TLR7 Agonist-Loaded Gadolinium Oxide Nanotubes Promote Anti-Tumor Immunity by Activation of Innate and Adaptive Immune Responses
Abstract
1. Introduction
2. Materials and Methods
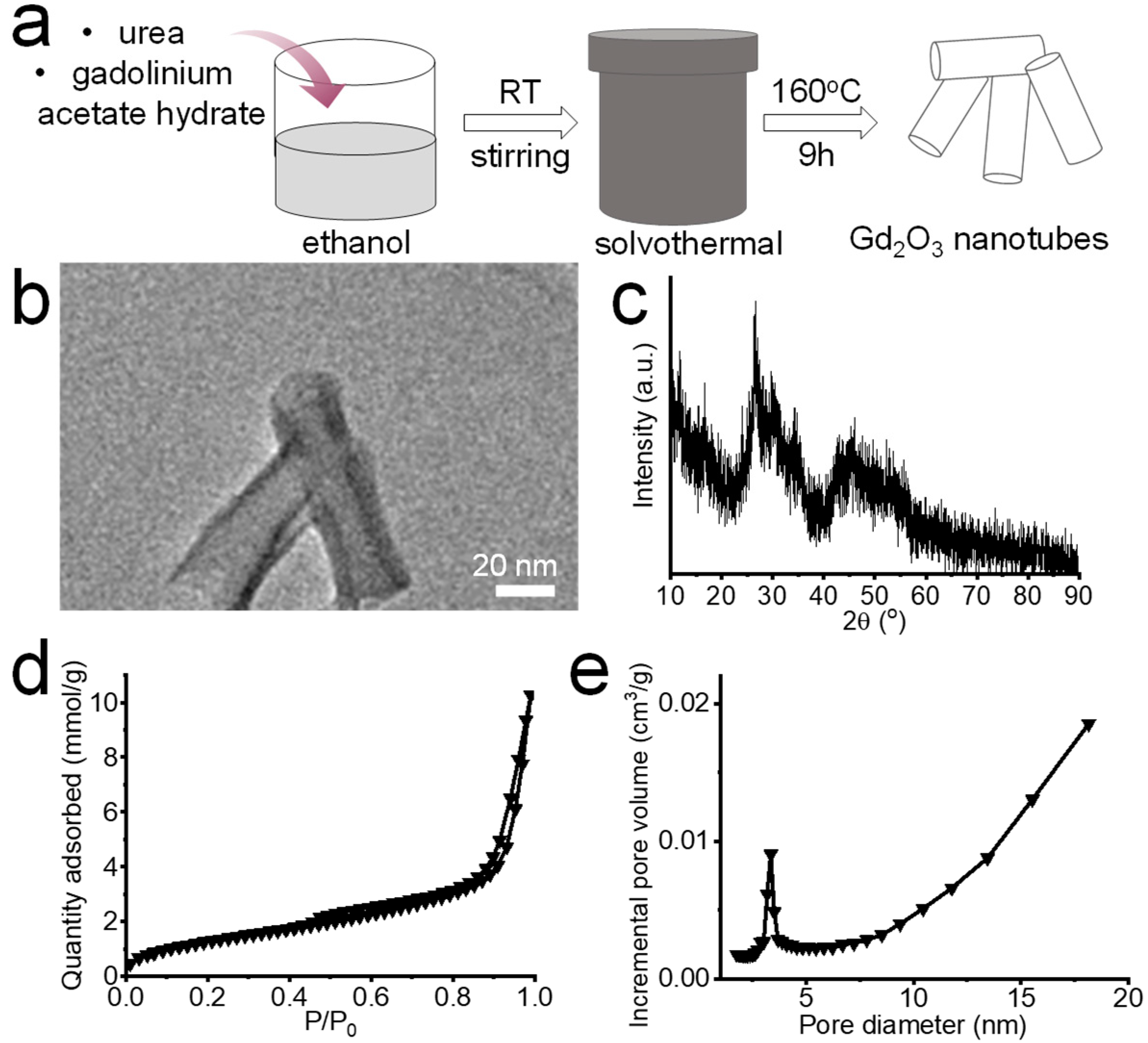
2.1. Synthesis of Gd2O3 Nanotubes
2.2. Characterization of Gd2O3 Nanotubes
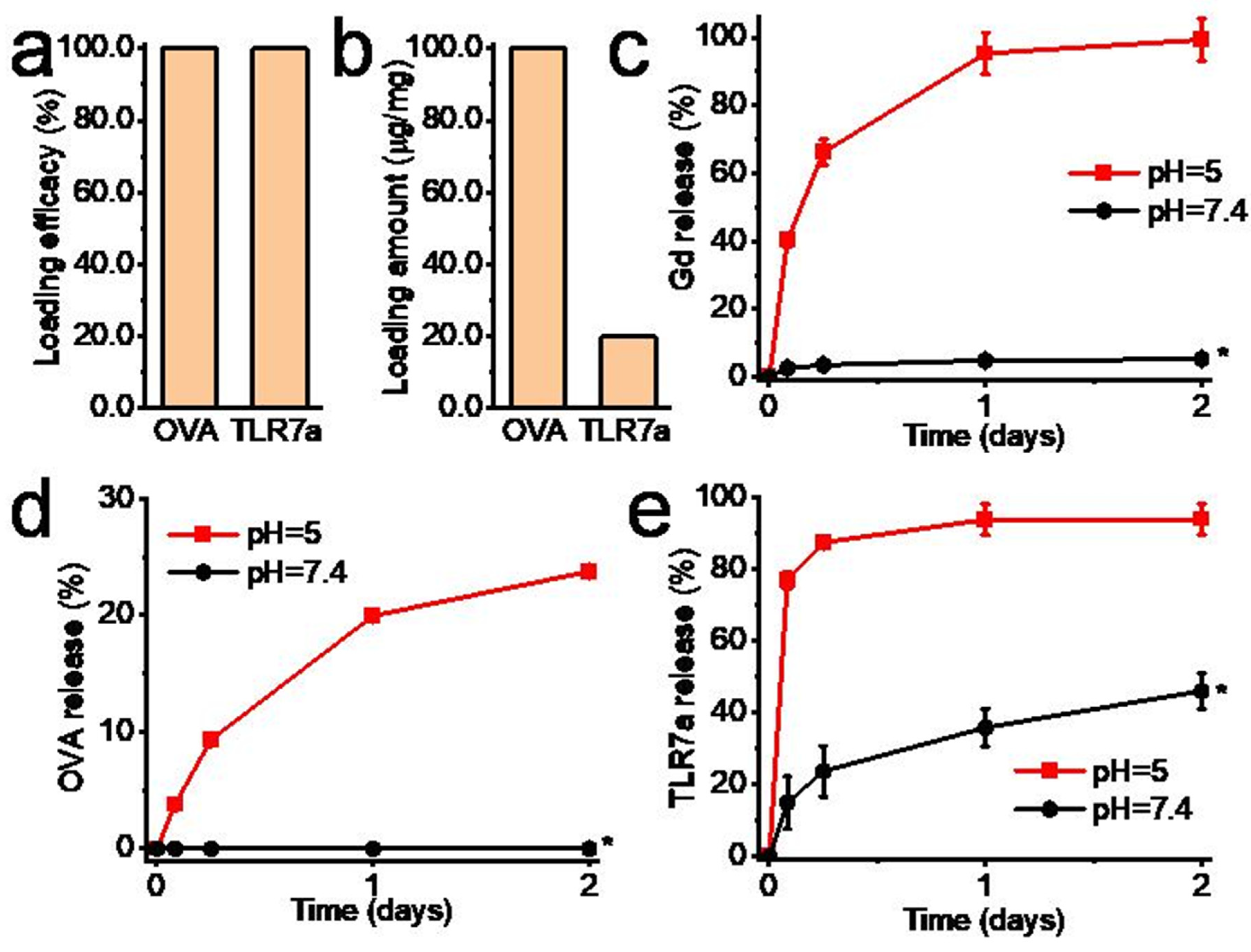
2.3. Biomolecule Loading and Release, and Gd2O3 Nanotube Degradation In Vitro
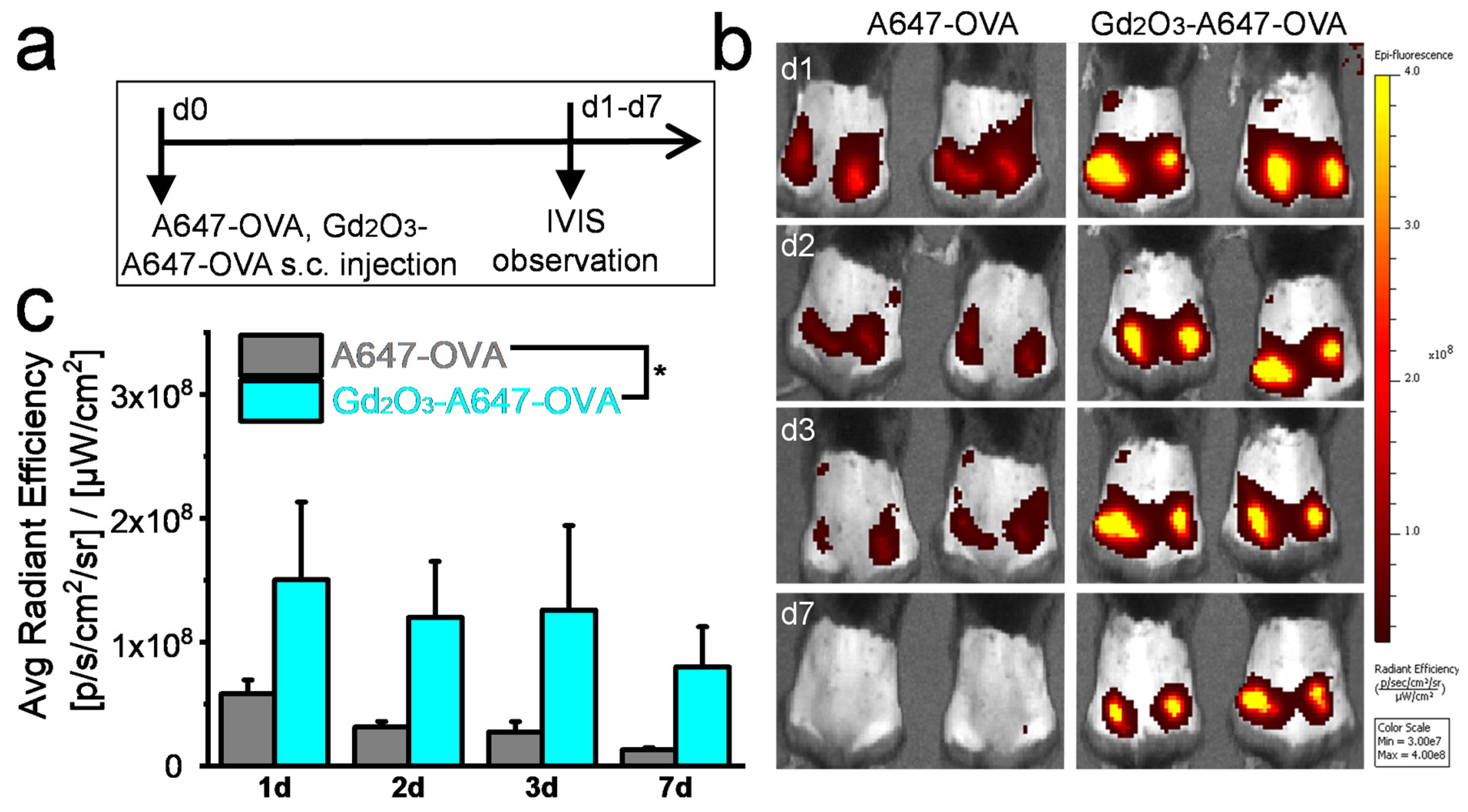
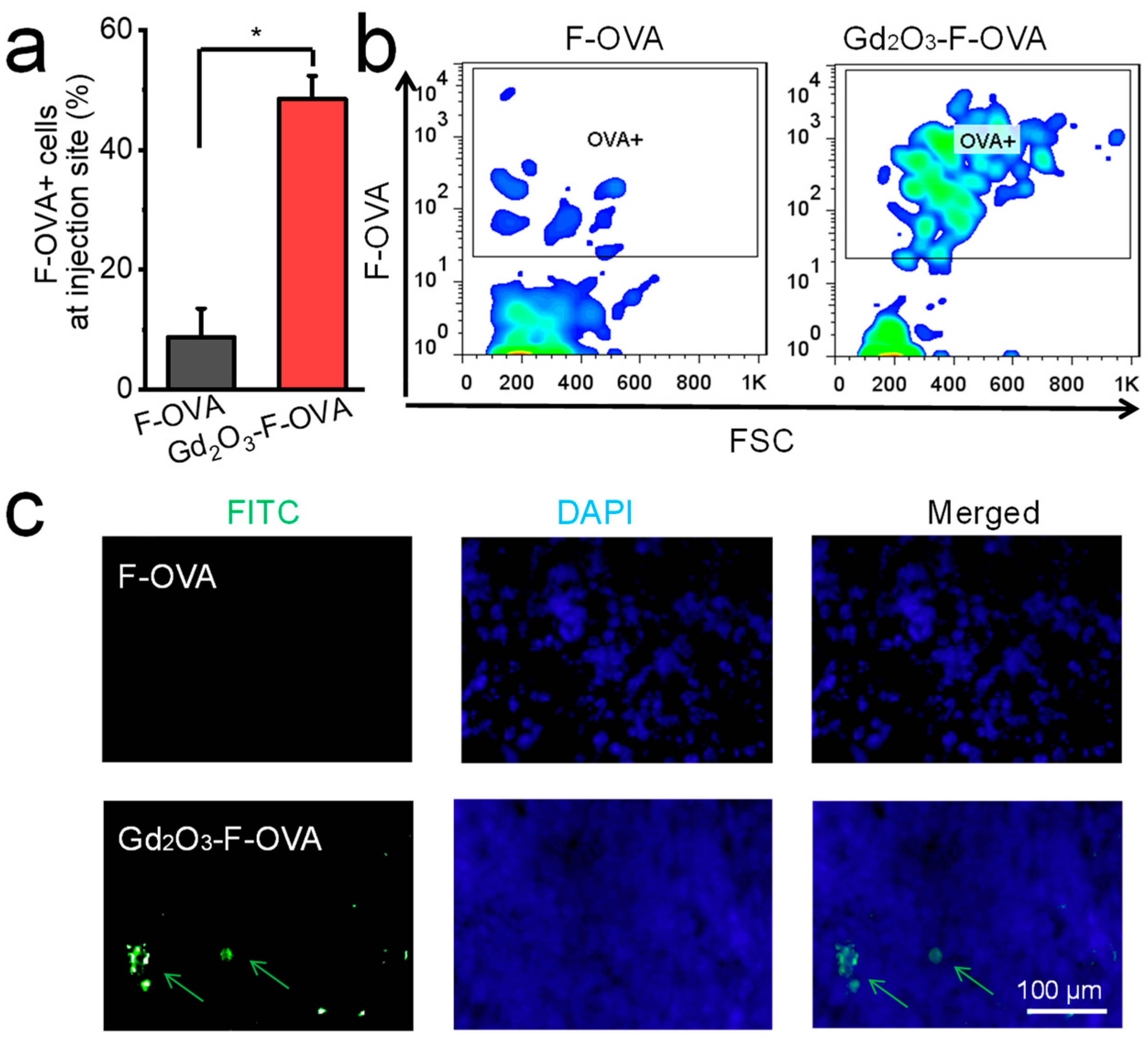
2.4. In Vivo Antigen Retention
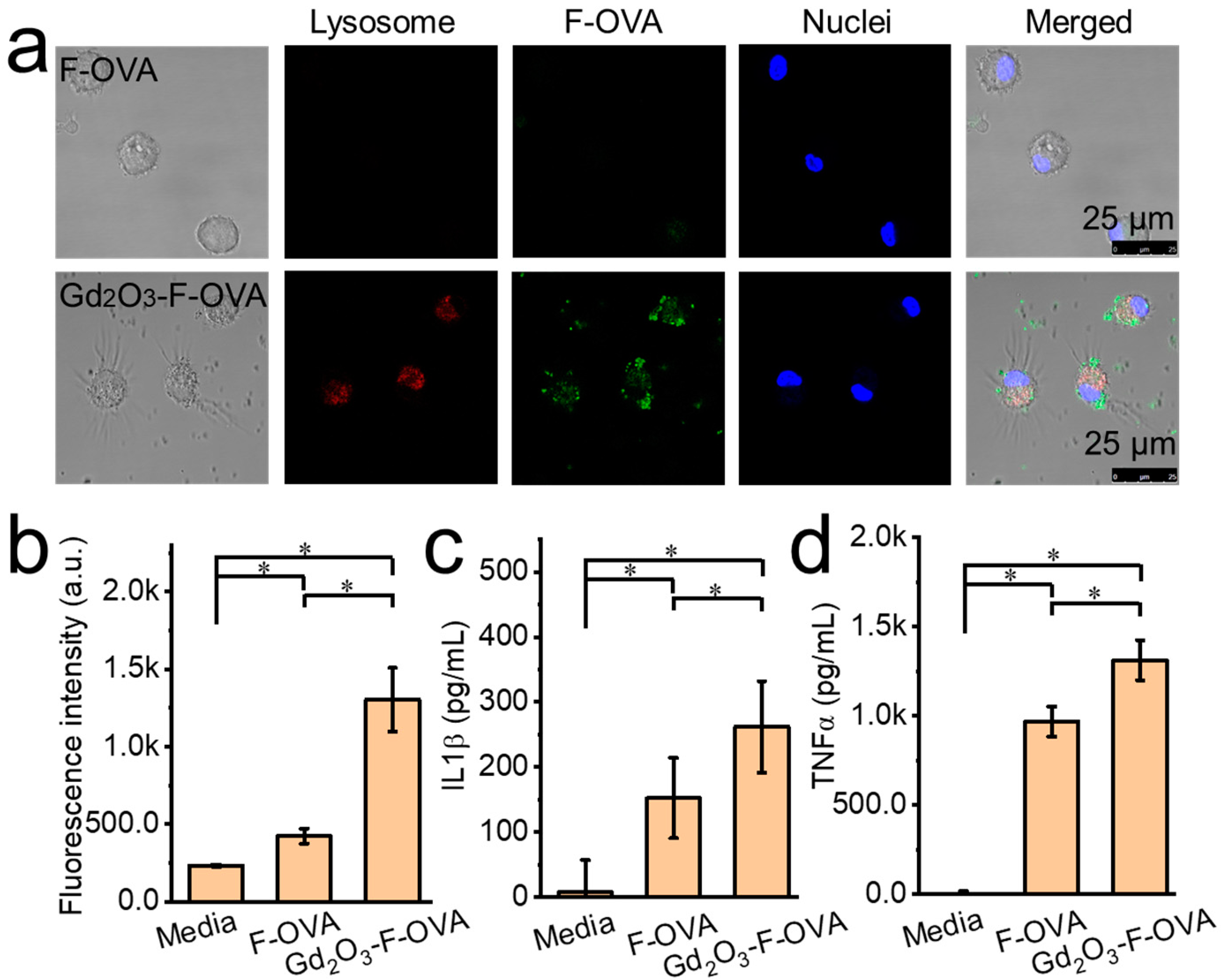
2.5. In Vitro Cellular Test
2.6. In Vivo Antigen Delivery in Lymph Nodes and In Vivo Safety
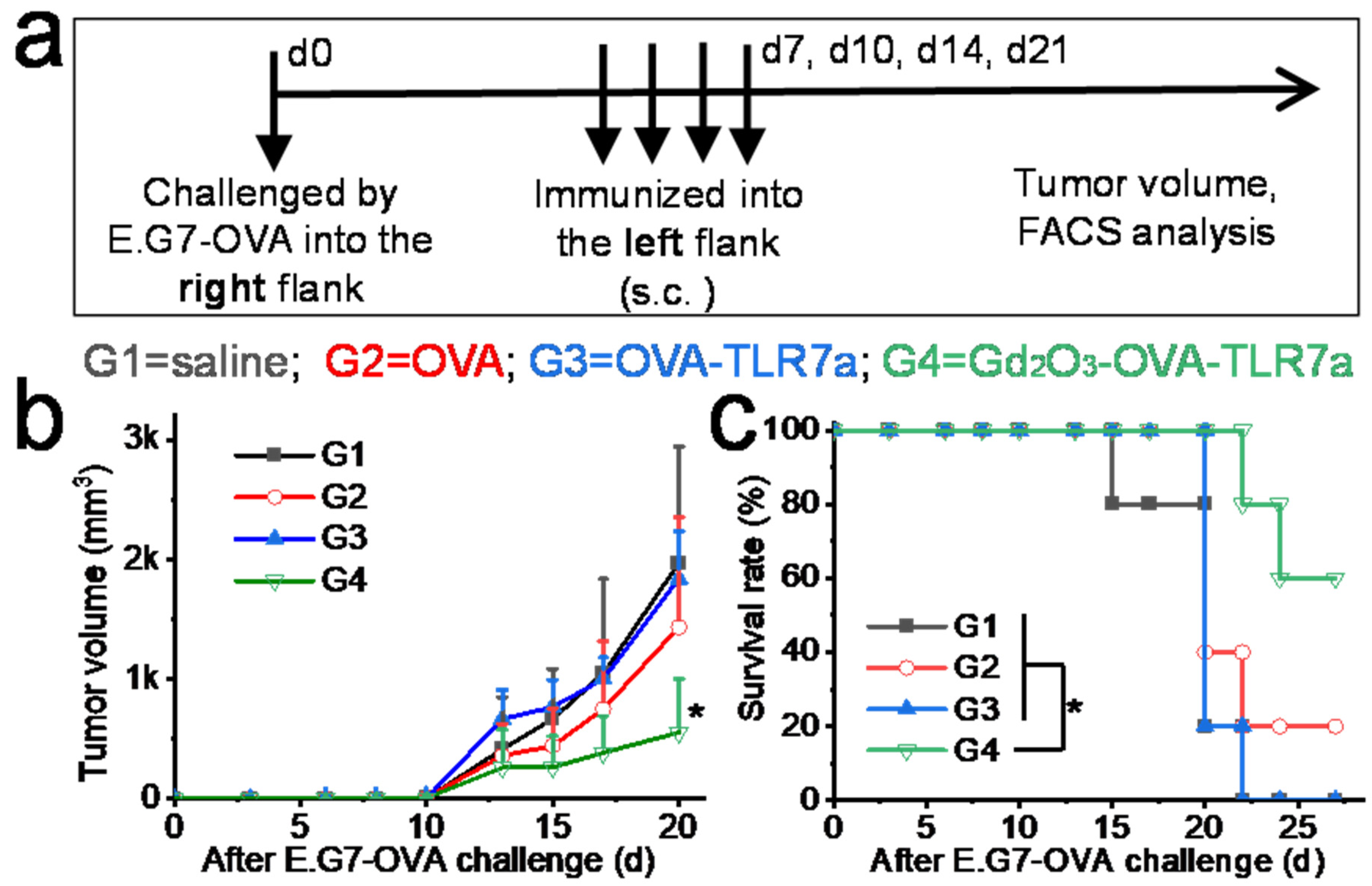
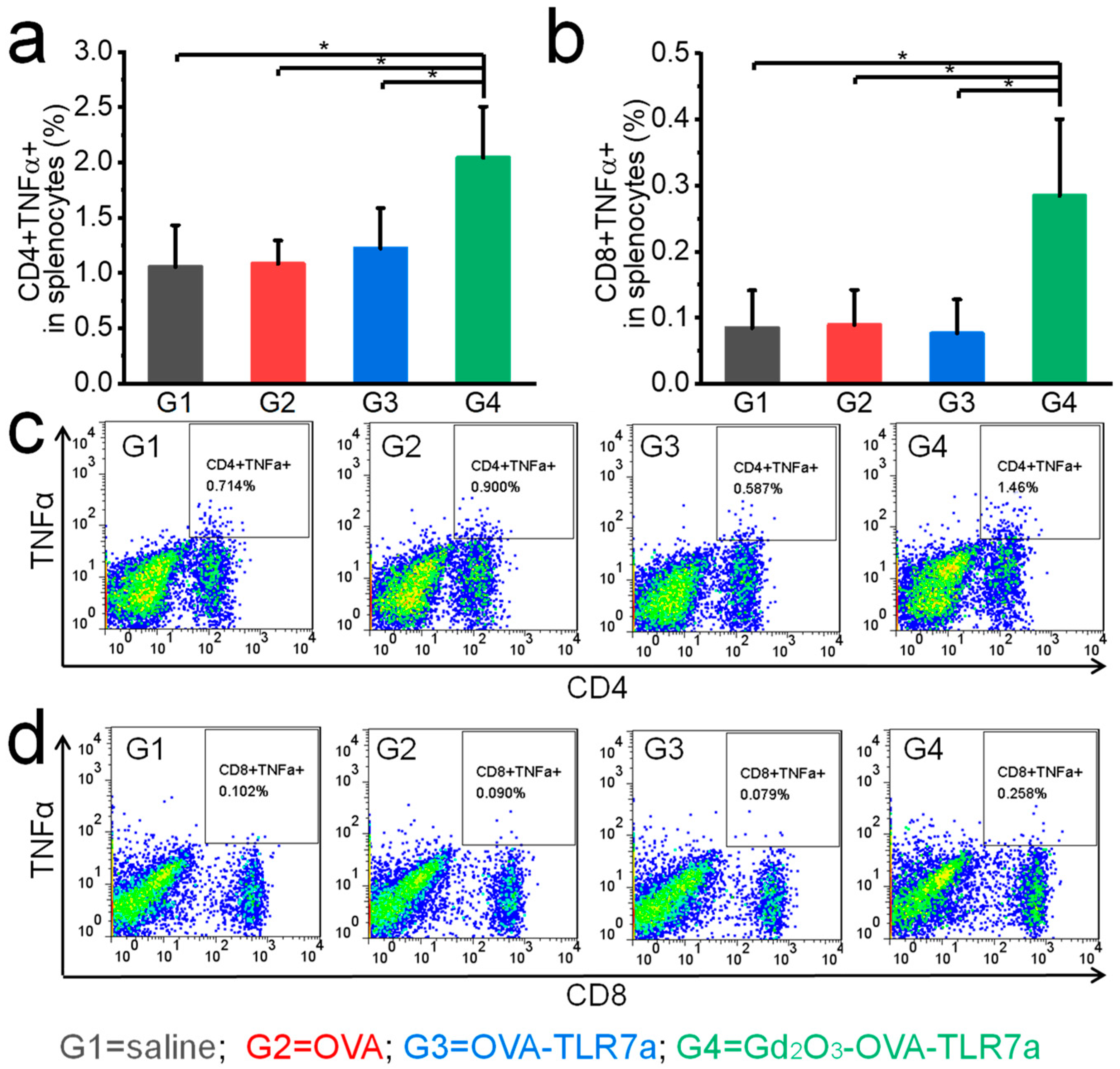
2.7. In Vivo Anti-Tumor Immunity
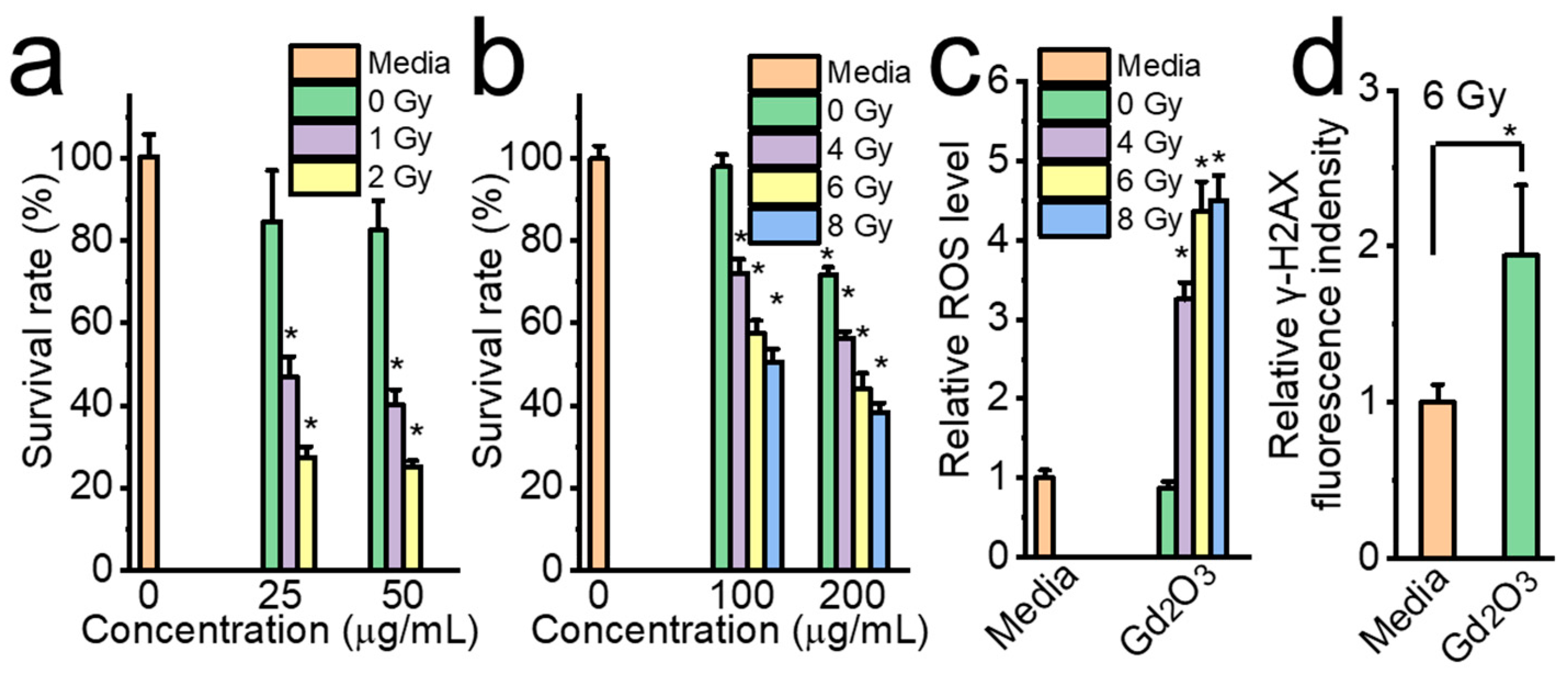
2.8. Radiation Sensitization Evaluation of Gd2O3 Nanotubes
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- McNutt, M. Cancer Immunotherapy. Science 2013, 342, 1417. [Google Scholar] [CrossRef] [PubMed]
- Elmusrati, A.; Wang, J.; Wang, C.Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral Sci. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Mascaux, C.; Angelova, M.; Vasaturo, A.; Beane, J.; Hijazi, K.; Anthoine, G.; Buttard, B.; Rothe, F.; Willard-Gallo, K.; Haller, A.; et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature 2019, 571, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Tureci, O. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Schuller, S.; Wisgrill, L.; Sadeghi, K.; Gindl, E.; Helmer, H.; Husslein, P.; Berger, A.; Spittler, A.; Forster-Waldl, E. The TLR-specific adjuvants R-848 and CpG-B endorse the immunological reaction of neonatal antigen-presenting cells. Pediatr. Res. 2016, 80, 311–318. [Google Scholar] [CrossRef]
- Lynn, G.M.; Laga, R.; Darrah, P.A.; Ishizuka, A.S.; Balaci, A.J.; Dulcey, A.E.; Pechar, M.; Pola, R.; Gerner, M.Y.; Yamamoto, A.; et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015, 33, 1201–1210. [Google Scholar] [CrossRef]
- Soria, I.; Myhre, P.; Horton, V.; Ellefson, P.; McCarville, S.; Schmitt, K.; Owens, M. Effect of food on the pharmacokinetics and bioavailability of oral imiquimod relative to a subcutaneous dose. Int. J. Clin. Pharmacol. Ther. 2000, 38, 476–481. [Google Scholar] [CrossRef]
- Stockfleth, E.; Trefzer, U.; Garcia-Bartels, C.; Wegner, T.; Schmook, T.; Sterry, W. The use of Toll-like receptor-7 agonist in the treatment of basal cell carcinoma: An overview. Br. J. Dermatol. 2003, 149, 53–56. [Google Scholar] [CrossRef]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef]
- Lee, J.; Chuang, T.H.; Redecke, V.; She, L.P.; Pitha, P.M.; Carson, D.A.; Raz, E.; Cottam, H.B. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: Activation of Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2003, 100, 6646–6651. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Choi, Y.; Kim, J. Mesoporous Silica as a Versatile Platform for Cancer Immunotherapy. Adv. Mater. 2019, 31, 1803953. [Google Scholar] [CrossRef]
- Li, X.; Yamazaki, T.; Ebara, M.; Shirahata, N.; Hanagata, N. Nanoengineered coordination polymers boost cancer immunotherapy. Mater. Today 2023, 67, 127–150. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, X.; Onuma, K.; Sogo, Y.; Ohno, T.; Ito, A. Zn- and Mg- Containing Tricalcium Phosphates-Based Adjuvants for Cancer Immunotherapy. Sci. Rep. 2013, 3, 2203. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, X.; Ito, A.; Sogo, Y.; Watanabe, Y.; Hashimoto, K.; Yamazaki, A.; Ohno, T.; Tsuji, N.M. Synergistic effects of stellated fibrous mesoporous silica and synthetic dsRNA analogues for cancer immunotherapy. Chem. Commun. 2018, 54, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Li, X.; Ito, A.; Sogo, Y.; Ohno, T. Pore-size dependent immunogenic activity of mesoporous silica-based adjuvants in cancer immunotherapy. J. Biomed. Mater. Res. A 2014, 102, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ihara, S.; Li, X.; Ito, A.; Sogo, Y.; Watanabe, Y.; Tsuji, N.M.; Yamazaki, A. Si-doping increases the adjuvant activity of hydroxyapatite nanorods. Colloids Surf. B Biointerfaces 2018, 174, 300–307. [Google Scholar] [CrossRef]
- Nuhn, L.; De Koker, S.; Van Lint, S.; Zhong, Z.F.; Catani, J.P.; Combes, F.; Deswarte, K.; Li, Y.P.; Lambrecht, B.N.; Lienenklaus, S.; et al. Nanoparticle-Conjugate TLR7/8 Agonist Localized Immunotherapy Provokes Safe Antitumoral Responses. Adv. Mater. 2018, 30, e1803397. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, J.; Xu, L.G.; Sun, X.Q.; Chen, Q.; Zhao, Y.H.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Z.; Tian, X.M.; Hu, W.Y.; Zhang, Y.Y.; Liu, H.; He, H.Q.; Shen, Y.Y.; Xie, F.K.; Li, L. The properties of Gd2O3-assembled silica nanocomposite targeted nanoprobes and their application in MRI. Biomaterials 2012, 33, 6438–6446. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kamisugi, R.; Narazaki, M.; Matsuda, T.; Tabata, Y.; Toshimitsu, A.; Kondo, T. Size-controlled and biocompatible Gd2O3 nanoparticles for dual photoacoustic and MR imaging. Adv. Healthc. Mater. 2012, 1, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Kosaka, A.; Yan, H.; Guo, Z.; Uchiyama, R.; Fukui, R.; Kaneko, D.; Kumagai, Y.; You, D.J.; Carreras, J.; et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-beta. Immunity 2013, 38, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.P.; Ito, A.; Tsuji, N.M. A nanoscale metal organic frameworks-based vaccine synergises with PD-1 blockade to potentiate anti-tumour immunity. Nat. Commun. 2020, 11, 3858. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, C.H.; Tian, S.M.; Perry, J.L.; Sailer, D.; Luft, J.C.; DeSimone, J.M. Extending antigen release from particulate vaccines results in enhanced antitumor immune response. J. Control. Release 2018, 269, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.M.; Lopez, S.C.B.; Irvine, D.J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl. Acad. Sci. USA 2011, 108, 15745–15750. [Google Scholar] [CrossRef] [PubMed]
- Johansen, P.; Storni, T.; Rettig, L.; Qiu, Z.Y.; Der-Sarkissian, A.; Smith, K.A.; Manolova, V.; Lang, K.S.; Senti, G.; Mullhaupt, B.; et al. Antigen kinetics determines immune reactivity. Proc. Natl. Acad. Sci. USA 2008, 105, 5189–5194. [Google Scholar] [CrossRef] [PubMed]
- de Titta, A.; Ballester, M.; Julier, Z.; Nembrini, C.; Jeanbart, L.; van der Vlies, A.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. USA 2013, 110, 19902–19907. [Google Scholar] [CrossRef]
- Murtaugh, M.P.; Foss, D.L. Inflammatory cytokines and antigen presenting cell activation. Vet. Immunol. Immunopathol. 2002, 87, 109–121. [Google Scholar] [CrossRef]
- Soto, J.A.; Galvez, N.M.S.; Andrade, C.A.; Pacheco, G.A.; Bohmwald, K.; Berrios, R.V.; Bueno, S.M.; Kalergis, A.M. The Role of Dendritic Cells during Infections Caused by Highly Prevalent Viruses. Front. Immunol. 2020, 11, 1513. [Google Scholar] [CrossRef]
- Martin-Gayo, E.; Yu, X.G. Role of Dendritic Cells in Natural Immune Control of HIV-1 Infection. Front. Immunol. 2019, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mooney, D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 2018, 17, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.J.; Swartz, M.A.; Szeto, G.L. Engineering synthetic vaccines using cues from natural immunity. Nat. Mater. 2013, 12, 978–990. [Google Scholar] [CrossRef]
- Phuengkham, H.; Song, C.; Um, S.H.; Lim, Y.T. Implantable Synthetic Immune Niche for Spatiotemporal Modulation of Tumor-Derived Immunosuppression and Systemic Antitumor Immunity: Postoperative Immunotherapy. Adv. Mater. 2018, 30, e1706719. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Kim, H.; Niu, L.; Larson, P.; Kucaba, T.A.; Murphy, K.A.; James, B.R.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials 2018, 164, 38–53. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-gamma: Teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- van Horssen, R.; Hagen, T.L.M.T.; Eggermont, A.M.M. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Curnis, F.; Sacchi, A.; Borgna, L.; Magni, F.; Gasparri, A.; Corti, A. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13). Nat. Biotechnol. 2000, 18, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, S.; Vormehr, M.; van de Roemer, N.; Diken, M.; Lower, M.; Diekmann, J.; Boegel, S.; Schrors, B.; Vascotto, F.; Castle, J.C.; et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015, 520, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Rojas, L.A.; Sethna, Z.; Soares, K.; Derhovanessian, E.; Mueller, F.; Yadav, M.; Basturk, O.; Gonen, M.; Wei, A.C.C.; et al. Phase I trial of adjuvant autogene cevumeran, an individualized mRNA neoantigen vaccine, for pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2022, 40, 2516. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hirose, M.; Li, X. TLR7 Agonist-Loaded Gadolinium Oxide Nanotubes Promote Anti-Tumor Immunity by Activation of Innate and Adaptive Immune Responses. Vaccines 2024, 12, 373. https://doi.org/10.3390/vaccines12040373
Wang X, Hirose M, Li X. TLR7 Agonist-Loaded Gadolinium Oxide Nanotubes Promote Anti-Tumor Immunity by Activation of Innate and Adaptive Immune Responses. Vaccines. 2024; 12(4):373. https://doi.org/10.3390/vaccines12040373
Chicago/Turabian StyleWang, Xiupeng, Motohiro Hirose, and Xia Li. 2024. "TLR7 Agonist-Loaded Gadolinium Oxide Nanotubes Promote Anti-Tumor Immunity by Activation of Innate and Adaptive Immune Responses" Vaccines 12, no. 4: 373. https://doi.org/10.3390/vaccines12040373
APA StyleWang, X., Hirose, M., & Li, X. (2024). TLR7 Agonist-Loaded Gadolinium Oxide Nanotubes Promote Anti-Tumor Immunity by Activation of Innate and Adaptive Immune Responses. Vaccines, 12(4), 373. https://doi.org/10.3390/vaccines12040373





