-CoV-2 Vaccine. J. Emerg. Med. 2021, 61, e71–e76. [Google Scholar] [CrossRef] [PubMed]
- Panthong, S.; Vimonsuntirungsri, T.; Thapanasuta, M.; Udayachalerm, W.; Ariyachaipanich, A. Acute Coronary Syndrome After Inactivated SARS-CoV-2 Vaccine A Case Report. Int. Heart J. 2022, 63, 388–392. [Google Scholar] [CrossRef]
- Parmar, K.; Mekraksakit, P.; Del Rio-Pertuz, G.; Sethi, P.; Motes, A.; Hughes, M.; Wischmeyer, J.; Carbajal, L.; Sosa, E.A. (Eds.) Myocarditis following COVID-19 mRNA vaccination. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon, UK, 2022. [Google Scholar]
- Patel, Y.R.; Louis, D.W.; Atalay, M.; Agarwal, S.; Shah, N.R. Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA COVID-19 vaccination: A case series. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2021, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Perna, F.; Verecchia, E.; Pinnacchio, G.; Gerardino, L.; Brucato, A.; Manna, R. Rapid resolution of severe pericardial effusion using anakinra in a patient with COVID-19 vaccine-related acute pericarditis relapse: A case report. Eur. Heart J.-Case Rep. 2022, 6, ytac123. [Google Scholar] [CrossRef]
- Puchalski, M.; Kamińska, H.; Bartoszek, M.; Brzewski, M.; Werner, B. COVID-19-vaccination-induced myocarditis in teenagers: Case series with further follow-up. Int. J. Environ. Res. Public Health 2022, 19, 3456. [Google Scholar] [CrossRef]
- Rosner, C.M.; Genovese, L.; Tehrani, B.N.; Atkins, M.; Bakhshi, H.; Chaudhri, S.; Damluji, A.A.; De Lemos, J.A.; Desai, S.S.; Emaminia, A.; et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation 2021, 144, 502–505. [Google Scholar] [CrossRef]
- Şancı, E.; Örçen, C.; Çelik, O.M.; Özen, M.T.; Bozyel, S. Kounis syndrome associated with BNT162b2 mRNA COVID-19 vaccine presenting as ST-elevation acute myocardial infarction. Anatol. J. Cardiol. 2022, 26, 72. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Murai, R.; Kim, K.; Furukawa, Y. Cardiac magnetic resonance findings in acute myocarditis after mRNA COVID-19 vaccination. J. Cardiol. Cases 2022, 26, 17–20. [Google Scholar] [CrossRef]
- Schauer, J.; Buddhe, S.; Colyer, J.; Sagiv, E.; Law, Y.; Chikkabyrappa, S.M.; Portman, M.A. Myopericarditis After the Pfizer Messenger Ribonucleic Acid Coronavirus Disease Vaccine in Adolescents. J. Pediatr. 2021, 238, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, P.; Demoulin, R.; Poyet, R.; Capilla, E.; Rohel, G.; Pons, F.; Jégo, C.; Sidibe, S.; Druelle, A.; Brocq, F.X.; et al. Acute Myocarditis after COVID-19 vaccination: A case report. La Rev. De Med. Interne 2021, 42, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Sciaccaluga, C.; D’Ascenzi, F.; Cameli, M.; Gallotta, M.; Menci, D.; Antonelli, G.; Banchi, B.; Mochi, V.; Valente, S.; Focardi, M. Case report: Two case reports of acute myopericarditis after mRNA COVID-19 vaccine. Front. Cardiovasc. Med. 2022, 9, 827237. [Google Scholar] [CrossRef]
- Sharbatdaran, A.; Chahal, Y.; Molaei, M.; Bhavsar, D. A rare case of COVID-19 vaccine-induced myopericarditis in a young adult. Radiol. Case Rep. 2022, 17, 1916–1920. [Google Scholar] [CrossRef]
- Shiyovich, A.; Witberg, G.; Aviv, Y.; Kornowski, R.; Hamdan, A. A case series of myocarditis following third (Booster) Dose of COVID-19 vaccination: Magnetic resonance imaging study. Front. Cardiovasc. Med. 2022, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Shumkova, M.; Vassilev, D.; Karamfiloff, K.; Ivanova, R.; Stoyanova, K.; Yaneva-Sirakova, T.; Gil, R.J. Acute myocarditis associated with the Pfizer/BioNTech vaccine. Kardiol. Pol. 2021, 79, 1282–1283. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, P.; Cedeno, L.; Brahimi, T.; Patel, P.; Virk, H.; Shamoon, F.; Bikkina, M. COVID-19 mRNA Vaccine and Myocarditis. Eur. J. Case Rep. Int. Med. 2021, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Snapiri, O.; Danziger, C.R.; Shirman, N.; Weissbach, A.; Lowenthal, A.; Ayalon, I.; Adam, D.; Yarden-Bilavsky, H.; Bilavsky, E. Transient Cardiac Injury in Adolescents Receiving the BNT162b2 mRNA COVID-19 Vaccine. Pediatr. Infect. Dis. J. 2021, 40, e360–e363. [Google Scholar] [CrossRef] [PubMed]
- Sokolska, J.M.; Kurcz, J.; Kosmala, W. Every rose has its thorns—Acute myocarditis following COVID-19 vaccination. Kardiol. Pol. 2021, 79, 1153–1154. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Albini, A.; Noonan, D.M.; Brucato, A.; Lombardo, M.; Santalucia, P. A Case of Acute Pericarditis after COVID-19 Vaccination. Front. Allergy 2021, 2, 733466. [Google Scholar] [CrossRef] [PubMed]
- Starekova, J.; Bluemke, D.A.; Bradham, W.S.; Grist, T.M.; Schiebler, M.L.; Reeder, S.B. Myocarditis associated with mRNA COVID-19 vaccination. Radiology 2021, 301, 409. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Gamble, D.T.; Dawson, D. Novel case of takotsubo cardiomyopathy following COVID-19 vaccination. BMJ Case Rep. CP 2022, 15, e247291. [Google Scholar] [CrossRef] [PubMed]
- Sulemankhil, I.; Abdelrahman, M.; Negi, S.I. Temporal association between the COVID-19 Ad26. COV2. S vaccine and acute myocarditis: A case report and literature review. Cardiovasc. Revascularization Med. 2022, 38, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.G.; Sobieszczyk, P.S.; Bhatt, D.L. Acute Myocardial Infarction Within 24 Hours After COVID-19 Vaccination. Am. J. Cardiol. 2021, 156, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Tailor, P.D.; Feighery, A.M.; El-Sabawi, B.; Prasad, A. Case report: Acute myocarditis following the second dose of mRNA-1273 SARS-CoV-2 vaccine. Eur. Heart J.-Case Rep. 2021, 5, ytab319. [Google Scholar] [CrossRef]
- Tano, E.; San Martin, S.; Girgis, S.; Martinez-Fernandez, Y.; Sanchez Vegas, C. Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19 vaccine. J. Pediatr. Infect. Dis. Soc. 2021, 10, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Camilli, M.; Ianni, U.; Tavecchia, G.; Palazzini, M.; Cartella, I.; Gentile, P.; Quattrocchi, G.; Spanò, F.M.; Cipriani, M.; et al. Takotsubo Syndrome after BNT162b2 mRNA COVID-19 vaccine: Emotional or causative relationship with vaccination? Int. J. Cardiol. Heart Vasc. 2022, 40, 101002. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, M.; Leite, S.; Faria, B.; Cardoso, S.; Von Hafe, P.; Dias, G.; Cardoso, F.; Pereira, T.; Machado, I.; Lourenço, A. Perimyocarditis following COVID-19 Vaccination. Clin. Med. Insights Cardiol. 2021, 15, 11795468211056634. [Google Scholar] [CrossRef]
- Toida, R.; Uezono, S.; Komatsu, H.; Toida, T.; Imamura, A.; Fujimoto, S.; Kaikita, K. Takotsubo cardiomyopathy after vaccination for coronavirus disease 2019 in a patient on maintenance hemodialysis. CEN Case Rep. 2022, 11, 220–224. [Google Scholar] [CrossRef]
- Ujueta, F.; Azimi, R.; Lozier, M.R.; Poppiti, R.; Ciment, A. Lymphohistocytic myocarditis after Ad26. COV2. S viral vector COVID-19 vaccination. Int. J. Cardiol. Heart Vasc. 2021, 36, 100869. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after COVID-19 mRNA vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Vidula, M.K.; Ambrose, M.; Glassberg, H.; Chokshi, N.; Chen, T.; Ferrari, V.A.; Han, Y. Myocarditis and Other Cardiovascular Complications of the mRNA-Based COVID-19 Vaccines. Cureus 2021, 13, e15576. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ashikaga, T.; Maejima, Y.; Tao, S.; Terui, M.; Kishigami, T.; Kaneko, M.; Nakajima, R.; Okata, S.; Lee, T.; et al. Case Report: Importance of MRI Examination in the Diagnosis and Evaluation of COVID-19 mRNA Vaccination Induced Myocarditis: Our Experience and Literature Review. Front. Cardiovasc. Med. 2022, 9, 844626. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Choi, J.-I.; Hosseini, F.; Roberts, J.; Ramanathan, K.; Ong, K. Acute myocarditis following mRNA-1273 SARS-CoV-2 vaccination. CJC Open 2021, 3, 1410–1412. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, H.; Ishikawa, H.; Otsuka, K.; Kasayuki, N. Reverse Takotsubo Cardiomyopathy as a Cause of Acute Chest Pain in a Young Woman Following COVID-19 Vaccination. Circ. Cardiovasc. Imaging 2022, 15, e013661. [Google Scholar] [CrossRef] [PubMed]
- Yen, K.-C.; Ho, C.-T.; Chin, S.-C.; Su, H.-C.; Lee, K.-T.; Chu, P.-H. Self-Limited Myocarditis after the First Dose of Coronavirus Disease 2019 Messenger RNA-1273 Vaccine in a Healthy Male. Acta Cardiol. Sin. 2022, 38, 210. [Google Scholar]
- Zaki, H.A.; Zahran, A.; Abdelrahim, M.; Elnabawy, W.A.; Kaber, Y. A Case of Acute Viral Pericarditis Complicated With Pericardial Effusion Induced by Third Dose of COVID Vaccination. Cureus 2022, 14, e21207. [Google Scholar] [CrossRef] [PubMed]
- Abičić, A.; Adamec, I.; Habek, M. Miller Fisher syndrome following Pfizer COVID-19 vaccine. Neurol. Sci. 2022, 43, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Salih, B.K.; Hussein, K.F.H.; Mikael, T.M.; Kakamad, F.H.; Salih, A.M. Aseptic meningoencephalitis after COVID-19 vaccination: A case report. Ann. Med. Surg. 2021, 71, 103028. [Google Scholar] [CrossRef]
- Alabkal, J.; Rebchuk, A.D.; Lyndon, D.; Randhawa, N. Incomplete subacute transverse myelitis following vaccination with Pfizer-BioNtech COVID-19 mRNA vaccine: A case report. Cureus 2021, 13, e20460. [Google Scholar] [CrossRef]
- Aladdin, Y.; Shirah, B. New-onset refractory status epilepticus following the ChAdOx1 nCoV-19 vaccine. J. Neuroimmunol. 2021, 357, 7. [Google Scholar] [CrossRef] [PubMed]
- Albokhari, A.; Alsawas, A.; Adnan, M.A.; Alasmari, A.; Aljuhani, S.; Almejalli, M.; Kedah, H. Acute inflammatory transverse myelitis post-Pfizer-BioNtech-COVID-19 vaccine in 16-year-old. J. Med. Res. Innov. 2021, 5, 47–50. [Google Scholar] [CrossRef]
- Alhashim, A.; Hadhiah, K.; Alhaddad, F.M.; Al ARhain, S.A.; Saif, F.H.B.; Abid, A.; Al Gamdi, O.; Alsulaiman, F.; AlQarni, M. Extensive Cerebral Venous Sinus Thrombosis (CVST) After the First Dose of Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine without Thrombotic Thrombocytopenia Syndrome (TTS) in a Healthy Woman. Am. J. Case Rep. 2022, 23, e934744-1. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.M.; Ramsamy, S.; Tarr, A.W.; Tighe, P.J.; Irving, W.L.; Tanasescu, R.; Evans, J.R. Guillain–Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann. Neurol. 2021, 90, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Al-Mashdali, A.F.; Ata, Y.M.; Sadik, N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: A case report. Ann. Med. Surg. 2021, 69, 102803. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayhani, T.; Saber, S.; Stubbs, M.J.; Losseff, N.A.; Perry, R.J.; Simister, R.J.; Gull, D.; Jäger, H.R.; Scully, M.A.; Werring, D.J. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Al-Midfai, Y.; Kujundzic, W.; Uppal, S.; Oakes, D.; Giezy, S. Acute Multiple Sclerosis Exacerbation after Vaccination With the Johnson & Johnson COVID-19 Vaccine: Novel Presentation and First Documented Case Report. Cureus 2022, 14, e24017. [Google Scholar] [PubMed]
- Al-Quliti, K.; Qureshi, A.; Quadri, M.; Abdulhameed, B.; Alanazi, A.; Alhujeily, R. Acute Demyelinating Encephalomyelitis Post-COVID-19 Vaccination: A Case Report and Literature Review. Diseases 2022, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Alsallamin, I.; Somoza-Cano, F.J.; Zakarna, L.; Aggarwal, P.; Karia, R.; Bawwab, A.; Chakhachiro, D.; Alsallamin, A. A Puzzling Diagnosis of Cerebral Vein Thrombosis in a COVID-19-Vaccinated Patient. Cureus 2022, 14, e25860. [Google Scholar] [CrossRef] [PubMed]
- Alshararni, A. Acute transverse myelitis associated with COVID-19 vaccine: A case report. Int. J. Res. Pharm. Sci. 2021, 12, 2083–2087. [Google Scholar] [CrossRef]
- Aly, A.S.; Alkolfat, F.; Mansour, E.R.; Salama, S. Guillain-Barre syndrome following COVID-19 vaccination: A case report and an updated review. Neuroimmunol. Rep. 2022, 2, 100083. [Google Scholar] [CrossRef]
- Amjad, M.A.; Hamid, Z.; Patel, Y.; Husain, M.; Saddique, A.; Liaqat, A.; Ochieng, P. COVID-19 Vaccine-Induced Parsonage-Turner Syndrome: A Case Report and Literature Review. Cureus 2022, 14, e25493. [Google Scholar] [CrossRef]
- Anamnart, C.; Tisavipat, N.; Owattanapanich, W.; Apiwattanakul, M.; Savangned, P.; Prayoonwiwat, N.; Siritho, S.; Rattanathamsakul, N.; Jitprapaikulsan, J. Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: Case report and systematic review. Mult. Scler. Relat. Disord. 2022, 58, 103414. [Google Scholar] [CrossRef] [PubMed]
- Ancau, M.; Liesche-Starnecker, F.; Niederschweiberer, J.; Krieg, S.M.; Zimmer, C.; Lingg, C.; Kumpfmüller, D.; Ikenberg, B.; Ploner, M.; Hemmer, B.; et al. Case series: Acute hemorrhagic encephalomyelitis after SARS-CoV-2 vaccination. Front. Neurol. 2022, 2669, 820049. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, V.; D’arco, B.; Pagliano, P.; Toriello, A.; Barone, P. Bilateral facial palsy after COVID-19 vaccination. Neurol. Sci. 2022, 43, 4069–4079. [Google Scholar] [CrossRef]
- Aravinda, R.; Tater, P.M.; Huliyappa, H.; Manual, C.J. Atypical facial onset Guillain-Barré syndrome following first dose of COVISHIELD vaccine. Rom. J. Rnal. Neurol. 2021, 20, 379. [Google Scholar] [CrossRef]
- Asif, S.; Kesireddy, M.; Koepsell, S.A.; Gonzalez-Castellon, M.A.; Gundabolu, K.; Baljevic, M. Cerebral venous sinus thrombosis due to thrombosis with thrombocytopenia syndrome following Ad26. COV2. S: A first real-world case report of a male subject. Neurohospitalist 2022, 12, 346–351. [Google Scholar] [CrossRef]
- Assiri, S.A.; Althaqafi, R.M.; Alswat, K.; Alghamdi, A.A.; Alomairi, N.E.; Nemenqani, D.M.; Ibrahim, Z.S.; Elkady, A. Post COVID-19 Vaccination-Associated Neurological Complications. Neuropsychiatr. Dis. Treat. 2022, 18, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Khalil, A.; Taha, A. Guillain-Barré syndrome in a 67-year-old male post COVID-19 vaccination (Astra Zeneca). Am. J. Med. Case Rep. 2021, 9, 424–427. [Google Scholar] [CrossRef]
- Bagella, C.F.; Corda, D.G.; Zara, P.; Elia, A.E.; Ruiu, E.; Sechi, E.; Solla, P. Chronic inflammatory demyelinating polyneuropathy after ChAdOx1 nCoV-19 vaccination. Vaccines 2021, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Ballout, A.A.; Babaie, A.; Kolesnik, M.; Li, J.Y.; Hameed, N.; Waldman, G.; Chaudhry, F.; Saba, S.; Harel, A.; Najjar, S. A Single-Health System Case Series of New-Onset CNS Inflammatory Disorders Temporally Associated With mRNA-Based SARS-CoV-2 Vaccines. Front. Neurol. 2022, 264, 796882. [Google Scholar] [CrossRef] [PubMed]
- Balloy, G.; Magot, A.; Fayet, G.; Bonnemain, B.; Péréon, Y. COVID-19, A putative trigger for neuralgic amyotrophy. Rev. Neurol. 2022, 178, 157. [Google Scholar] [CrossRef]
- Bax, F.; Gigli, G.L.; Belgrado, E.; Brunelli, L.; Valente, M. Guillain–Barré syndrome following COVID-19 immunization: A report of two cases. Acta Neurol. Belg. 2021, 122, 1365–1367. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, H.; Jahromi, L.S.M.; Parvin, R.; Ashraf, A. A case of Guillain-Barre syndrome after the second dose of AstraZeneca COVID-19 vaccination. Turk. J. Phys. Med. Rehabil. 2022, 68, 295. [Google Scholar] [CrossRef] [PubMed]
- Bensaidane, M.R.; Picher-Martel, V.; Émond, F.; De Serres, G.; Dupré, N.; Beauchemin, P. Case Report: Acute Necrotizing Encephalopathy Following COVID-19 Vaccine. Front. Neurol. 2022, 758, 872734. [Google Scholar] [CrossRef] [PubMed]
- Berlot, G.; Tomasini, A.; La Fata, C.; Pintacuda, S.; Rigutti, S.; Falanga, A. Widespread Arterial Thrombosis after ChAdOx1 nCov-19 Vaccination. Case Rep. Crit. Care 2022, 2022, 6804456. [Google Scholar] [CrossRef] [PubMed]
- Bernheimer, J.H.; Gasbarro, G. Parsonage Turner Syndrome Following Vaccination With mRNA-1273 SARS-CoV-2 Vaccine. J. Clin. Neuromuscul. Dis. 2022, 23, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Pandey, S.K.; Kumar, D.; Vardhan, H. Post coronavirus disease-2019 vaccination Guillain-Barré syndrome. Indian J. Public Health 2021, 65, 422–424. [Google Scholar] [PubMed]
- Bogs, T.; Saleh, N.; Yavuz, S.T.; Fazeli, W.; Ganschow, R.; Schreiner, F. Aseptic Meningitis, Mucocutaneous Lesions and Arthritis after COVID-19 Vaccination in a 15-Year-Old Boy. Vaccines 2022, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Bollo, L.; Marinari, A.; Iaffaldano, P. Mild Symptomatic Guillain-Barre Syndrome after SARS-CoV-2 Vaccine. Open J. Case Rep. 2021, 2, 141. [Google Scholar]
- Bonifacio, G.B.; Patel, D.; Cook, S.; Purcaru, E.; Couzins, M.; Domjan, J.; Ryan, S.; Alareed, A.; Tuohy, O.; Slaght, S.; et al. Bilateral facial weakness with paraesthesia variant of Guillain-Barré syndrome following Vaxzevria COVID-19 vaccine. J. Neurol. Neurosurg. Psychiatry 2022, 93, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Borelli, W.V.; da Silveira, A.L.M.; Finkelsztejn, A.; Hansel, G.; Sato, D.K.; Saute, J.A.M. Simultaneous bilateral optic neuritis and longitudinally extensive transverse myelitis following vaccination against COVID-19, A case report. Neuroimmunol. Rep. 2021, 1, 100041. [Google Scholar] [CrossRef]
- Bouattour, N.; Hdiji, O.; Sakka, S.; Fakhfakh, E.; Moalla, K.; Daoud, S.; Farhat, N.; Damak, M.; Mhiri, C. Guillain-Barré syndrome following the first dose of Pfizer-BioNTech COVID-19 vaccine: Case report and review of reported cases. Neurol. Sci. 2022, 43, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Bramer, S.; Jaffe, Y.; Sivagnanaratnam, A. Vestibular neuronitis after COVID-19 vaccination. BMJ Case Rep. CP 2022, 15, e247234. [Google Scholar] [CrossRef] [PubMed]
- Burillo, J.C.; Martínez, C.L.; Arguedas, C.G.; Pueyo, F.M. Amyotrophic neuralgia secondary to Vaxzevri (AstraZeneca) COVID-19 vaccine. Neurologia 2021, 36, 571. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.; Bartholomew, T.; Rudd, J.; Walker, D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021, 14, e243829. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Coebergh, J.; Safavi, F.; Carson, A.; Hallett, M.; Michael, B.; Pollak, T.A.; Solomon, T.; Stone, J.; Nicholson, T.R.; et al. Functional Neurological Disorder After SARS-CoV-2 Vaccines: Two Case Reports and Discussion of Potential Public Health Implications. J. Neuropsychiatry Clin. Neurosci. 2021, 33, 345–348. [Google Scholar] [CrossRef]
- Cabral, G.; Gonçalves, C.; Serrazina, F.; Sá, F. MRI negative myelitis induced by pfizer-biontech COVID-19 vaccine. J. Clin. Neurol. 2022, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ren, L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: A case report. Acta Neurol. Belg. 2022, 122, 793–795. [Google Scholar] [CrossRef]
- Carneiro, D.R.; Matos, A.; Morgadinho, A. Steroid-responsive aseptic meningitis after BNT162b2 SARS-CoV-2 vaccine. Rev. Neurol. 2022, 178, 160. [Google Scholar] [CrossRef] [PubMed]
- Carranza, O.; Babici, D.; Waheed, S.; Yousuf, F. Neurologic Sequela of COVID-19, Guillain-Barré Syndrome Following Johnson & Johnson COVID-19 Vaccination. Cureus 2022, 14, e24252. [Google Scholar] [PubMed]
- Castan, M.; Damin-Pernik, M.; Thiéry, G.; Page, D.; Smadja, D.M.; Bertoletti, L. A case report of vaccine-induced immune thrombocytopenia and thrombosis syndrome after Ad26. COV2. S vaccine (Janssen/Johnson & Johnson). Therapie 2022, 77, 734. [Google Scholar] [PubMed]
- Castiglione, J.I.; Crespo, J.M.; Lecchini, L.; Silveira, F.O.; Luis, M.B.; Cotti, N.; Simison, C.J.; Aguirre, F.; Piedrabuena, M.A.; Alonso, R.N.; et al. Bilateral facial palsy with paresthesias, variant of Guillain-Barré syndrome following COVID-19 vaccine: A case series of 9 patients. Neuromuscul. Disord. 2022, 32, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; D’Arrigo, A.; Floridi, C.; Oliva, G.; Carrafiello, G. Left Bell’s palsy following the first dose of mRNA-1273 SARS-CoV-2 vaccine: A case report. Clin. Imaging 2022, 82, 1–4. [Google Scholar] [CrossRef]
- Čenščák, D.; Ungermann, L.; Štětkářová, I.; Ehler, E. Guillan-Barré Syndrome after First Vaccination Dose against COVID-19, Case Report. Acta Medica 2021, 64, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.S.; Tiwari, A.; Jaiswal, S.; Kaur, U.; Kumar, I.; Mittal, A.; Singh, A.; Jin, K.; Chakrabarti, S. Rapidly progressive dementia with asymmetric rigidity following ChAdOx1 nCoV-19 vaccination. Aging Dis. 2022, 13, 633. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Tan, B.Y.; Goh, Y.; Tan, S.S.; Tambyah, P.A. Aseptic meningitis after BNT-162b2 COVID-19 vaccination. Brain Behav. Immun.-Health 2022, 19, 100406. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Chavda, V.; Lu, B.; Garg, K.; Montemurro, N. Cognitive deficits and memory impairments after COVID-19 (Covishield) vaccination. Brain Behav. Immun.-Health 2022, 22, 100463. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fan, X.-R.; He, S.; Zhang, J.-W.; Li, S.-J. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021, 42, 3537–3539. [Google Scholar] [CrossRef]
- Chua, M.M.J.; Hayes, M.T.; Cosgrove, R. Parsonage-Turner syndrome following COVID-19 vaccination and review of the literature. Surg. Neurol. Int. 2022, 13, 152. [Google Scholar] [CrossRef]
- Chun, J.Y.; Park, S.; Jung, J.; Kim, S.H.; Kim, T.S.; Choi, Y.J.; Kim, H.J.; Eom, H.S.; Hyun, J.W. Guillain-Barré syndrome after vaccination against COVID-19. Lancet Neurol. 2022, 21, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, M.P.; Ferrua, F.; Barzaghi, F.; Cerri, F.; Moro, M.; Aiuti, A.; Silvani, P. Third cranial nerve palsy in an 88-year-old man after SARS-CoV-2 mRNA vaccination: Change of injection site and type of vaccine resulted in an uneventful second dose with humoral immune response. BMJ Case Rep. CP 2022, 15, e246485. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Orlandi, M.; Cirillo, N. Bell’s palsy following COVID-19 vaccination. J. Neurol. 2021, 268, 3589–3591. [Google Scholar] [CrossRef] [PubMed]
- Consoli, S.; Dono, F.; Evangelista, G.; D’Apolito, M.; Travaglini, D.; Onofrj, M.; Bonanni, L. Status migrainosus: A potential adverse reaction to Comirnaty (BNT162b2, BioNtech/Pfizer) COVID-19 vaccine-a case report. Neurol. Sci. 2022, 43, 767–770. [Google Scholar] [CrossRef]
- Corrêa, D.G.; Cañete, L.A.Q.; Dos Santos, G.A.C.; de Oliveira, R.V.; Brandão, C.O.; da Cruz, L.C.H., Jr. Neurological symptoms and neuroimaging alterations related with COVID-19 vaccine: Cause or coincidence? Clin. Imaging 2021, 80, 348–352. [Google Scholar] [CrossRef]
- Costentin, G.; Ozkul-Wermester, O.; Triquenot, A.; Le Cam-Duchez, V.; Massy, N.; Benhamou, Y.; Massardier, E. Acute Ischemic Stroke Revealing ChAdOx1 nCov-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia: Impact on Recanalization Strategy. J. Stroke Cerebrovasc. Dis. 2021, 30, 105942. [Google Scholar] [CrossRef] [PubMed]
- Daher, M.; Mansour, J.; Haikal, E.; Estephan, M.; Sebaaly, A. Unilateral Lower Limb Paralysis: A Case Report of An Uncommon Side Effect of COVID-19 Mrna Vaccine. J. Med. Res. Case Rep. 2022, 4, 1–8. [Google Scholar]
- Dalwadi, V.; Hancock, D.; Ballout, A.A.; Geraci, A. Axonal-variant guillian-Barre syndrome temporally associated with mRNA-based Moderna SARS-CoV-2 vaccine. Cureus 2021, 13, e18291. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.L.; Bryson, A. Miller-Fisher syndrome and Guillain-Barre syndrome overlap syndrome in a patient post Oxford-AstraZeneca SARS-CoV-2 vaccination. BMJ Case Rep. CP 2021, 14, e246701. [Google Scholar] [CrossRef] [PubMed]
- Desai, U.G. Myasthenia Gravis Exacerbation Following Second Dose of mRNA-1273 Vaccine. RRNMF Neuromuscul. J. 2021, 2, 46–47. [Google Scholar] [CrossRef]
- Diaz-Segarra, N.; Edmond, A.; Gilbert, C.; Mckay, O.; Kloepping, C.; Yonclas, P. Painless idiopathic neuralgic amyotrophy after COVID-19 vaccination: A case report. Pm & R 2021, 14, 889. [Google Scholar]
- Doi, K.; Ohara, Y.; Ouchi, T.; Sasaki, R.; Maki, F.; Mizuno, J. Cervical Transverse Myelitis Following COVID-19 Vaccination. NMC Case Rep. J. 2022, 9, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Einstein, E.H.; Shahzadi, A.; Desir, L.; Katz, J.; Boockvar, J.; D’Amico, R. New-Onset Neurologic Symptoms and Related Neuro-Oncologic Lesions Discovered After COVID-19 Vaccination: Two Neurosurgical Cases and Review of Post-Vaccine Inflammatory Responses. Cureus 2021, 13, e15664. [Google Scholar] [CrossRef] [PubMed]
- Eom, H.; Kim, S.W.; Kim, M.; Kim, Y.E.; Kim, J.H.; Shin, H.Y.; Lee, H.L. Case reports of acute transverse myelitis associated with mRNA vaccine for COVID-19. J. Korean Med. Sci. 2022, 37, e52. [Google Scholar] [CrossRef]
- Erdem, N.Ş.; Demirci, S.; Özel, T.; Mamadova, K.; Karaali, K.; Çelik, H.T.; Uslu, F.I.; Özkaynak, S.S. Acute transverse myelitis after inactivated COVID-19 vaccine. Ideggyogy. Szle. 2021, 74, 273–276. [Google Scholar] [CrossRef]
- Etemadifar, M.; Sigari, A.A.; Sedaghat, N.; Salari, M.; Nouri, H. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum. Vaccines Immunother. 2021, 17, 3481–3483. [Google Scholar] [CrossRef]
- Fan, H.T.; Lin, Y.Y.; Chiang, W.F.; Lin, C.Y.; Chen, M.H.; Wu, K.A.; Chan, J.S.; Kao, Y.H.; Shyu, H.Y.; Hsiao, P.J. COVID-19 vaccine-induced encephalitis and status epilepticus. QJM Int. J. Med. 2022, 115, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Jaggernauth, S.; Ramnarine, V.; Mohammed, S.R.; Khan, C.; Panday, A. Neurological conditions following COVID-19 vaccinations: Chance or association? Cureus 2022, 14, 21919. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Exacerbating guillain–barré syndrome eight days after vector-based COVID-19 vaccination. Case Rep. Infect. Dis. 2021, 2021, 3619131. [Google Scholar] [CrossRef] [PubMed]
- Franco, S.V.; Fonollosa, A. Ischemic Optic Neuropathy After Administration of a SARS-CoV-2 Vaccine: A Report of 2 Cases. Am. J. Case Rep. 2022, 23, e935095-1. [Google Scholar]
- Fujimori, J.; Miyazawa, K.; Nakashima, I. Initial clinical manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neuroimmunol. 2021, 361, 577755. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Tomita, M.; Ikeda, S.; Hattori, N. A case of sensory ataxic Guillain–Barré syndrome with immunoglobulin G anti-GM1 antibodies following the first dose of mRNA COVID-19 vaccine BNT162b2 (Pfizer). QJM Int. J. Med. 2021, 115, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, L.A.; Abadía, L.R.; de los Reyes Guevara, C.A.; Orozco, J.H. Guillain-Barre syndrome after vaccination for COVID-19. The first report in Latin America. Neurol. Perspect. 2021, 1, 236. [Google Scholar] [CrossRef] [PubMed]
- Gama, P.D.D.; Alcantara, T.G.D.; Smaniotto, R.R.; Petuco, P.D.L.; Almeida, W.A.D.; Gama, R.A.D.D.; Fragoso, Y.D. Extensive Longitudinal Transverse Myelitis Temporally Related to the Use of AZD1222, AstraZeneca COVID-19 Vaccine: Cerebrospinal Fluid Analysis and Recent Data Review. Case Rep. Neurol. Med. 2022, 2022, 8999853. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-J.; Tseng, H.-P.; Lin, C.-L.; Hsu, R.-F.; Lee, M.-H.; Liu, C.-H. Acute encephalitis after COVID-19 vaccination: A case report and literature review. Human vaccines & immunotherapeutics 2022, 18, 2082206. [Google Scholar]
- Gao, J.-J.; Tseng, H.-P.; Lin, C.-L.; Shiu, J.-S.; Lee, M.-H.; Liu, C.-H. Acute transverse myelitis following COVID-19 vaccination. Vaccines 2021, 9, 1008. [Google Scholar] [CrossRef]
- García-Grimshaw, M.; Michel-Chávez, A.; Vera-Zertuche, J.M.; Galnares-Olalde, J.A.; Hernández-Vanegas, L.E.; Figueroa-Cucurachi, M.; Paredes-Ceballos, O.; Reyes-Terán, G.; Carbajal-Sandoval, G.; Arauz, A.; et al. Guillain-Barré syndrome is infrequent among recipients of the BNT162b2 mRNA COVID-19 vaccine. Clin. Immunol. 2021, 230, 108818. [Google Scholar] [CrossRef]
- Garg, R.K.; Malhotra, H.S.; Kumar, N.; Pandey, S.; Patil, M.R.; Uniyal, R.; Rizvi, I. Tumefactive Demyelinating Brain Lesion Developing after Administration of Adenovector-Based COVID-19 Vaccine: A Case Report. Neurol. India 2022, 70, 409. [Google Scholar] [CrossRef] [PubMed]
- George, T.B.; Kainat, A.; Pachika, P.S.; Arnold, J. Rare occurrence of Guillain-Barré syndrome after Moderna vaccine. BMJ Case Rep. CP 2022, 15, e249749. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Dubey, S.; Roy, D.; Mandal, A.; Naga, D.; Benito-León, J. Focal onset non-motor seizure following COVID-19 vaccination: A mere coincidence? Diabetes Metab. Syndr. 2021, 15, 1023. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after ChAdOx1 nCOV-19 vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, J.; Creed, M.; Muriel, S. Transverse myelitis as a first event of multiple sclerosis precipitated by Pfizer-BioNTech COVID-19 vaccination. Neuroimmunol. Rep. 2022, 2, 100074. [Google Scholar] [CrossRef]
- Guditi, S.; Setty, G.; Verma, M.; Reddy, R.; Devraj, R.; Raju, S.B.; Gokhale, G.K. Vaccine-Induced Thrombotic Thrombocytopenia Due to Coronavirus Disease 2019 Vaccine From a Deceased Donor: A Case Report. Transplant. Proc. 2021, 54, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Khan, M.; Khan, F.; Hamza, G. Case of Guillain-Barré syndrome following COVID-19 vaccine. BMJ Case Rep. CP 2021, 14, e243629. [Google Scholar] [CrossRef] [PubMed]
- Havla, J.; Schultz, Y.; Zimmermann, H.; Hohlfeld, R.; Danek, A.; Kümpfel, T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neurol. 2022, 269, 55–58. [Google Scholar] [CrossRef]
- Hemalatha, S.; Karishma, M.; Bera, J.; Blessy, S.; Thirumaran, J.; Loganathan, T.; Gopala Krishnan, A. A Case Report on Post COVID-19 Vaccine Associated Guillain–Barré Syndrome. 2021, 33, 454–458.
- Hirose, S.; Hara, M.; Koda, K.; Natori, N.; Yokota, Y.; Ninomiya, S.; Nakajima, H. Acute autoimmune transverse myelitis following COVID-19 vaccination: A case report. Medicine 2021, 100, e28423. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-T.; Tsai, M.-J.; Chen, Y.-H.; Hsu, C.-F. Acute transverse myelitis after COVID-19 vaccination. Medicina 2021, 57, 1010. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L.; Brunn, J.A.; Jacobs, J.; Todd, P.K.; Askari, F.K.; Fontana, R.J. Guillain-Barré Syndrome AFTER COVID-19 mRNA Vaccination in a Liver Transplantation Recipient With Favorable Treatment Response. Liver Transplant. 2022, 28, 134–137. [Google Scholar] [CrossRef]
- Iftikhar, H.; Noor, S.M.U.; Masood, M.; Bashir, K. Bell’s palsy after 24 hours of mRNA-1273 SARS-CoV-2 vaccine. Cureus 2021, 13, e15935. [Google Scholar] [CrossRef] [PubMed]
- Introna, A.; Caputo, F.; Santoro, C.; Guerra, T.; Ucci, M.; Mezzapesa, D.M.; Trojano, M. Guillain-Barré syndrome after AstraZeneca COVID-19-vaccination: A causal or casual association? Clin. Neurol. Neurosurg. 2021, 208, 106887. [Google Scholar] [CrossRef] [PubMed]
- Ish, S.; Ish, P. Facial nerve palsy after COVID-19 vaccination–A rare association or a coincidence. Indian J. Ophthalmol. 2021, 69, 2550. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Jose, J.; Gafoor, V.A.; Smita, B.; Balaram, N. Guillain-Barré syndrome following ChAdOx1 nCoV-19 COVID-19 vaccination: A case series. Neurol. Clin. Neurosci. 2021, 9, 402–405. [Google Scholar] [CrossRef] [PubMed]
- John, V.; Koshy, J.T.; Gladson, N.; Wills, V.K. Guillain-Barré syndrome associated with COVID-19 vaccination: A case report. Int. J. Res. Med. Sci. 2022, 10, 268. [Google Scholar] [CrossRef]
- Kanabar, G.; Wilkinson, P. Guillain-Barré syndrome presenting with facial diplegia following COVID-19 vaccination in two patients. BMJ Case Rep. CP 2021, 14, e244527. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, J.E.; Yoo, J.R.; Oh, H.; Kim, M.; Kim, Y.R.; Heo, S.T. Aseptic meningitis following second dose of an mRNA coronavirus disease 2019 vaccine in a healthy male: Case report and literature review. Infect. Chemother. 2022, 54, 189. [Google Scholar] [CrossRef] [PubMed]
- Kania, K.; Ambrosius, W.; Tokarz Kupczyk, E.; Kozubski, W. Acute disseminated encephalomyelitis in a patient vaccinated against SARS-CoV-2. Ann. Clin. Transl. Neurol. 2021, 8, 2000–2003. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Boostani, R.; Fatehi, F.; Panahi, A.; Okhovat, A.A.; Ziaadini, B.; Basiri, K.; Abdi, S.; Sinaei, F.; Rezaei, M.; et al. Guillain-Barré syndrome and COVID-19 vaccine: A report of nine patients. Basic Clin. Neurosci. 2021, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Katada, E.; Toyoda, T.; Yamada, G.; Morishima, A.; Matsukawa, N. A case of chronic inflammatory demyelinating polyneuropathy following COVID-19 vaccine. Neurol. Clin. Neurosci. 2022, 10, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Rogers, S.; Bilal, U.; Baktashi, H.; Singh, R. Multiple Sclerosis Relapse following COVID-19 Vaccination: A Case Report and Literature Review. Cureus 2022, 14, e21374. [Google Scholar] [CrossRef]
- Kavya, S.; Kubra, K.; Vaidyanathan, R.; Adarsh, S. An Interesting Case of Acute Disseminated Encephalomyelitis Post COVID-19 Vaccine. RGUHS J. Allied Health Sci. 2021, 1, 35–38. [Google Scholar]
- Kawtharani, A.A.; Fakhry, B.; Serhan, A. Longitudinal extensive transverse myelitis with sixth nerve palsy post ChAdOx1 nCov-19 vaccine: A case report and literature review. World J. Adv. Res. Rev. 2021, 12, 526–538. [Google Scholar] [CrossRef]
- Kaya, A.; Kaya, S.Y. A case of trigeminal neuralgia developing after a COVID-19 vaccination. J. Neurovirology 2022, 28, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Keir, G.; Maria, N.I.; Kirsch, C.F. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: A case report. Top. Magn. Reson. Imaging 2021, 30, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kenda, J.; Lovrič, D.; Škerget, M.; Milivojević, N. Treatment of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia related acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 106072. [Google Scholar] [CrossRef] [PubMed]
- Khadka, B.; Khanal, K. Post COVID-19 vaccine Guillain-Barré syndrome. J. Nepal Health Res. Counc. 2022, 19, 852–854. [Google Scholar] [PubMed]
- Khajavirad, N.; Salehi, M.; Haji Ghadery, A.; Khalili, H.; Arab Ahmadi, M.; Dehghan Manshadi, S.A.; Zare Dehnavi, A. Serious events following COVID-19 vaccination with ChAdOx1 nCoV-19 vaccine (Vaxzevria): A short case series from Iran. Clin. Case Rep. 2022, 10, e05390. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.; Shrestha, A.K.; Colantonio, M.A.; Liberio, R.N.; Sriwastava, S. Acute transverse myelitis following SARS-CoV-2 vaccination: A case report and review of literature. J. Neurol. 2022, 269, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Khayat-Khoei, M.; Bhattacharyya, S.; Katz, J.; Harrison, D.; Tauhid, S.; Bruso, P.; Houtchens, M.K.; Edwards, K.R.; Bakshi, R. COVID-19 mRNA vaccination leading to CNS inflammation: A case series. J. Neurol. 2022, 269, 1093–1106. [Google Scholar] [CrossRef]
- Kılıç, R.; Kılıç, E.; Dinç, Y.; Demir, A. New onset refractory status epilepticus after BNT162b2 nCoV-19. J. Clin. Images Med. Case Rep. 2022, 3, 1855. [Google Scholar] [CrossRef]
- Kim, J.E.; Min, Y.G.; Shin, J.Y.; Kwon, Y.N.; Bae, J.S.; Sung, J.J.; Hong, Y.H. Guillain–Barré Syndrome and Variants following COVID-19 Vaccination: Report of 13 Cases. Front. Neurol. 2022, 12, 820723. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, Y.G.; Park, Y.C.; Choi, S.; Lee, S.; Min, H.J.; Kim, M.J. Guillain-Barre syndrome after two COVID-19 vaccinations: Two case reports with follow-up electrodiagnostic study. J. Korean Med. Sci. 2022, 37, e58. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, J.-H.; Park, J.-S. Guillain–Barré syndrome associated with BNT162b2 COVID vaccination: A first case report from South Korea. Neurol. Sci. 2022, 43, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Seok, H.Y.; Yi, J.; Cho, J.H. Leg paralysis after AstraZeneca COVID-19 vaccination diagnosed as neuralgic amyotrophy of the lumbosacral plexus: A case report. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zhu, Z.; Kochar, P.; Gavigan, P.; Kaur, D.; Kumar, A. A pediatric case of sensory predominant Guillain-Barré syndrome following COVID-19 vaccination. Child Neurol. Open 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Karasawa, S.; Ohashi, N.; Yamamoto, K. A case of encephalitis following COVID-19 vaccine. J. Infect. Chemother. 2022, 28, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.S.; Goh, Y.; Tan, B.Q.; Hui, A.F.; Hoe, R.H.M.; Makmur, A.; Kei, P.L.; Vijayan, J.; Ng, K.W.P.; Quek, A.M.L.; et al. Neuralgic amyotrophy following COVID-19 mRNA vaccination. QJM Int. J. Med. 2021, 114, 503–505. [Google Scholar] [CrossRef]
- Kotal, R.; Jacob, I.; Rangappa, P.; Rao, K.; Hosurkar, G.; Anumula, S.K.; Kuberappa, A.M. A rare case of vaccine-induced immune thrombosis and thrombocytopenia and approach to management. Surg. Neurol. Int. 2021, 12, 408. [Google Scholar] [CrossRef]
- Kripalani, Y.; Lakkappan, V.; Parulekar, L.; Shaikh, A.; Singh, R.; Vyas, P. A Rare Case of Guillain-Barré Syndrome following COVID-19 Vaccination. Eur. J. Case Rep. Intern. Med. 2021, 8, 002707. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Hasegawa, T.; Ikeda, K.; Aoki, M. Case Report: Isolated, unilateral oculomotor palsy with anti-GQ1b antibody following COVID-19 vaccination. F1000Research 2021, 10, 1142. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, T. Autoimmune encephalitis following ChAdOx1-S SARS-CoV-2 vaccination. Neurol. Sci. 2022, 43, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Kyle, K.; Martinez-Lage, M.; Lang-Orsini, M.; Venna, N. An Unusually Fulminant Case of Encephalomyelitis in an 80 Year Old. Neuroimmunol. Rep. 2022, 2, 100102. [Google Scholar] [CrossRef]
- Łagosz, P.; Biegus, J.; Gruszka, E.; Zymliński, R. The surprising course of multiple sclerosis relapse in a patient after SARS-CoV-2 vaccination. Kardiol. Pol. 2022, 80, 237–238. [Google Scholar] [CrossRef]
- Lee, S.; Oh, S.-I. Guillain-Barré Syndrome Following the ChAdOx1-S/nCoV-19 Vaccine: A Case Report. J. Korean Assoc. EMG-Electrodiagn. Med. 2022, 24, 7–10. [Google Scholar] [CrossRef]
- Leemans, W.; Antonis, S.; De Vooght, W.; Lemmens, R.; Van Damme, P. Neuromuscular complications after COVID-19 vaccination: A series of eight patients. Acta Neurol. Belg. 2022, 122, 753–761. [Google Scholar] [CrossRef]
- Ling, L.; Bagshaw, S.M.; Villeneuve, P.-M. Guillain–Barré syndrome after SARS-CoV-2 vaccination in a patient with previous vaccine-associated Guillain–Barré syndrome. CMAJ 2021, 193, E1766–E1769. [Google Scholar] [CrossRef]
- Liu, B.D.; Ugolini, C.; Jha, P. Two Cases of Post-Moderna COVID-19 Vaccine Encephalopathy Associated With Nonconvulsive Status Epilepticus. Cureus 2021, 13, e16172. [Google Scholar] [CrossRef]
- Lohmann, L.; Glaser, F.; Möddel, G.; Lünemann, J.D.; Wiendl, H.; Klotz, L. Severe disease exacerbation after mRNA COVID-19 vaccination unmasks suspected multiple sclerosis as neuromyelitis optica spectrum disorder: A case report. BMC Neurol. 2022, 22, 185. [Google Scholar] [CrossRef]
- Luca, A.; Squillaci, R.; Terravecchia, C.; Contrafatto, F.; Reggio, E.; Nicoletti, A.; Zappia, M. Pure sensitive chronic inflammatory axonal polyneuropathy following Pfizer COVID-19 vaccine. Neurol. Sci. 2022, 43, 1431–1433. [Google Scholar] [CrossRef]
- Luciano, P.Q.; Binatti, R.; Sodré, A.R.; Zajac, S.R.; Marson, F.A.L.; Ortega, M.M. Vaccine-induced immune thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccine in an older patient: Minireview and a case report. J. Infect. Public Health 2022, 15, 638–642. [Google Scholar] [CrossRef]
- Luitel, P.; Poudel, B.; Upadhyay, D.; Paudel, S.; Tiwari, N.; Gajurel, B.P.; Karn, R.; Rajbhandari, R.; Shrestha, A.; Gautam, N.; et al. Guillain–Barré syndrome following coronavirus disease vaccine: First report from Nepal. SAGE Open Med. Case Rep. 2022, 10. [Google Scholar] [CrossRef]
- Mahajan, S.; Zhang, F.; Mahajan, A.; Zimnowodzki, S. Parsonage Turner syndrome after COVID-19 vaccination. Muscle Nerve 2021, 64, E3. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, H.S.; Gupta, P.; Prabhu, V.; Garg, R.K.; Dandu, H.; Agarwal, V. COVID-19 vaccination-associated myelitis. QJM Int. J. Med. 2021, 114, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Malik, B.; Kalantary, A.; Rikabi, K.; Kunadi, A. Pulmonary embolism, transient ischaemic attack and thrombocytopenia after the Johnson & Johnson COVID-19 vaccine. BMJ Case Rep. CP 2021, 14, e243975. [Google Scholar]
- Mancuso, M.; Lauretti, D.L.; Cecconi, N.; Santini, M.; Lami, V.; Orlandi, G.; Casagli, S.; Ghetta, A.; Liberti, G.; Elena, B.M.; et al. Arterial intracranial thrombosis as the first manifestation of vaccine-induced immune thrombotic thrombocytopenia (VITT): A case report. Neurol. Sci. 2022, 43, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Manea, M.M.; Dragoș, D.; Enache, I.; Sirbu, A.G.; Tuta, S. Multiple cranial nerve palsies following COVID-19 vaccination-Case report. Acta Neurol. Scand. 2022, 145, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, G.T.; Manzo, V.; Di Battista, M.E.; Salvatore, S.; Moreggia, O.; Scavone, C.; Capuano, A. Severe multiple sclerosis relapse after COVID-19 vaccination: A case report. Front. Neurol. 2021, 12, 721502. [Google Scholar] [CrossRef] [PubMed]
- Maramattom, B.V.; Krishnan, P.; Paul, R.; Padmanabhan, S.; Cherukudal Vishnu Nampoothiri, S.; Syed, A.A.; Mangat, H.S. Guillain-Barré Syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann. Neurol. 2021, 90, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Maramattom, B.V.; Lotlikar, R.S.; Sukumaran, S. Central nervous system adverse events after ChAdOx1 vaccination. Neurol. Sci. 2022, 43, 3503–3507. [Google Scholar] [CrossRef] [PubMed]
- Maramattom, B.V.; Moidu, F.M.; Varikkottil, S.; Syed, A.A. Cerebral venous sinus thrombosis after ChAdOx1 vaccination: The first case of definite thrombosis with thrombocytopenia syndrome from India. BMJ Case Rep. CP 2021, 14, e246455. [Google Scholar] [CrossRef] [PubMed]
- Maroufi, S.F.; Naderi Behdani, F.; Rezania, F.; Tanhapour Khotbehsara, S.; Mirzaasgari, Z. Longitudinally extensive transverse myelitis after COVID-19 vaccination: Case report and review of literature. Hum. Vaccines Immunother. 2022, 18, 2040239. [Google Scholar] [CrossRef] [PubMed]
- Martin-Villares, C.; Vazquez-Feito, A.; Gonzalez-Gimeno, M.J.; de la Nogal-Fernandez, B. Bell’s palsy following a single dose of mRNA SARS-CoV-2 vaccine: A case report. J. Neurol. 2022, 269, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Masuccio, F.G.; Comi, C.; Solaro, C. Guillain–Barrè syndrome following COVID-19 vaccine mRNA-1273, a case report. Acta Neurol. Belg. 2021, 122, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- Matarneh, A.S.; Al-Battah, A.H.; Farooqui, K.; Ghamoodi, M.; Alhatou, M. COVID-19 vaccine causing Guillain-Barre syndrome, a rare potential side effect. Clin. Case Rep. 2021, 9, e04756. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.T.; Noto, A.; Asmundo, A.; Granata, F.; Galletta, K.; Mallamace, R.; De Gregorio, C.; Puliatti, F.; Fazio, M.C.; Germano’, A.; et al. Cerebral venous sinus thrombosis (CVST) associated with SARS-CoV-2 vaccines: Clues for an immunopathogenesis common to CVST observed in COVID-19. J. Anesth. Analg. Crit. Care 2021, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- McKean, N.; Chircop, C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. CP 2021, 14, e244125. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.R.; Mangion, S.A.; Benger, M.; Stanton, B.R.; Czuprynska, J.; Arya, R.; Sztriha, L.K. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination—A report of two UK cases. Brain Behav. Immun. 2021, 95, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Mélo-Silva, M.L., Jr.; Lopes, D.P. Large hemorrhagic stroke after ChAdOx1 nCoV-19 vaccination: A case report. Acta Neurol. Scand. 2021, 144, 717–718. [Google Scholar] [CrossRef] [PubMed]
- De Michele, M.; Iacobucci, M.; Chistolini, A.; Nicolini, E.; Pulcinelli, F.; Cerbelli, B.; Merenda, E.; Schiavo, O.G.; Sbardella, E.; Berto, I.; et al. Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: A catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 2021, 12, 4663. [Google Scholar] [CrossRef]
- Min, Y.G.; Ju, W.; Ha, Y.E.; Ban, J.J.; Lee, S.A.; Sung, J.J.; Shin, J.Y. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: Report of two cases and review of literature. J. Neuroimmunol. 2021, 359, 577691. [Google Scholar] [CrossRef] [PubMed]
- Mirmosayyeb, O.; Barzegar, M.; Rezaei, M.; Baharlouie, N.; Shaygannejad, V. Bell’s palsy after Sputnik V COVID-19 (Gam-COVID-Vac) vaccination. Clin. Case Rep. 2022, 10, e05468. [Google Scholar] [CrossRef]
- Miyamoto, K.; Koh, J.; Takahashi, M.; Niwa, M.; Ito, H. A case of anti-MOG antibody-positive ADEM following COVID-19 mRNA vaccination. Neurol. Sci. 2022, 43, 3513–3514. [Google Scholar] [CrossRef] [PubMed]
- Miyaue, N.; Yoshida, A.; Yamanishi, Y.; Tada, S.; Ando, R.; Hosokawa, Y.; Yabe, H.; Nagai, M. A case of refractory longitudinally extensive transverse myelitis after severe acute respiratory syndrome coronavirus 2 vaccination in a Japanese man. Intern. Med. 2022, 61, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Morehouse, Z.P.; Paulus, A.; Jasti, S.A.; Bing, X. A Rare Variant of Guillain-Barre Syndrome following Ad26. COV2. S Vaccination. Cureus 2021, 13, e18153. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic thrombocytopenia after Ad26. COV2. S vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef] [PubMed]
- Mumoli, L.; Vescio, V.; Pirritano, D.; Russo, E.; Bosco, D. ADEM anti-MOG antibody-positive after SARS-CoV-2 vaccination. Neurol. Sci. 2022, 43, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, C.C.; Sokol, J.; Alapati, N. Bell’s palsy following COVID-19 vaccine administration in HIV+ patient. Am. J. Ophthalmol. Case Rep. 2022, 25, 101259. [Google Scholar] [CrossRef] [PubMed]
- Nagalli, S.; Shankar Kikkeri, N. Sub-acute onset of Guillain-Barré syndrome post-mRNA-1273 vaccination: A case report. SN Compr. Clin. Med. 2022, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Nagaratnam, S.A.; Ferdi, A.C.; Leaney, J.; Lee, R.L.K.; Hwang, Y.T.; Heard, R. Acute disseminated encephalomyelitis with bilateral optic neuritis following ChAdOx1 COVID-19 vaccination. BMC Neurol. 2022, 22, 54. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, A.; Haddad, Q.; Alazwary, N.; Ghozzi, I.; Elkarouri, M. Guillain-Barre Syndrome Post COVID-19 Vaccination: Case Report. Int. J. Infect. Dis. Ther. 2022, 7, 8–12. [Google Scholar] [CrossRef]
- Nasuelli, N.A.; De Marchi, F.; Cecchin, M.; De Paoli, I.; Onorato, S.; Pettinaroli, R.; Savoini, G.; Godi, L. A case of acute demyelinating polyradiculoneuropathy with bilateral facial palsy after ChAdOx1 nCoV-19 vaccine. Neurol. Sci. 2021, 42, 4747–4749. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.B.; Raigam, W.A. Parsonage Turner Syndrome Following COVID-19 Vaccine. Eur. J. Med. Health Sci. 2022, 4, 13–14. [Google Scholar] [CrossRef]
- Netravathi, M.; Dhamija, K.; Gupta, M.; Tamborska, A.; Nalini, A.; Holla, V.V.; Nitish, L.K.; Menon, D.; Pal, P.K.; Seena, V.; et al. COVID-19 vaccine associated demyelination & its association with MOG antibody. Mult. Scler. Relat. Disord. 2022, 60, 103739. [Google Scholar] [PubMed]
- Nishiguchi, Y.; Matsuyama, H.; Maeda, K.; Shindo, A.; Tomimoto, H. Miller Fisher syndrome following BNT162b2 mRNA coronavirus 2019 vaccination. BMC Neurol. 2021, 21, 452. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, Y.; Hoshina, Y.; Baker, V. Bell’s palsy following the Ad26. COV2. S COVID-19 vaccination. QJM An Int. J. Med. 2021, 114, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Nistri, R.; Barbuti, E.; Rinaldi, V.; Tufano, L.; Pozzilli, V.; Ianniello, A.; Marinelli, F.; De Luca, G.; Prosperini, L.; Tomassini, V.; et al. Case report: Multiple sclerosis relapses after vaccination against SARS-CoV2, a series of clinical cases. Front. Neurol. 2021, 12, 765954. [Google Scholar] [CrossRef] [PubMed]
- Ogbebor, O.; Seth, H.; Min, Z.; Bhanot, N. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: A temporal occurrence, not a causal association. IDCases 2021, 24, e01143. [Google Scholar] [CrossRef] [PubMed]
- Onoda, K.; Sashida, R.; Fujiwara, R.; Wakamiya, T.; Michiwaki, Y.; Tanaka, T.; Shimoji, K.; Suehiro, E.; Yamane, F.; Kawashima, M.; et al. Trigeminal neuropathy after tozinameran vaccination against COVID-19 in postmicrovascular decompression for trigeminal neuralgia: Illustrative case. J. Neurosurg. Case Lessons 2022, 3, CASE22101. [Google Scholar] [CrossRef] [PubMed]
- Oo, W.M.; Giri, P.; De Souza, A. AstraZeneca COVID-19 vaccine and Guillain-Barré Syndrome in Tasmania: A causal link? J. Neuroimmunol. 2021, 360, 577719. [Google Scholar] [CrossRef] [PubMed]
- Oshida, S.; Akamatsu, Y.; Matsumoto, Y.; Suzuki, T.; Sasaki, T.; Kondo, Y.; Fujiwara, S.; Kashimura, H.; Kubo, Y.; Ogasawara, K. Intracranial aneurysm rupture within three days after receiving mRNA anti-COVID-19 vaccination: Three case reports. Surg. Neurol. Int. 2022, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Ozgen Kenangil, G.; Ari, B.C.; Guler, C.; Demir, M.K. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol. Belg. 2021, 121, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Pagenkopf, C.; Südmeyer, M. A case of longitudinally extensive transverse myelitis following vaccination against COVID-19. J. Neuroimmunol. 2021, 358, 577606. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Garg, R.K.; Malhotra, H.S.; Kumar, N.; Uniyal, R. Accelerated hypertension and intracerebral haemorrhage following first dose of ChAdOx1-nCoV-19 vaccination. Ann. Indian Acad. Neurol. 2022, 25, 322. [Google Scholar] [CrossRef] [PubMed]
- Pang, E.; Ghosh, S.; Chemmanam, T.; Grove, C.; Phillips, T. Cerebral arterial and venous thrombosis due to COVID-19 vaccine-induced immune thrombotic thrombocytopenia. BMJ Case Rep. CP 2022, 15, e245445. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.U.; Khurram, R.; Lakhani, A.; Quirk, B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. CP 2021, 14, e242956. [Google Scholar] [CrossRef] [PubMed]
- Permezel, F.; Borojevic, B.; Lau, S.; de Boer, H.H. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci. Med. Pathol. 2021, 18, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Pothiawala, S. Bell’s Palsy After Second Dose of Moderna COVID-19 Vaccine: Coincidence or Causation? Acta Medica Litu. 2021, 28, 298. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Nepali, P.; Baniya, S.; Shah, S.; Bogati, S.; Nepal, G.; Ojha, R.; Edaki, O.; Lazovic, G.; Kara, S. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann. Med. Surg. 2022, 78, 103897. [Google Scholar] [CrossRef]
- Prasad, A.; Hurlburt, G.; Podury, S.; Tandon, M.; Kingree, S.; Sriwastava, S. A novel case of bifacial diplegia variant of guillain-barré syndrome following janssen COVID-19 vaccination. Neurol. Int. 2021, 13, 404–409. [Google Scholar] [CrossRef]
- Queler, S.C.; Towbin, A.J.; Milani, C.; Whang, J.; Sneag, D.B. Parsonage-Turner syndrome following COVID-19 vaccination: MR neurography. Radiology 2022, 302, 84. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Bordás, C.; Gascón-Gimenez, F.; Alcalá, C.; Payá, M.; Mallada, J.; Silla, R.; Carratalà-Boscà, S.; Gasque-Rubio, R.; Castillo, J.; Casanova, B. Case Report: Exacerbation of Relapses Following mRNA COVID-19 Vaccination in Multiple Sclerosis: A Case Series. Front. Neurol. 2022, 13, 897275. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.J.; Khurana, S.; Murthy, G.; Dawson, E.T.; Jazebi, N.; Haas, C.J. A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 597–600. [Google Scholar] [CrossRef]
- Rastogi, A.; Bingeliene, A.; Strafella, A.P.; Tang-Wai, D.F.; Wu, P.E.; Mandell, D.M. Reversible neurological and brain MRI changes following COVID-19 vaccination: A case report. J. Neuroradiol. 2022, 1, 1–3. [Google Scholar] [CrossRef]
- Razok, A.; Shams, A.; Almeer, A.; Zahid, M. Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Ann. Med. Surg. 2021, 67, 102540. [Google Scholar] [CrossRef]
- Repajic, M.; Lai, X.L.; Xu, P.; Liu, A. Bell’s Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav. Immun.-Health 2021, 13, 100217. [Google Scholar] [CrossRef]
- Reyes-Capo, D.P.; Stevens, S.M.; Cavuoto, K.M. Acute abducens nerve palsy following COVID-19 vaccination. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2021, 25, 302–303. [Google Scholar] [CrossRef]
- Rinaldi, V.; Bellucci, G.; Romano, A.; Bozzao, A.; Salvetti, M. ADEM after ChAdOx1 nCoV-19 vaccine: A case report. Mult. Scler. 2021, 28, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Garg, R.K.; Gupta, P.; Malhotra, H.S.; Kumar, N.; Uniyal, R.; Pandey, S. Acute hemorrhagic leukoencephalitis after administration of the first dose of ChAdOx1 nCoV-19 SARS-CoV-2 (COVISHIELD™) vaccine. Neuroimmunol. Rep. 2022, 2, 100089. [Google Scholar] [CrossRef]
- Rizzo, A.C.; Giussani, G.; Agostoni, E.C. Ischemic Stroke and Vaccine-Induced Immune Thrombotic Thrombocytopenia following COVID-19 Vaccine: A Case Report with Systematic Review of the Literature. Cerebrovasc. Dis. 2022, 51, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Gravina, D.; Marra, C.; Della Rosa, N.; Adani, R. Cubital Tunnel Syndrome Temporally after COVID-19 Vaccination. Trop. Med. Infect. Dis. 2022, 7, 62. [Google Scholar] [CrossRef]
- Rossetti, A.; Gheihman, G.; O’Hare, M.; Kosowsky, J.M. Guillain-Barré syndrome presenting as facial diplegia after COVID-19 vaccination: A case report. J. Emerg. Med. 2021, 61, e141–e145. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Shimizu, T.; Suzuki-Inoue, K.; Ishida, T.; Wada, Y. Aseptic meningitis after vaccination of the BNT162b2 mRNA COVID-19 vaccine. Neurol. Sci. 2021, 42, 4433–4435. [Google Scholar] [CrossRef] [PubMed]
- Salbas, E.; Özge, O.; Levent, Ö.; Karahan, A.Y. Facial paralysis following messenger RNA COVID-19 Vaccines: The report of two Cases. Aegean J. Med. Sci. 2021, 4, 73–77. [Google Scholar] [CrossRef]
- Saleh, M.; Zimmermann, J.; Lehnen, N.C.; Pötzsch, B.; Weller, J.M. Late-Onset Vaccine-Induced Immune Thombotic Thrombocytopenia (VITT) with Cerebral Venous Sinus Thrombosis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2022, 31, 106311. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, S.; Acharya, I.; Rawoof, A.; Shetty, R. Non-arteritic anterior ischaemic optic neuropathy (NA-AION) and COVID-19 vaccination. BMJ Case Rep. CP 2022, 15, e248415. [Google Scholar] [CrossRef] [PubMed]
- Sansen, P.; Calaras, E.; Weynants, N.; Glorieux, P. Miller-Fisher Syndrome following the first dose of Comirnaty (Pfizer COVID-19 vaccination). J. Med. Case Rep. Case Ser. 2021, 2, 16. [Google Scholar]
- Saraswat, B.; Kumar, S.; Rawal, D.; Johri, S.; Rai, P.K.; Grover, A. Acute Onset Quadriparesis Post COVID-19 Vaccination: A Case Report. East Afr. Sch. J. Med. Sci. 2021, 4, 181–182. [Google Scholar]
- Scendoni, R.; Petrelli, C.; Scaloni, G.; Logullo, F.O. Electromyoneurography and laboratory findings in a case of Guillain-Barré syndrome after second dose of Pfizer COVID-19 vaccine. Hum. Vaccines Immunother. 2021, 17, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Senda, J.; Ashida, R.; Sugawara, K.; Kawaguchi, K. Acute Meningoencephalitis after COVID-19 Vaccination in an Adult Patient with Rheumatoid Vasculitis. Intern. Med. 2022, 61, 8815–8821. [Google Scholar] [CrossRef]
- Sepahvand, M.; Yazdi, N.; Rohani, M.; Emamikhah, M. Cervical longitudinally extensive myelitis after vaccination with inactivated virus-based COVID-19 vaccine. Radiol. Case Rep. 2022, 17, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Shalilahmadi, D.; Porkar, N.F. Acute Disseminated Encephalomyelitis Following Sputnik V COVID-19 Vaccine: A. Int. J. Neurol. Disord. 2021, 5, 7–10. [Google Scholar]
- Sharma, A.; Gupta, A. A Rare Case of Brachial Plexus Neuropraxia after COVID-19 Vaccination. Cureus 2022, 14, e21244. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.B.; Iyer, V.G.; Zhang, Y.P.; Burger, J.T.; Shields, C.B. Parsonage-Turner Syndrome following COVID-19 Vaccination: Clinical and Electromyographic Findings in 6 Patients. Case Rep. Neurol. 2022, 14, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Ogaki, K.; Nakamura, R.; Kado, E.; Nakajima, S.; Kurita, N.; Watanabe, M.; Yamashiro, K.; Hattori, N.; Urabe, T. An 88-year-old woman with acute disseminated encephalomyelitis following messenger ribonucleic acid-based COVID-19 vaccination. Eneurologicalsci 2021, 25, 100381. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.R.; Khan, T.; Tahir, M.J.; Asghar, M.S.; Islam, M.S.; Yousaf, Z. Miller Fisher syndrome after COVID-19 vaccination: Case report and review of literature. Medicine 2022, 101, e29333. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.F.; da Silva, C.F.; Oliveira, R.E.N.D.N.; Romancini, F.; Mendes, R.M.; Locks, A.; Longo, M.F.M.; Moro, C.H.C.; Longo, A.L.; Braatz, V.L. Guillain-Barré syndrome after coronavirus disease 2019 vaccine: A temporal association. Clin. Exp. Neuroimmunol. 2022, 13, 92–94. [Google Scholar] [CrossRef]
- Silva, G.F.; Silva, C.F.; Oliveira, R.E.N.D.N.; Silva, F.W.D.; Baptista, J.P.R.; Loz, S.H.; Moro, C.H.C.; Longo, A.L.; Ribas, G.D.C. Transverse myelitis and coronavirus disease 2019 vaccine: A temporal association. Clin. Exp. Neuroimmunol. 2022, 13, 75–79. [Google Scholar] [CrossRef]
- Singh, S.; Sanna, F.; Adhikari, R.; Akella, R.; Gangu, K. Chronic Inflammatory Demyelinating Polyneuropathy Post-mRNA-1273 Vaccination. Cureus 2022, 14, e24528. [Google Scholar] [CrossRef]
- Siriratnam, P.; Buzzard, K.; Yip, G. Acute haemorrhagic necrotizing encephalopathy in the setting of SARS-CoV-2 vaccination: A case report and literature review. Acta Neurol. Belg. 2022, 123, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Sivji, M.; Vidya, M.; Pratap, T.; Jalal, M.J.A. Acute disseminated encephalomyelitis post-SARS-CoV-2 vaccination with Chadox1 nCov-19 (AZD1222)–A rare case report. East. J. Med. Sci. 2022, 7, 47–50. [Google Scholar] [CrossRef]
- Sluyts, Y.; Arnst, Y.; Vanhemelryck, T.; De Cauwer, H. COVID-19-booster vaccine-induced encephalitis. Acta Neurol. Belg. 2022, 122, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Sobh, O.; AlSoofi, N.; Alatifi, A.; Alsulaim, L.; Dahhan, H.; Abuselmiya, M.; AlJarallah, A.; Elmaghrabi, M.M.; SobH, O.; Alatifi, A.; et al. A Rare Case of COVID-19 Vaccine-Induced Thrombotic Thrombocytopenia in a Young Patient. Cureus 2022, 14, e24355. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.; Oo, W.M.; Giri, P. Inflammatory demyelinating polyneuropathy after the ChAdOx1 nCoV-19 vaccine may follow a chronic course. J. Neurol. Sci. 2022, 436, 120231. [Google Scholar] [CrossRef] [PubMed]
- Sriwastava, S.; Shrestha, A.K.; Khalid, S.H.; Colantonio, M.A.; Nwafor, D.; Srivastava, S. Spectrum of neuroimaging findings in post-COVID-19 vaccination: A case series and review of literature. Neurol. Int. 2021, 13, 622–639. [Google Scholar] [CrossRef]
- Stefanou, M.I.; Karachaliou, E.; Chondrogianni, M.; Moschovos, C.; Bakola, E.; Foska, A.; Melanis, K.; Andreadou, E.; Voumvourakis, K.; Papathanasiou, M.; et al. Guillain-Barré syndrome and fulminant encephalomyelitis following Ad26. COV2. S vaccination: Double jeopardy. Neurol. Res. Pract. 2022, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-C.; Lyu, R.-K.; Chang, C.-W.; Tseng, W.-E.J. The First Guillain-Barr? Syndrome After SARS-CoV-2 Vaccination in Taiwan. Acta Neurol. Taiwanica 2022, 31, 46–51. [Google Scholar]
- Suri, V.; Pandey, S.; Singh, J.; Jena, A. Acute-onset chronic inflammatory demyelinating polyneuropathy after COVID-19 infection and subsequent ChAdOx1 nCoV-19 vaccination. BMJ Case Rep. 2021, 14, e245816. [Google Scholar] [CrossRef] [PubMed]
- Syed, K.; Chaudhary, H.; Donato, A. Central Venous Sinus Thrombosis with Subarachnoid Hemorrhage Following an mRNA COVID-19 Vaccination: Are These Reports Merely Co-Incidental? Am. J. Case Rep. 2021, 22, e933397. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, S.; Rezania, F.; Alwedaie, S.M.J.; Malekdar, E.; Badi, Z.; Tabatabaei, S.M.; Mirzaasgari, Z. Post COVID-19 vaccination Guillain-Barre syndrome: Three cases. Hum. Vaccines Immunother. 2022, 18, 2045153. [Google Scholar] [CrossRef]
- Tagliaferri, A.R.; Horani, G.; Stephens, K.; Michael, P. A rare presentation of undiagnosed multiple sclerosis after the COVID-19 vaccine. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tomoda, Y.; Kadena, S.; Kanbayashi, T.; Kobayashi, S.; Kato, R. Guillain–Barré syndrome after BNT162b2 (Pfizer-BioNTec) vaccination. QJM Int. J. Med. 2022, 115, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.Y.; Yusof Khan, A.H.K.; Mohd Yaakob, M.N.; Abdul Rashid, A.M.; Loh, W.C.; Baharin, J.; Ibrahim, A.; Ismail, M.R.; Inche Mat, L.N.; Wan Sulaiman, W.A.; et al. Longitudinal extensive transverse myelitis following ChAdOx1 nCOV-19 vaccine: A case report. BMC Neurol. 2021, 21, 395. [Google Scholar] [CrossRef] [PubMed]
- Tapdia, M.; Kumar, A.; Singh, V.K.; Pathak, A.; Joshi, D. Post ChAdOx1 nCoV-19 vaccination frontal lobe syndrome. Neurol. Sci. 2022, 43, 4099–4101. [Google Scholar] [CrossRef] [PubMed]
- Terreros-Caro, G.G.; Díaz, S.G.; Alé, M.P.; Gimeno, M.M. Bell’s palsy following COVID-19 vaccination: A case report. Neurologia 2021, 36, 567. [Google Scholar]
- Thant, H.L.; Morgan, R.; Paese, M.M.; Persaud, T.; Diaz, J.; Hurtado, L. Guillain-Barré Syndrome after Ad26. COV2. S Vaccination. Am. J. Case Rep. 2022, 23, e935275-1. [Google Scholar] [CrossRef]
- Tiede, A.; Sachs, U.J.; Czwalinna, A.; Werwitzke, S.; Bikker, R.; Krauss, J.K.; Donnerstag, F.; Weißenborn, K.; Höglinger, G.; Maasoumy, B.; et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood J. Am. Soc. Hematol. 2021, 138, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Toljan, K.; Amin, M.; Kunchok, A.; Ontaneda, D. New diagnosis of multiple sclerosis in the setting of mRNA COVID-19 vaccine exposure. J. Neuroimmunol. 2022, 362, 577785. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, M.; Zoleo, P.; Arabia, G.; Gambardella, A. Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Neurol. Sci. 2021, 42, 4401–4402. [Google Scholar] [CrossRef]
- Vegezzi, E.; Ravaglia, S.; Buongarzone, G.; Bini, P.; Diamanti, L.; Gastaldi, M.; Prunetti, P.; Rognone, E.; Marchioni, E. Acute myelitis and ChAdOx1 nCoV-19 vaccine: Casual or causal association? J. Neuroimmunol. 2021, 359, 577686. [Google Scholar] [CrossRef]
- Vitturi, B.K.; Grandis, M.; Beltramini, S.; Orsi, A.; Schenone, A.; Icardi, G.; Durando, P. Parsonage–Turner syndrome following coronavirus disease 2019 immunization with ChAdOx1-S vaccine: A case report and review of the literature. J. Med. Case Rep. 2021, 15, 589. [Google Scholar] [CrossRef]
- Vogrig, A.; Janes, F.; Gigli, G.L.; Curcio, F.; Del Negro, I.; D’Agostini, S.; Fabris, M.; Valente, M. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin. Neurol. Neurosurg. 2021, 208, 106839. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Fuchs, M.; Grossmann, A.; Walter, M.; Thiele, T.; Storch, A.; Wittstock, M. Adenovirus-vectored COVID-19 vaccine–induced immune thrombosis of carotid artery: A case report. Neurology 2021, 97, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Nakahara, K.; Nakanishi, T.; Nomura, T.; Ueda, M. Miller Fisher Syndrome Following Vaccination against SARS-CoV-2. Intern. Med. 2022, 61, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, F.; Iranpour, P.; Haseli, S.; Poursadeghfard, M.; Yarmahmoodi, F. Acute disseminated encephalomyelitis (ADEM) after SARS-CoV-2 vaccination: A case report. Radiol. Case Rep. 2022, 17, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.-Y.; Cen, L.-S.; Chen, T.; Yang, T.-H. Bell’s palsy after inactivated COVID-19 vaccination in a patient with history of recurrent Bell’s palsy: A case report. World J. Clin. Cases 2021, 9, 8274–8279. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Jonguitud, L.F.; Pérez-García, C.C. Delirium triggered by COVID-19 vaccine in an elderly patient. Geriatr. Gerontol. Int. 2021, 21, 540. [Google Scholar] [CrossRef] [PubMed]
- Zavari, A.; Hamidabad, N.M.; Hassanzadeh, M. A case of aseptic meningitis following AZD1222 COVID-19 vaccination. Am. J. Emerg. Med. 2021, 55, 225.e5–225.e6. [Google Scholar]
- Zlotnik, Y.; Gadoth, A.; Abu-Salameh, I.; Horev, A.; Novoa, R.; Ifergane, G. Case Report: Anti-LGI1 Encephalitis Following COVID-19 Vaccination. Front. Immunol. 2022, 12, 813487. [Google Scholar] [CrossRef] [PubMed]
- Zubair, A.S.; Bae, J.Y.; Desai, K. Facial Diplegia Variant of Guillain-Barré Syndrome in Pregnancy FOLLOWING COVID-19 Vaccination: A Case Report. Cureus 2022, 14, e22341. [Google Scholar] [CrossRef] [PubMed]
- Zuhorn, F.; Graf, T.; Klingebiel, R.; Schäbitz, W.R.; Rogalewski, A. Postvaccinal encephalitis after ChAdOx1 nCoV-19. Ann. Neurol. 2021, 90, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Purkayastha, B.; Alam, M.J.; Chakraborty, S.R.; Roy, S.; Ahmed, N. COVID-19 mRNA vaccine-associated encephalopathy, myocarditis, and thrombocytopenia with excellent response to methylprednisolone: A case report. J. Neuroimmunol. 2022, 368, 577883. [Google Scholar] [CrossRef] [PubMed]
- Barsha, S.Y.; Haque, M.M.A.; Rashid, M.U.; Rahman, M.L.; Hossain, M.A.; Zaman, S.; Bhuiyan, E.; Sultana, R.; Hossian, M.; Nabi, M.H.; et al. A case of acute encephalopathy and non-ST segment elevation myocardial infarction following mRNA-1273 vaccination: Possible adverse effect? Clin. Exp. Vaccine Res. 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Porcari, A.; Merlo, M.; Roncon, L.; Sinagra, G. One-Year Risk of myocarditis after COVID-19 infection: A systematic review and meta-analysis. Can. J. Cardiol. 2022, 39, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Voleti, N.; Reddy, S.P.; Ssentongo, P. Myocarditis in SARS-CoV-2 infection vs. COVID-19 vaccination: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 2059. [Google Scholar] [CrossRef] [PubMed]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschöpe, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis following COVID-19 vaccine: Incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2022, 24, 2000–2018. [Google Scholar] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Costa, L.; Ruscitti, P.; Navarini, L.; Del Puente, A.; Giacomelli, R.; Scarpa, R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020, 19, 102524. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population incidence of Guillain-Barré syndrome: A systematic review and meta-analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Palaiodimou, L.; Stefanou, M.I.; Katsanos, A.H.; Fragkou, P.C.; Papadopoulou, M.; Moschovos, C.; Michopoulos, I.; Kokotis, P.; Bakirtzis, C.; Naska, A.; et al. Prevalence, clinical characteristics and outcomes of Guillain− Barré syndrome spectrum associated with COVID-19, a systematic review and meta-analysis. Eur. J. Neurol. 2021, 28, 3517–3529. [Google Scholar] [CrossRef] [PubMed]
- Ogunjimi, O.B.; Tsalamandris, G.; Paladini, A.; Varrassi, G.; Zis, P. Guillain-Barré Syndrome Induced by Vaccination Against COVID-19, A Systematic Review and Meta-Analysis. Cureus 2023, 15, e37578. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R. What Is Guillain-Barré Syndrome? JAMA 2023, 329, 602. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.B. Guillain-Barré syndrome. Bmj 2008, 337, 227–231. [Google Scholar] [CrossRef]
- Arami, M.A.; Yazdchi, M.; Khandaghi, R. Epidemiology and characteristics of Guillain-Barré syndrome in the northwest of Iran. Ann. Saudi Med. 2006, 26, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.; Félix, R.; Da Silva, W.; Queiroz, J.; Jeronimo, S. Clinical characteristics of Guillain–Barré syndrome in a tropical country: A Brazilian experience. Acta Neurol. Scand. 2012, 125, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Gómez, Á.; Díaz, A.; Carrión-Penagos, J.; Reyes, J.; Reyes, S. Clinical and electrophysiological characteristics of Guillain-Barré syndrome in Colombia. J. Peripher. Nerv. Syst. 2019, 24, 268–271. [Google Scholar] [CrossRef]
- Hanson, K.E.; Goddard, K.; Lewis, N.; Fireman, B.; Myers, T.R.; Bakshi, N.; Weintraub, E.; Donahue, J.G.; Nelson, J.C.; Xu, S.; et al. Incidence of Guillain-Barré syndrome after COVID-19 vaccination in the vaccine safety datalink. JAMA Netw. Open 2022, 5, e228879-e. [Google Scholar] [CrossRef]
- WHO. The Top 10 Causes of Death; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death#:~:text=Stroke%20and%20chronic%20obstructive%20pulmonary,4th%20leading%20cause%20of%20death (accessed on 23 September 2023).
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019, a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Luo, W.; Liu, X.; Bao, K.; Huang, C. Ischemic stroke associated with COVID-19, a systematic review and meta-analysis. J. Neurol. 2022, 269, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, B.; Li, T.; Yang, Z.; Li, S.; Le, W. Risk of ischemic stroke in patients with COVID-19 infection: A systematic review and meta-analysis. Brain Res. Bull. 2022, 180, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Stefanou, M.I.; Palaiodimou, L.; Aguiar de Sousa, D.; Theodorou, A.; Bakola, E.; Katsaros, D.E.; Halvatsiotis, P.; Tzavellas, E.; Naska, A.; Coutinho, J.M.; et al. Acute arterial ischemic stroke following COVID-19 vaccination: A systematic review and meta-analysis. Neurology 2022, 99, e1465–e1474. [Google Scholar] [CrossRef] [PubMed]
- Krzywicka, K.; Heldner, M.R.; Sánchez van Kammen, M.; van Haaps, T.; Hiltunen, S.; Silvis, S.M.; Levi, M.; Kremer Hovinga, J.A.; Jood, K.; Lindgren, E.; et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: An analysis of cases notified to the European Medicines Agency. Eur. J. Neurol. 2021, 28, 3656–3662. [Google Scholar] [CrossRef] [PubMed]
- Kakovan, M.; Shirkouhi, S.G.; Zarei, M.; Andalib, S. Stroke associated with COVID-19 vaccines. J. Stroke Cerebrovasc. Dis. 2022, 31, 106440. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, W.; Zhou, H.; Duan, W.; Li, S.; Huo, X.; Xu, W.; Zheng, H.; Liu, J.; Liu, H.; et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc. Neurol. 2020, 5, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Al-Mufti, F.; Amuluru, K.; Sahni, R.; Bekelis, K.; Karimi, R.; Ogulnick, J.; Cooper, J.; Overby, P.; Nuoman, R.; Tiwari, A.; et al. Cerebral venous thrombosis in COVID-19, a New York metropolitan cohort study. Am. J. Neuroradiol. 2021, 42, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Hinduja, A.; Nalleballe, K.; Onteddu, S.; Kovvuru, S.; Hussein, O. Impact of cerebral venous sinus thrombosis associated with COVID-19. J. Neurol. Sci. 2021, 425, 117448. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Husain, M.; Geddes, J.R.; Luciano, S.; Harrison, P.J. Cerebral venous thrombosis: A retrospective cohort study of 513,284 confirmed COVID-19 cases and a comparison with 489,871 people receiving a COVID-19 mRNA vaccine. Cent. Open Sci. Prepr. 2021. medRxiv:2021.04.27.21256153. [Google Scholar]
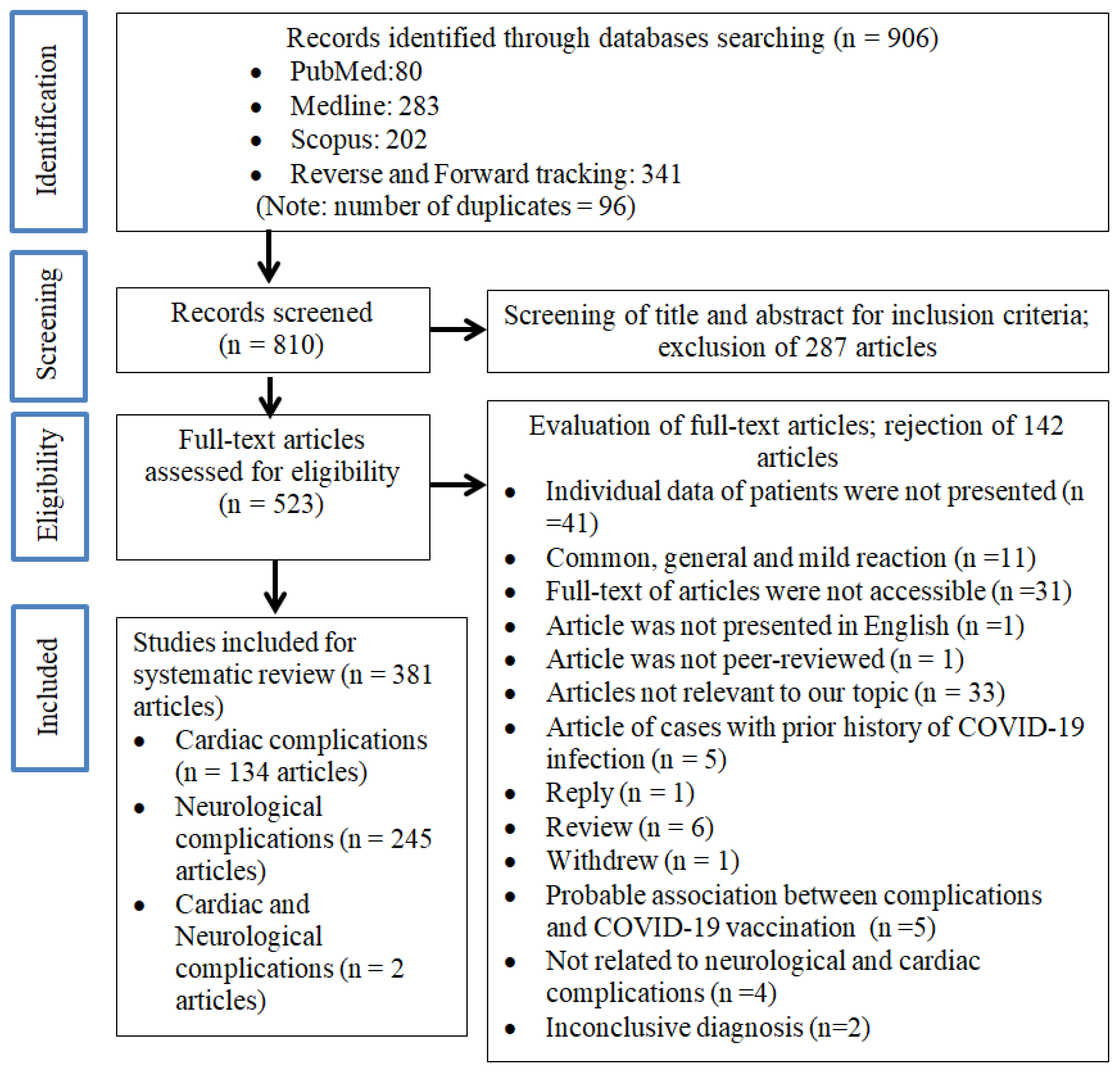
| Complications | Total Cases, n (%) | Demography, n (%) | Geographical Area, n (%) | Vaccine Type, n (%) | Clinical Course/Outcomes n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, Mean ± SD 33.8 ± 19.1 | F 120 (46.3) | M 139 (53.7) | Asia 100 (38.6) | Africa 1 (0.4) | America 107 (41.3) | Europe 49 (18.9) | Australasia 2 (0.8) | mRNA 233 (90.0) * | Viral vector 22 (8.5) | Attenuated virus 2 (1.5) | † 214 (82.6) | †† 27 (10.9) | Fatal 6 (2.4) | ||
| Cardiac arrhythmia | 3 (1.2) | 44.7 ± 13.1 | 1 (33.3) | 2 (66.7) | 2 (66.7) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 0 (0.0) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 0 (0.0) |
| Cardiomyopathy | 11 (4.2) | 54.5 ± 19.3 | 10 (90.9) | 1 (9.1) | 3 (27.3) | 0 (0.0) | 3 (27.3) | 4 (36.4) | 1 (9.1) | 9 (81.8) | 2 (18.2) | 0 (0.0) | 11 (100.0) | 0 (0.0) | 0 (0.0) |
| Inflammatory conditions of the heart | 221 (85.3) | 30.8 ± 16.5 | 93 (42.1) | 128 (57.9) | 75 (33.9) | 1 (0.5) | 100 (45.0) | 44 (19.9) | 1 (0.5) | 207 (93.7) | 13 (5.9) | 1 (0.5) | 181 (85.4) | 24 (11.4) | 6 (2.8) |
| 2 | 42.3 ± 21.0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 |
| 1 | NA | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 152 | 28.0 ± 13.8 | 49 | 103 | 64 | 1 | 59 | 27 | 1 | 142 | 10 | 0 | 121 | 16 | 5 |
| 38 | 19.7 ± 8.4 | 27 | 11 | 4 | 0 | 31 | 3 | 0 | 37 | 1 | 0 | 34 | 4 | 0 |
| 22 | 54.1 ± 20.5 | 14 | 8 | 4 | 0 | 5 | 13 | 0 | 20 | 2 | 0 | 17 | 4 | 1 |
| 6 | 25.3 ± 6.8 | 1 | 5 | 0 | 0 | 5 | 1 | 0 | 6 | 0 | 0 | 6 | 0 | 0 |
| Acute myocardial infarction | 15 (5.8) | 66.0 ± 18.0 | 8 (53.3) | 7 (46.7) | 12 (80.0) | 0 (0.0) | 3 (20.0) | 0 (0.3) | 0 (0.0) | 6 (40.0) | 7 (46.7) | 2 (13.3) | 12 (85.7) | 2 (14.3) | 0 (0.0) |
| Myocardial injury | 9 (3.5) | 24.8 ± 18.4 | 8 (88.9) | 1 (11.1) | 8 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (100.0) | 0 (100.0) | 0 (100.0) | 8 (100.0) | 0 (0.0) | 0 (0.0) |
| 2 | 54.0 ± 22.6 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| 7 | 16.4 ± 0.5 | 7 | 0 | 7 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 7 | 0 | 0 |
| Complications | Total Cases, n (%) | Dose of Vaccine, n (%) | Brand of Vaccine, n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First 65 (25.1) | Second 179 (69.1) | First Booster 6 (2.3) | NA 9 (3.5) | Astrazeneca 17 (6.6) | Jassen 4 (1.5) | Moderna 66 (25.5) | Pfizer 161 (62.2) | Sinopharm 1 (0.4) | Sinovac 3 (1.2) | Sputnik V 1 (0.4) | Nonspecific mRNA 4 (1.5) | Heterologous 2 (0.8) | ||
| Cardiac arrhythmia | 3 (1.2) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7 | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cardiomyopathy | 11 (4.2) | 6 (54.5) | 5 (45.5) | 0 (0.0) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 4 (36.4) | 5 (45.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Inflammatory conditions of the heart | 221 (85.3) | 45 (20.4) | 166 (75.1) | 5 (2.3) | 5 (2.3) | 8 (3.6) | 4 (1.8) | 58 (26.2) | 143 (64.7) | 0 (0.0) | 1 (0.5) | 1 (0.5) | 4 (1.8) | 2 (0.9) |
| 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 152 | 29 | 114 | 5 | 4 | 5 | 4 | 48 | 90 | 0 | 0 | 1 | 2 | 2 |
| 38 | 2 | 36 | 0 | 0 | 1 | 0 | 6 | 31 | 0 | 0 | 0 | 0 | 0 |
| 22 | 9 | 12 | 0 | 1 | 2 | 0 | 3 | 15 | 0 | 0 | 0 | 2 | 0 |
| 6 | 2 | 4 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 |
| Acute myocardial infarction | 15 (5.8) | 10 (66.7) | 1 (6.7) | 0 (0.0) | 4 (26.7) | 7 (46.7) | 0 (0.0) | 4 (26.7) | 2 (13.3) | 0 (0.0) | 2 (13.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Myocardial injury | 9 (3.5) | 3 (33.3) | 6 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 7 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 |
| Complications | Total Cases, n (%) | Demography, n (%) | Geographical Area, n (%) | Vaccine Type, n (%) | Clinical Course/Outcomes, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, Mean ± SD 49.7 ± 17.0 | F 230 (53.5) | M 200 (46.5) | Asia 195 (44.4) | Africa 2 (0.5) | America 99 (22.6) | Europe 130 (29.6) | Australasia 13 (3.0) | mRNA 183 (42.1) | Viral Vector 233 (53.6) * | Attenuated Virus 19 (4.4) | Non-Fatal 414 (94.3) | Fatal 25 (5.7) | ||
| CNS—Cerebrovascular disorders | 65 (14.8) | 47.5 ± 15.5 | 41 (73.2) | 15 (26.8) | 22 (33.8) | 0 (0.0) | 8 (12.3) | 34 (52.3) | 1 (1.5) | 10 (15.6) | 54 (84.4) | 0 (0.0) | 48 (73.8) | 17 (26.2) |
| 7 | 57.4 ± 13.7 | 7 | 0 | 4 | 0 | 1 | 2 | 0 | 3 | 4 | 0 | 6 | 1 |
| 58 | 46.0 ± 15.4 | 34 | 15 | 18 | 0 | 7 | 32 | 1 | 7 | 50 | 0 | 42 | 16 |
| CNS—Demyelinating disorders | 109 (24.8) | 43.6 ± 16.5 | 74 (67.9) | 35 (32.1) | 51 (46.8) | 0 (0.0) | 24 (22.0) | 32 (29.4) | 2 (1.8) | 51 (47.2) | 50 (46.3) | 7 (6.5) | 105 (96.3) | 4 (3.7) |
| 26 | 46.2 ± 17.4 | 15 | 11 | 19 | 0 | 1 | 4 | 2 | 5 | 17 | 4 | 24 | 1 |
| 4 | 42.3 ± 18.4 | 3 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 4 | 0 | 3 | 1 |
| 21 | 45.3 ± 20.9 | 13 | 8 | 15 | 0 | 4 | 2 | 0 | 11 | 8 | 2 | 21 | 0 |
| 14 | 44.9 ± 16.1 | 7 | 7 | 10 | 0 | 3 | 1 | 0 | 2 | 11 | 1 | 14 | 0 |
| 41 | 41.0 ± 13.6 | 33 | 8 | 3 | 0 | 16 | 22 | 0 | 33 | 7 | 0 | 40 | 1 |
| 3 | 40.3 ± 16.6 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 |
| CNS—Inflammatory disorders | 22 (5.0) | 47.9 ± 18.3 | 15 (68.2) | 7 (31.8) | 13 (59.1) | 0 (0.0) | 3 (13.6) | 6 (27.3) | 0 (0.0) | 15 (68.2) | 7 (31.8) | 0 (0.0) | 22 (100.0) | 0 (0.0) |
| 11 | 52.1 ± 18.1 | 6 | 5 | 6 | 0 | 1 | 4 | 0 | 6 | 5 | 0 | 11 | 0 |
| 9 | 42.3 ± 16.8 | 7 | 2 | 6 | 0 | 1 | 2 | 0 | 7 | 2 | 0 | 9 | 0 |
| 2 | 49.5 ± 31.8 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 |
| CNS—Neuroimmunological disorders | 18 (4.1) | 43.2 ± 13.5 | 10 (55.6) | 8 (44.4) | 13 (72.2) | 0 (0.0) | 4 (22.2) | 1 (5.6) | 0 (0.0) | 5 (27.8) | 11 (61.1) | 2 (11.1) | 18 (100.0) | 0 (0.0) |
| 4 | 41.8 ± 10.5 | 2 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 0 |
| 9 | 49.4 ± 15.4 | 7 | 2 | 5 | 0 | 3 | 1 | 0 | 4 | 3 | 2 | 9 | 0 |
| 5 | 34.4 ± 7.8 | 1 | 4 | 4 | 0 | 1 | 0 | 0 | 1 | 4 | 0 | 5 | 0 |
| CNS—Others | 19 (4.3) | 56.1 ± 20.4 | 10 (52.6) | 9 (47.4) | 10 (52.6) | 0 (0.0) | 5 (26.3) | 3 (15.8) | 1 (5.3) | 10 (52.6) | 9 (47.4) | 0 (0.0) | 17 (89.5) | 2 (10.5) |
| 1 | NA | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 1 | NA | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 1 | NA | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 7 | 65.4 ± 18.6 | 4 | 3 | 3 | 0 | 3 | 0 | 1 | 4 | 3 | 0 | 6 | 1 |
| 2 | 37.0 ± 1.4 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 0 |
| 1 | NA | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| 1 | NA | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 5 | 43.6 ± 20.4 | 2 | 3 | 4 | 0 | 1 | 0 | 0 | 2 | 3 | 0 | 5 | 0 |
| MIX CNS/PNS | 2 (0.5) | 51.5 ± 6.4 | 0 (0.0) | 2 (100.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) |
| PNS—Polyneuropathy | 146 (33.3) | 55.1 ± 17.0 | 56 (38.4) | 90 (61.6) | 65 (44.5) | 2 (1.4) | 35 (24.0) | 35 (24.0) | 9 (6.2) | 49 (33.6) | 89 (61.0) | 8 (5.4) | 144 (98.6) | 2 (1.4) |
| 7 | 59.0 ± 14.9 | 2 | 5 | 1 | 0 | 2 | 3 | 1 | 3 | 4 | 0 | 7 | 0 |
| 139 | 54.9 ± 17.2 | 54 | 85 | 64 | 2 | 33 | 32 | 8 | 46 | 85 | 8 | 139 | 0 |
| PNS—Cranial nerve disorders | 32 (7.3) | 49.6 ± 14.9 | 16 (50.0) | 16 (50.0) | 14 (43.8) | 0 (0.0) | 6 (18.8) | 12 (37.5) | 0 (0.0) | 23 (74.2) | 6 (19.4) | 2 (6.5) | 32 (100.0) | 0 (0.0) |
| 21 | 45.2 ± 12.7 | 10 | 11 | 10 | 0 | 4 | 7 | 0 | 12 | 6 | 2 | 21 | 0 |
| 11 | 58.0 ± 15.9 | 6 | 5 | 4 | 0 | 2 | 5 | 0 | 11 | 0 | 0 | 11 | 0 |
| PNS—Plexopathy | 23 (5.2) | 51.8 ± 13.4 | 6 (26.1) | 17 (73.9) | 5 (21.7) | 0 (0.0) | 13 (56.5) | 5 (21.7) | 0 (0.0) | 17 (77.3) | 5 (22.7) | 0 (0.0) | 23 (100.0) | 0 (0.0) |
| 17 | 51.5 ± 11.6 | 4 | 13 | 3 | 0 | 9 | 5 | 0 | 13 | 3 | 0 | 17 | 0 |
| 5 | 54.4 ± 20.7 | 1 | 4 | 1 | 0 | 4 | 0 | 0 | 4 | 1 | 0 | 5 | 0 |
| 1 | NA | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| PNS—Others | 3 (0.7) | 47.7 ± 20.6 | 2 (66.7) | 1 (33.3) | 1 (33.3) | 0 (0.0) | 1 (33.3) | 1 (33.3) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) |
| 1 | NA | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| 1 | NA | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 1 | NA | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Complications | Total Cases, n (%) | Dose of Vaccine n (%) | Brand of Vaccine n (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First 279 (63.6) | Second 104 (23.7) | First Booster 1 (0.2) | NA 55 (12.5) | Astrazeneca 202 (46.0) | Jassen 16 (3.6) | Moderna 45 (10.3) | Pfizer 135 (30.8) | Sinopharm 9 (2.1) | Sinovac 6 (1.4) | Sputnik V 13 (3.0) | Nonspecific mRNA 9 (2.1) | Heterologous 1 (0.2) | Covaxin Bharat 3 (0.7) | ||
| CNS—Cerebrovascular disorders | 65 (14.8) | 39 (60.0) | 4 (6.2) | 1 (1.5) | 21 (32.3) | 49 (75.4) | 5 (7.7) | 1 (1.5) | 9 (13.8) | 0 (0.0) | 0 0.0) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) |
| 7 | 6 | 1 | 0 | 0 | 4 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 58 | 33 | 3 | 1 | 21 | 45 | 5 | 1 | 6 | 0 | 0 | 0 | 1 | 0 | 0 |
| CNS—Demyelinating disorders | 109 (24.8) | 69 (63.3) | 35 (32.1) | 0 (0.0) | 5 (4.6) | 47 (43.1) | 1 (0.9) | 15 (13.8) | 36 (33.0) | 3 (2.8) | 2 (1.8) | 2 (1.8) | 1 (0.9) | 0 (0.0) | 2 (1.8) |
| 26 | 18 | 7 | 0 | 1 | 16 | 0 | 2 | 3 | 2 | 1 | 1 | 0 | 0 | 1 |
| 4 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21 | 15 | 5 | 0 | 1 | 8 | 0 | 4 | 7 | 0 | 1 | 0 | 0 | 0 | 1 |
| 14 | 12 | 2 | 0 | 0 | 11 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| 41 | 18 | 20 | 0 | 3 | 5 | 1 | 9 | 24 | 0 | 0 | 1 | 1 | 0 | 0 |
| 3 | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CNS—Inflammatory disorders | 22 (5.0) | 10 (45.5) | 9 (40.9) | 0 (0.0) | 3 (13.6) | 7 (3.18) | 0 (0.0) | 3 (13.6) | 11 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.5) | 0 (0.0) |
| 11 | 3 | 5 | 0 | 1 | 5 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 1 | 0 |
| 9 | 5 | 4 | 0 | 0 | 2 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| CNS—Neuroimmunological disorders | 18 (4.1) | 15 (83.3) | 3 (16.7) | 0 (0.0) | 0 (0.0) | 11 (61.1) | 0 (0.0) | 1 (5.6) | 4 (22.2) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 0 (0.0) |
| 4 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 7 | 2 | 0 | 0 | 3 | 0 | 1 | 3 | 0 | 1 | 0 | 1 | 0 | 0 |
| 5 | 4 | 1 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| CNS—Others | 19 (4.3) | 14 (73.7) | 5 (26.3) | 0 (0.0) | 0 (0.0) | 9 (47.4) | 0 (0.0) | 4 (21.1) | 6 (31.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 6 | 1 | 0 | 0 | 3 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 5 | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| MIX CNS/PNS | 2 (0.4) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PNS—Polyneuropathy | 146 (33.3) | 105 (71.9) | 25 (17.1) | 0 (0.0) | 16 (11.0) | 70 (47.9) | 8 (5.5) | 7 (4.8) | 41 (28.1) | 6 (4.1) | 2 (1.4) | 9 (6.2) | 3 (2.1) | 0 (0.0) | 0 (0.0) |
| PNS—Cranial nerve disorders | 32 (7.3) | 18 (56.3) | 9 (28.1) | 0 (0.0) | 5 (15.6) | 3 (9.4) | 1 (3.1) | 6 (18.8) | 17 (53.1) | 0 (0.0) | 1 (3.1) | 2 (6.3) | 1 (3.1) | 0 (0.0) | 1 (3.1) |
| 21 | 11 | 6 | 0 | 4 | 3 | 1 | 5 | 7 | 0 | 1 | 2 | 1 | 0 | 1 |
| 11 | 7 | 3 | 0 | 1 | 0 | 0 | 1 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| PNS—Plexopathy | 23 (5.2) | 7 (30.4) | 12 (52.2) | 0 (0.0) | 4 (17.4) | 5 (21.7) | 0 (0.0) | 6 (26.1) | 11 (47.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.3) | 0 (0.0) | 0 (0.0) |
| 17 | 6 | 9 | 0 | 2 | 3 | 0 | 5 | 8 | 0 | 0 | 0 | 1 | 0 | 0 |
| 5 | 1 | 3 | 0 | 1 | 1 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PNS—Others | 3 (0.7) | 1 (33.3) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) |
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.W.; Yap, S.F.; Amin-Nordin, S.; Ngeow, Y.F. Cardiac and Neurological Complications Post COVID-19 Vaccination: A Systematic Review of Case Reports and Case Series. Vaccines 2024, 12, 575. https://doi.org/10.3390/vaccines12060575
Lee KW, Yap SF, Amin-Nordin S, Ngeow YF. Cardiac and Neurological Complications Post COVID-19 Vaccination: A Systematic Review of Case Reports and Case Series. Vaccines. 2024; 12(6):575. https://doi.org/10.3390/vaccines12060575
Chicago/Turabian StyleLee, Kai Wei, Sook Fan Yap, Syafinaz Amin-Nordin, and Yun Fong Ngeow. 2024. "Cardiac and Neurological Complications Post COVID-19 Vaccination: A Systematic Review of Case Reports and Case Series" Vaccines 12, no. 6: 575. https://doi.org/10.3390/vaccines12060575
APA StyleLee, K. W., Yap, S. F., Amin-Nordin, S., & Ngeow, Y. F. (2024). Cardiac and Neurological Complications Post COVID-19 Vaccination: A Systematic Review of Case Reports and Case Series. Vaccines, 12(6), 575. https://doi.org/10.3390/vaccines12060575






