The Equal Neutralizing Effectiveness of BNT162b2, ChAdOx1 nCoV-19, and Sputnik V Vaccines in the Palestinian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Blood Sampling and Data Collection
2.3. Cell Lines
2.4. Neutralization Assay
2.5. SARS-CoV-2 CLIA Assay
2.6. Statistical Analysis
2.7. Ethical Approval, Registration and Patient Consent Procedures
2.8. Data Availability
3. Results
3.1. Demographic Characteristics of the Participants
3.2. Post-Vaccine SARS-CoV-2 Total Antibodies
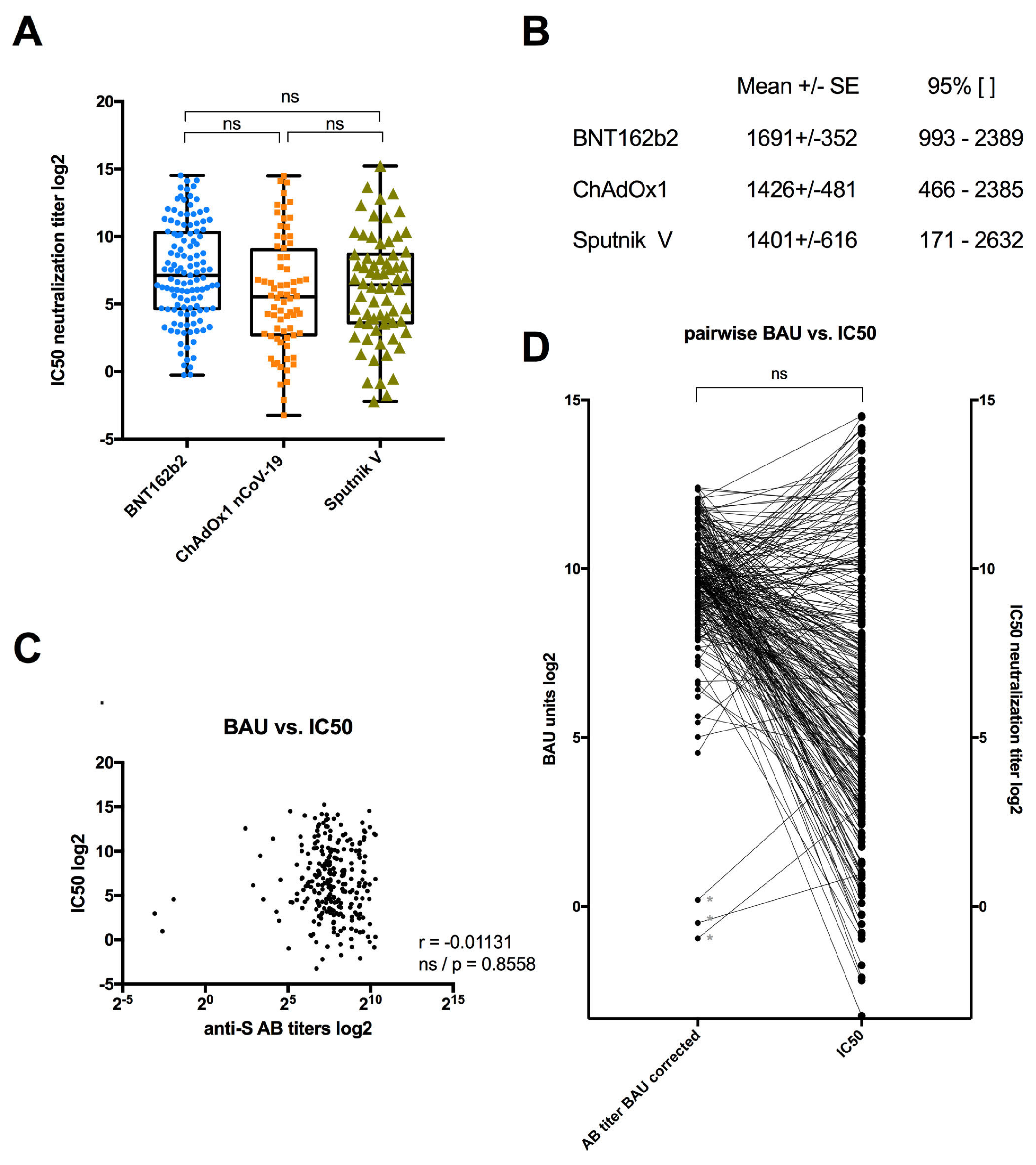
3.3. Serum Neutralization among Individuals Vaccinated with BNT162b2, ChAdOx1 or Sputnik V
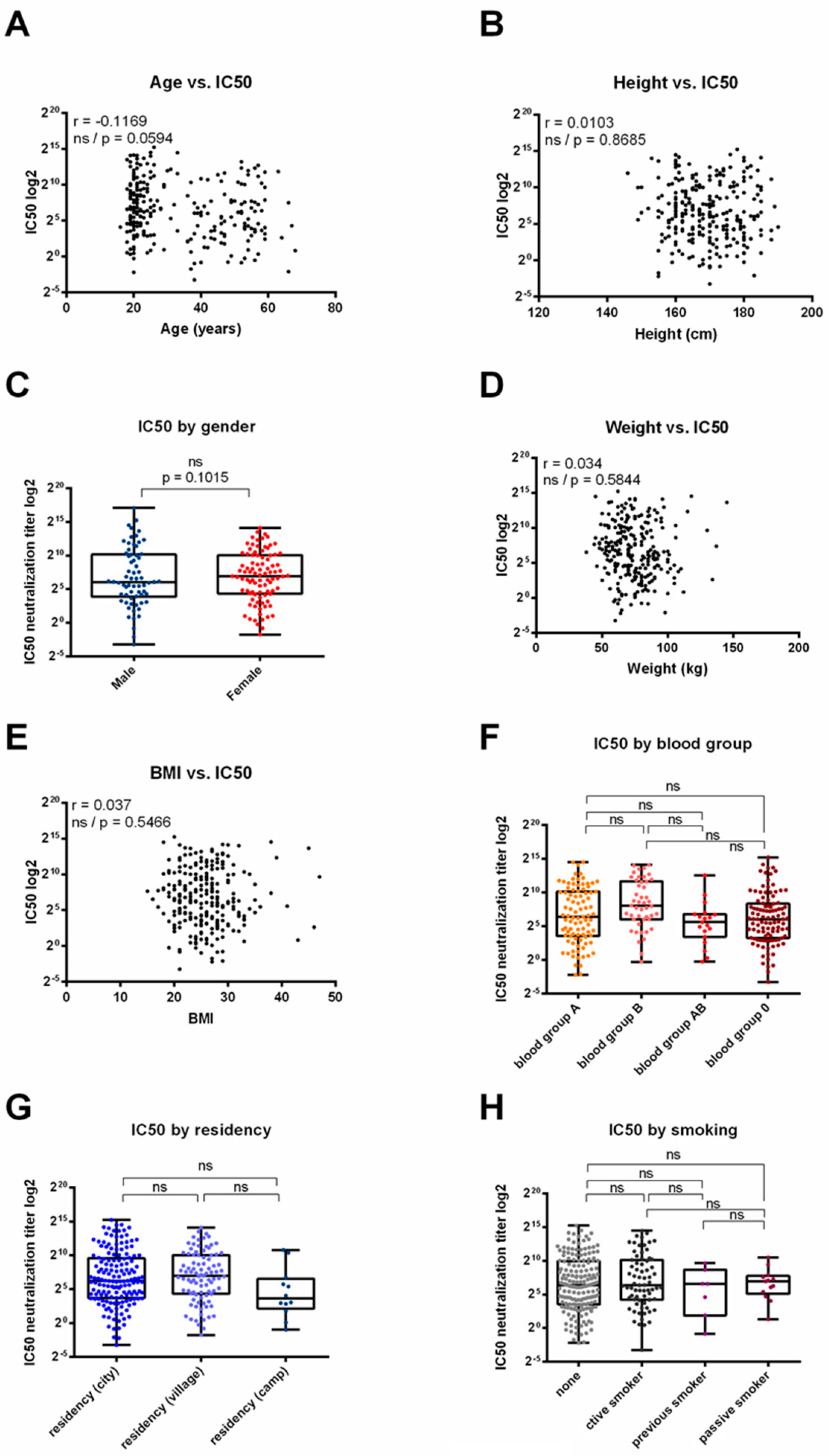
3.4. Correlation between Serum Neutralization Efficacy and Demographic Parameters across BNT162b2, ChAdOx1 or Sputnik V Vaccinated Individuals
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, E.P.K.; Shrotri, M.; Kampmann, B. Keeping track of the SARS-CoV-2 vaccine pipeline. Nat. Rev. Immunol. 2020, 20, 650. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.Y.; Lu, Q.B.; Cui, F. Vaccination with the Inactivated Vaccine (Sinopharm BBIBP-CorV) Ensures Protection against SARS-CoV-2 Related Disease. Vaccines 2022, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. “Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety” [Response To Letter]. Infect. Drug Resist. 2021, 14, 4501–4502. [Google Scholar] [CrossRef] [PubMed]
- D’Apice, L.; Trovato, M.; Gramigna, G.; Colavita, F.; Francalancia, M.; Matusali, G.; Meschi, S.; Lapa, D.; Bettini, A.; Mizzoni, K.; et al. Comparative analysis of the neutralizing activity against SARS-CoV-2 Wuhan-Hu-1 strain and variants of concern: Performance evaluation of a pseudovirus-based neutralization assay. Front. Immunol. 2022, 13, 981693. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H. Development of Corona-virus-disease-19 Vaccines. JMA J. 2021, 4, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Pallett, S.J.C.; Heskin, J.; Groppelli, E.; Mazzella, A.; Moore, L.S.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants. Lancet Microbe 2022, 3, e167. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- WHO. COVID-19 in Occupied Palestinian Territory, Including East Jerusalem [Internet]. 2023. Available online: https://covid19.who.int/region/emro/country/ps (accessed on 17 October 2023).
- Maraqa, B.; Alkarajeh, M.; Almahareeq, M.; Al-Shakhra, K.; Al-Kalia, M. Palestinian analysis of COVID-19 vaccine compliance and reported death by vaccination type. J. Family Med. Prim. Care 2022, 11, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Damour, A.; Delalande, P.; Cordelieres, F.; Lafon, M.E.; Faure, M.; Segovia-Kueny, S.; Stalens, C.; Mathis, S.; Spinazzi, M.; Violleau, M.H.; et al. Anti-SARS-CoV-2 (COVID-19) vaccination efficacy in patients with severe neuromuscular diseases. Rev. Neurol. 2023, 179, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; et al. The WHO International Standard for COVID-19 serological tests: Towards harmonization of anti-spike assays. Int. Immunopharmacol. 2021, 100, 108095. [Google Scholar] [CrossRef] [PubMed]
- Muecksch, F.; Wise, H.; Batchelor, B.; Squires, M.; Semple, E.; Richardson, C.; McGuire, J.; Clearly, S.; Furrie, E.; Greig, N.; et al. Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J. Infect. Dis. 2021, 223, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, J.C.; Goldstein, D.R. How aging impacts vaccine efficacy: Known molecular and cellular mechanisms and future directions. Trends Mol. Med. 2022, 28, 1100–1111. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Beck, M.A.; Alwarawrah, Y.; MacIver, N.J. Emerging mechanisms of obesity-associated immune dysfunction. Nat. Rev. Endocrinol. 2024, 20, 136–148. [Google Scholar] [CrossRef]
- Nasr, M.C.; Geerling, E.; Pinto, A.K. Impact of Obesity on Vaccination to SARS-CoV-2. Front. Endocrinol. 2022, 13, 898810. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, T.; Wagener, D.; Chowdhury, G.; Majumder, P.P. A large study on immunological response to a whole-cell killed oral cholera vaccine reveals that there are significant geographical differences in response and that O blood group individuals do not elicit a higher response. Clin. Vaccine Immunol. 2010, 17, 1232–1237. [Google Scholar] [CrossRef]
- Richie, E.E.; Punjabi, N.H.; Sidharta, Y.Y.; Peetosutan, K.K.; Sukandar, M.M.; Wasserman, S.S.; Lesmana, M.M.; Wangsasaputra, F.F.; Pandam, S.S.; Levine, M.M.; et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 2000, 18, 2399–2410. [Google Scholar] [CrossRef]
- Kabagenyi, J.; Natukunda, A.; Nassuuna, J.; Sanya, R.E.; Nampijja, M.; Webb, E.L.; Elliott, A.M.; Nkurunungi, G. Urban-rural differences in immune responses to mycobacterial and tetanus vaccine antigens in a tropical setting: A role for helminths? Parasitol. Int. 2020, 78, 102132. [Google Scholar] [CrossRef] [PubMed]
- Damour, A. (CNRS UMR 5234, Fundamental Microbiology and Pathogenicity, University of Bordeaux, 33076 Bordeaux, France); Wodrich, H. (CNRS UMR 5234, Fundamental Microbiology and Pathogenicity, University of Bordeaux, 33076 Bordeaux, France). Personal communication, 2023.
- Sadeghalvad, M.; Mansourabadi, A.H.; Noori, M.; Nejadghaderi, S.A.; Masoomikarimi, M.; Alimohammadi, M.; Rezaei, N. Recent developments in SARS-CoV-2 vaccines: A systematic review of the current studies. Rev. Med. Virol. 2023, 33, e2359. [Google Scholar] [CrossRef]
- Karbiener, M.; Farcet, M.R.; Zollner, A.; Masuda, T.; Mori, M.; Moschen, A.R.; Kreil, T.R. Calibrated comparison of SARS-CoV-2 neutralizing antibody levels in response to protein-, mRNA-, and vector-based COVID-19 vaccines. NPJ Vaccines 2022, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Zhou, H.; Samanovic, M.I.; Dcosta, B.M.; Cornelius, A.; Herati, R.S.; Mulligan, M.J.; Landau, N.R. Neutralization of SARS-CoV-2 Variants by mRNA and Adenoviral Vector Vaccine-Elicited Antibodies. Front. Immunol. 2022, 13, 797589. [Google Scholar] [CrossRef]
- Stuart, A.S.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022, 399, 36–49. [Google Scholar] [CrossRef]
- Liu, Y.; Sanchez-Ovando, S.; Carolan, L.; Dowson, L.; Khvorov, A.; Jessica Hadiprodjo, A.; Tseng, Y.Y.; Delahunty, C.; Khatami, A.; Macnish, M.; et al. Superior immunogenicity of mRNA over adenoviral vectored COVID-19 vaccines reflects B cell dynamics independent of anti-vector immunity: Implications for future pandemic vaccines. Vaccine 2023, 41, 7192–7200. [Google Scholar] [CrossRef]
- van den Hoogen, L.L.; Verheul, M.K.; Vos, E.R.A.; van Hagen, C.C.E.; van Boven, M.; Wong, D.; Wijmenga-Monsuur, A.J.; Smits, G.; Kuijer, M.; van Rooijen, D.; et al. SARS-CoV-2 Spike S1-specific IgG kinetic profiles following mRNA or vector-based vaccination in the general Dutch population show distinct kinetics. Sci. Rep. 2022, 12, 5935. [Google Scholar] [CrossRef]
- Brunner, W.M.; Freilich, D.; Victory, J.; Krupa, N.; Scribani, M.B.; Jenkins, P.; Lasher, E.G.; Fink, A.; Shah, A.; Cross, P.; et al. Comparison of antibody response durability of mRNA-1273, BNT162b2, and Ad26.COV2.S SARS-CoV-2 vaccines in healthcare workers. Int. J. Infect. Dis. 2022, 123, 183–191. [Google Scholar] [CrossRef] [PubMed]
| Frequency | Percent | Mean of Antibody Titer | p Value | |
|---|---|---|---|---|
| (AU/mL) | ||||
| Age | ||||
| 18–39 | 769 | 68.7 | 285.5 | 0.591 |
| 40–49 | 119 | 10.6 | 294.8 | |
| 50 and above | 232 | 20.7 | 372.0 | |
| Body Mass Index (BMI) | ||||
| Underweight | 53 | 4.7 | 245.4 | 0.602 |
| Normal | 537 | 47.9 | 316.7 | |
| Overweight | 384 | 34.3 | 306.8 | |
| Obese | 146 | 13.0 | 276.5 | |
| Gender | ||||
| Male | 580 | 51.8 | 332.3 | 0.105 |
| Female | 540 | 48.2 | 274.9 | |
| Smoking | ||||
| Non-Smoker | 788 | 70.4 | 313.7 | 0.087 |
| Current Smoker | 332 | 29.6 | 283.0 | |
| Blood Group | ||||
| A | 403 | 36.0 | 321.2 | 0.072 |
| B | 190 | 17.0 | 322.7 | |
| AB | 88 | 7.9 | 312.1 | |
| O | 439 | 39.2 | 280.1 | |
| Type of Vaccine | ||||
| Pfizer | 727 | 64.9 | 322.1 | 0.126 |
| AstraZeneca | 185 | 16.5 | 227.1 | |
| Sputnik V | 208 | 18.6 | 312.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damour, A.; Faure, M.; Landrein, N.; Ragues, J.; Ardah, N.; Dhaidel, H.; Lafon, M.-E.; Wodrich, H.; Basha, W. The Equal Neutralizing Effectiveness of BNT162b2, ChAdOx1 nCoV-19, and Sputnik V Vaccines in the Palestinian Population. Vaccines 2024, 12, 493. https://doi.org/10.3390/vaccines12050493
Damour A, Faure M, Landrein N, Ragues J, Ardah N, Dhaidel H, Lafon M-E, Wodrich H, Basha W. The Equal Neutralizing Effectiveness of BNT162b2, ChAdOx1 nCoV-19, and Sputnik V Vaccines in the Palestinian Population. Vaccines. 2024; 12(5):493. https://doi.org/10.3390/vaccines12050493
Chicago/Turabian StyleDamour, Alexia, Muriel Faure, Nicolas Landrein, Jessica Ragues, Narda Ardah, Haneen Dhaidel, Marie-Edith Lafon, Harald Wodrich, and Walid Basha. 2024. "The Equal Neutralizing Effectiveness of BNT162b2, ChAdOx1 nCoV-19, and Sputnik V Vaccines in the Palestinian Population" Vaccines 12, no. 5: 493. https://doi.org/10.3390/vaccines12050493
APA StyleDamour, A., Faure, M., Landrein, N., Ragues, J., Ardah, N., Dhaidel, H., Lafon, M.-E., Wodrich, H., & Basha, W. (2024). The Equal Neutralizing Effectiveness of BNT162b2, ChAdOx1 nCoV-19, and Sputnik V Vaccines in the Palestinian Population. Vaccines, 12(5), 493. https://doi.org/10.3390/vaccines12050493






