Oncolytic Adenovirus Armed with a Novel Agonist of the CD137 Immune Checkpoint Stimulator Suppresses Tumor Growth
Abstract
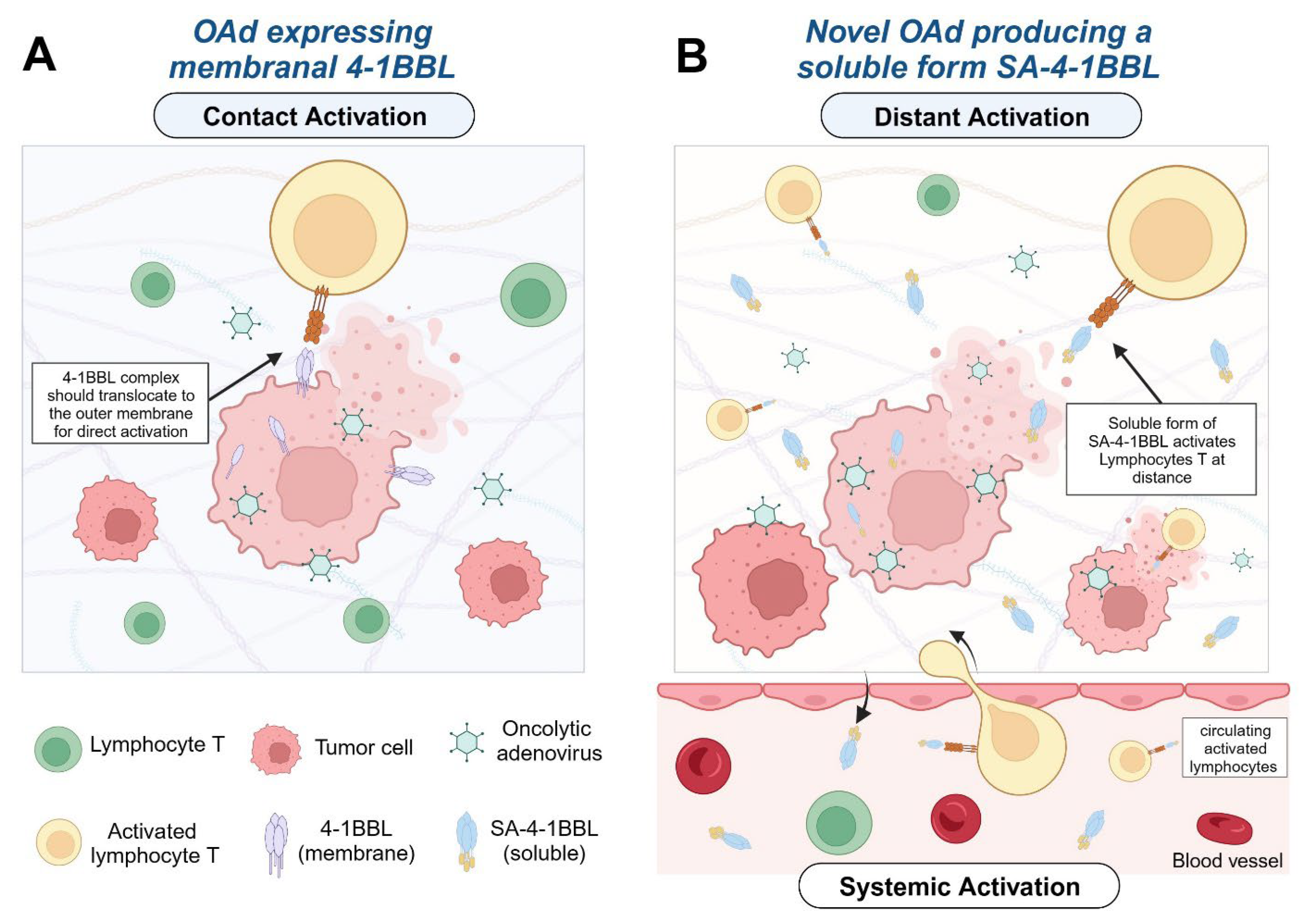
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Recombinant Adenoviral Vectors
2.3. SA-4-1BBL Splenocyte Co-Stimulation In Vitro Assay
2.4. Evaluation of OAd-Mediated Oncolytic Cell Death
2.5. Western Blot Analysis
2.6. Quantification of Activated Autophagy-Mediated Adenosine Triphosphate (ATP) Release
2.7. Evaluation of OAds-Mediated Cytotoxicity and Virus Replication in Lung Non-Cancerous Cells
2.8. Oncolytic Ads Anti-Tumor Therapeutic Efficacy Study
2.9. Immunohistochemistry
2.10. Flow Cytometry and Phenotyping
2.11. Statistical Analysis
3. Results
3.1. Recombinant Oncolytic Adenovirus Expresses an Active form of SA-4-1BBL
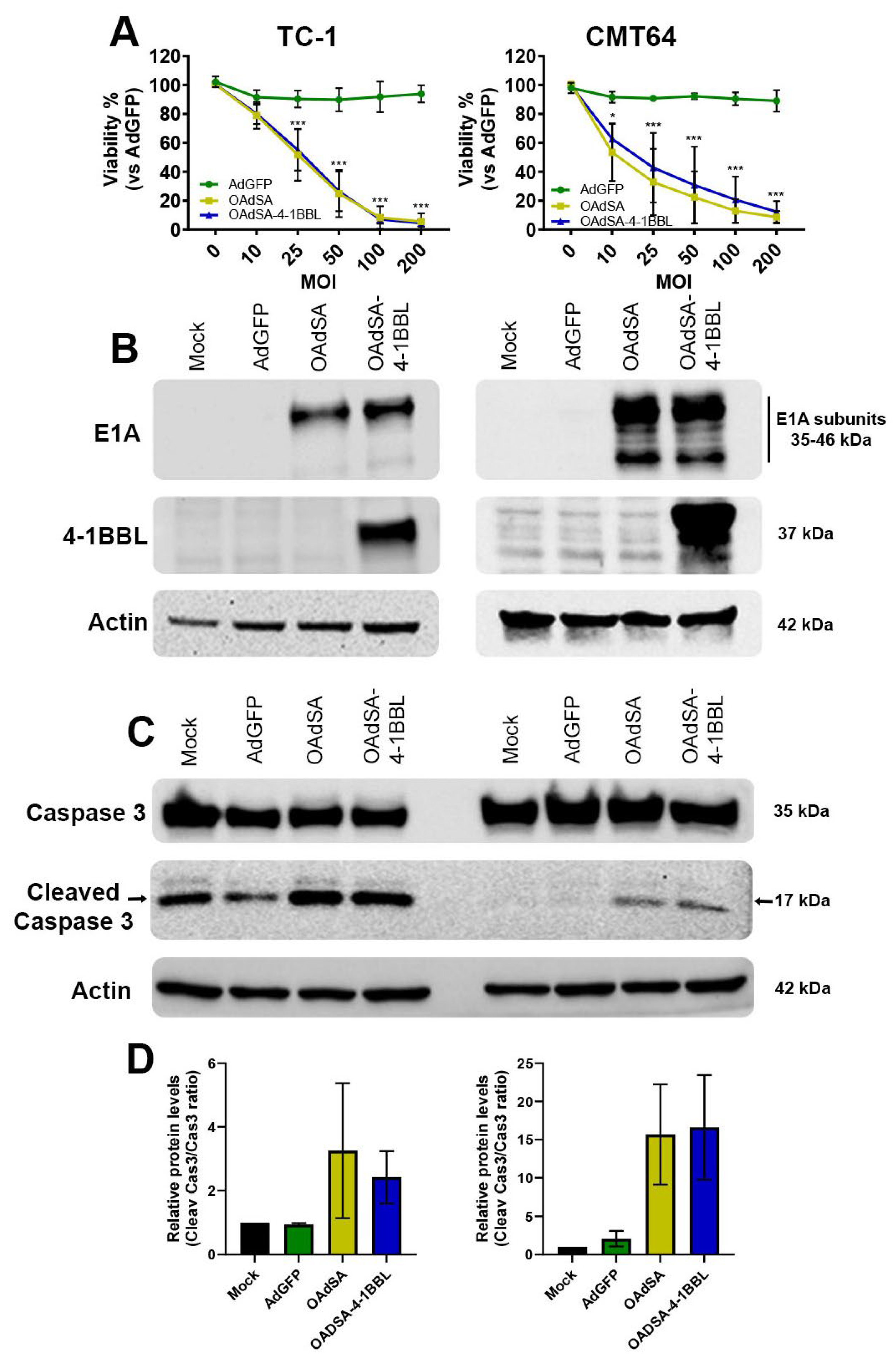
3.2. OAdSA and OAdSA-4-1BBL Induce Cytotoxic Effect on Lung Cancer Cell Lines In Vitro
3.3. Activation of Apoptotic Cell Death Is Associated with rOAds-Mediated Oncolytic Cell Death
3.4. rOAds Trigger Autophagy-Mediated Adenosine Triphosphate (ATP) Release in Lung Cancer Cell Lines In Vitro
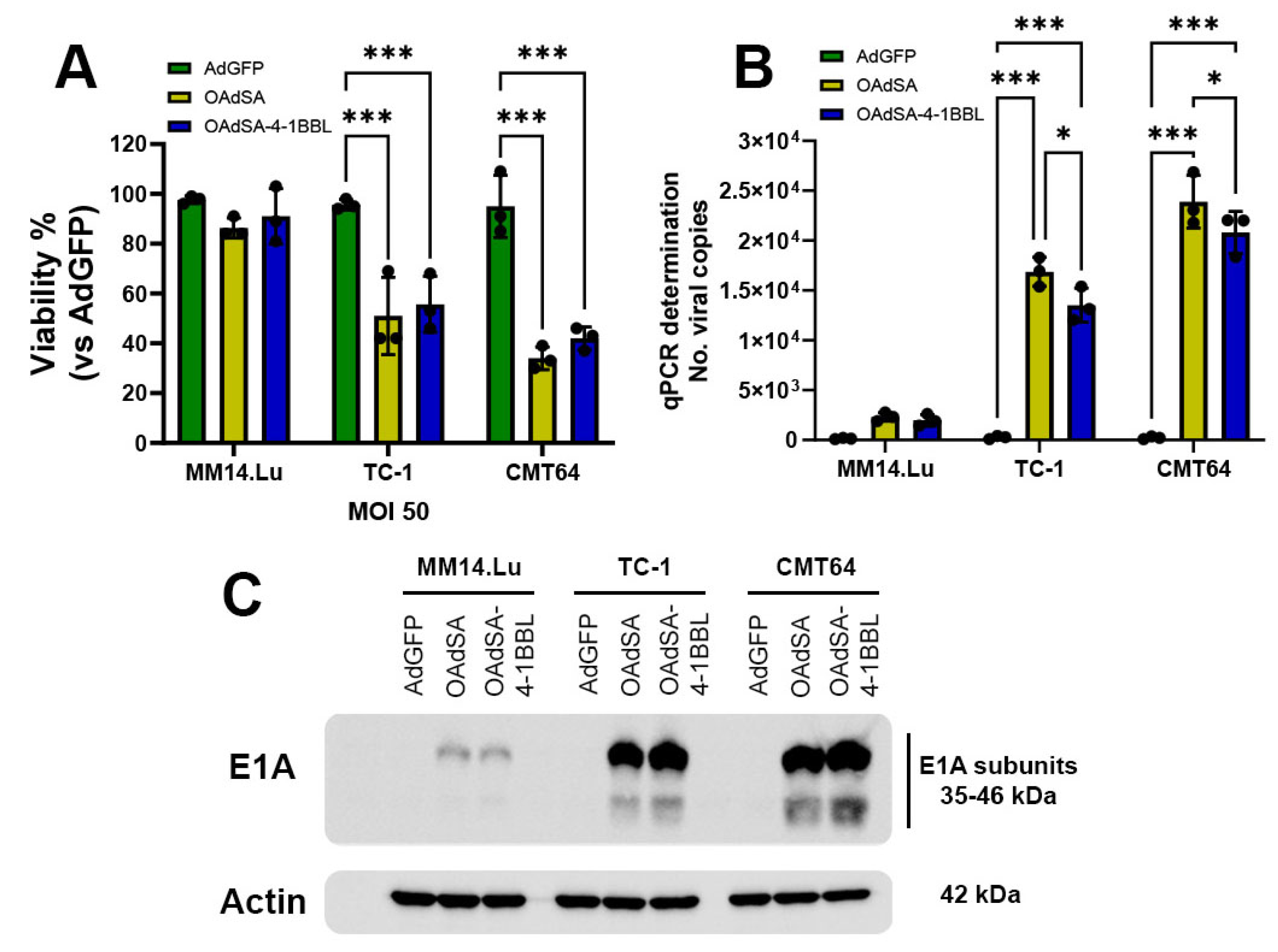
3.5. rOAds Preferentially Infect, Replicate, and Kill Murine Lung Cancer Cell Lines, but Not Non-Cancerous Lung Cells
3.6. OAdSA-4-1BBL Efficiently Delivers SA-4-1BBL, Replicates, and Has a Potent Therapeutic Efficacy against Established TC-1 Tumors
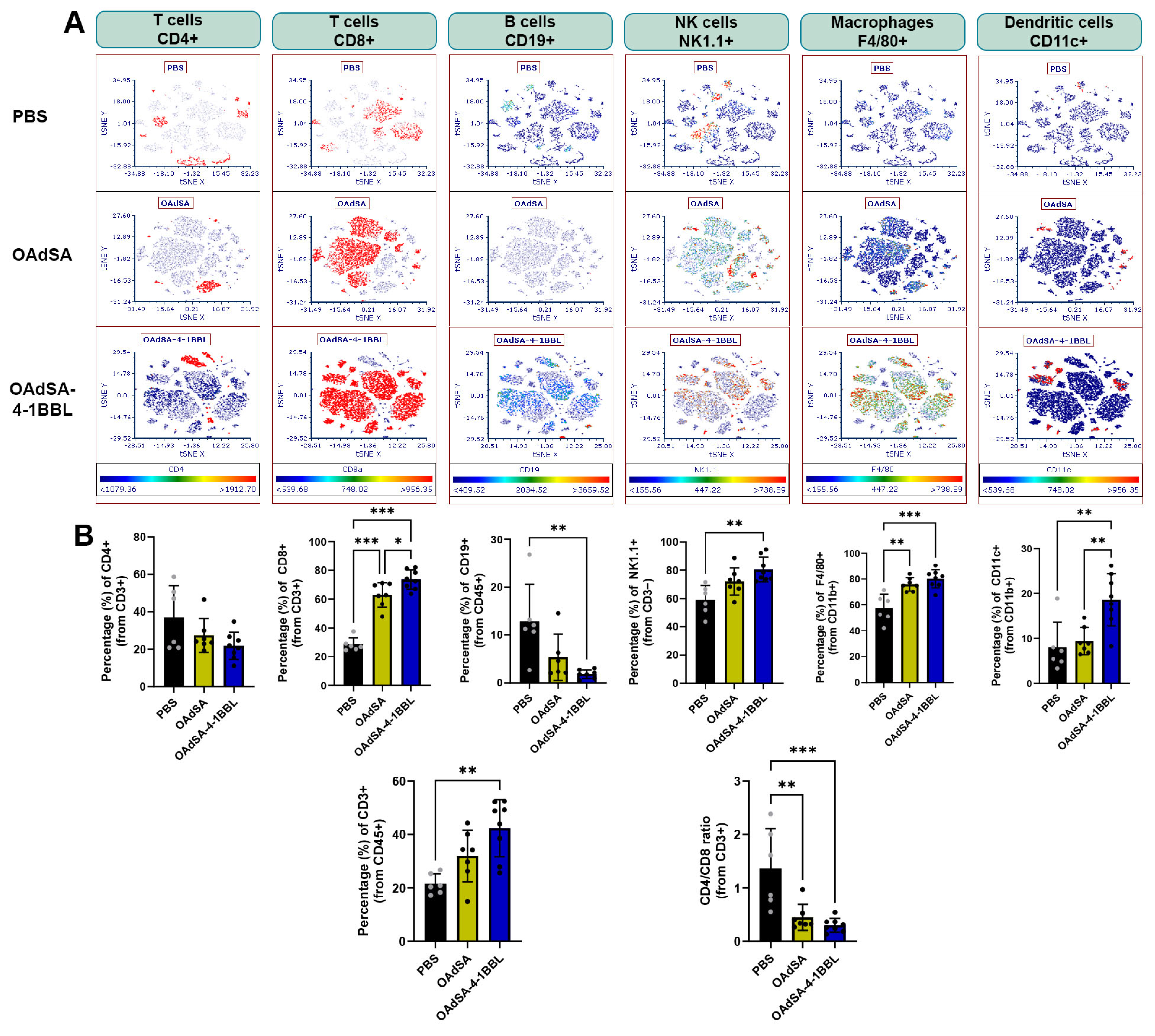
3.7. Immune Cell Phenotyping of Tumors Demonstrates the Activation of the Innate and Adaptive Immune Response by OAd Expressing the Costimulatory Protein SA-4-1BBL
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melero, I.; Shuford, W.W.; Newby, S.A.; Aruffo, A.; Ledbetter, J.A.; Hellstrom, K.E.; Mittler, R.S.; Chen, L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997, 3, 682–685. [Google Scholar] [CrossRef]
- Rabu, C.; Quemener, A.; Jacques, Y.; Echasserieau, K.; Vusio, P.; Lang, F. Production of recombinant human trimeric CD137L (4-1BBL). Cross-linking is essential to its T cell co-stimulation activity. J. Biol. Chem. 2005, 280, 41472–41481. [Google Scholar] [CrossRef]
- Sharma, R.K.; Elpek, K.G.; Yolcu, E.S.; Schabowsky, R.-H.; Zhao, H.; Bandura-Morgan, L.; Shirwan, H. Costimulation as a Platform for the Development of Vaccines: A Peptide-Based Vaccine Containing a Novel Form of 4-1BB Ligand Eradicates Established Tumors. Cancer Res. 2009, 69, 4319–4326. [Google Scholar] [CrossRef]
- Sharma, R.K.; Schabowsky, R.H.; Srivastava, A.K.; Elpek, K.G.; Madireddi, S.; Zhao, H.; Zhong, Z.; Miller, R.W.; Macleod, K.J.; Yolcu, E.S.; et al. 4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res. 2010, 70, 3945–3954. [Google Scholar] [CrossRef]
- Barsoumian, H.B.; Batra, L.; Shrestha, P.; Bowen, W.S.; Zhao, H.; Egilmez, N.K.; Gomez-Gutierrez, J.G.; Yolcu, E.S.; Shirwan, H. A Novel Form of 4-1BBL Prevents Cancer Development via Nonspecific Activation of CD4+ T and Natural Killer Cells. Cancer Res. 2019, 79, 783–794. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Galivo, F.; Diaz, R.M.; Thanarajasingam, U.; Jevremovic, D.; Wongthida, P.; Thompson, J.; Kottke, T.; Barber, G.N.; Melcher, A.; Vile, R.G. Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum. Gene Ther. 2010, 21, 439–450. [Google Scholar] [CrossRef]
- Tsun, A.; Miao, X.N.; Wang, C.M.; Yu, D.C. Oncolytic Immunotherapy for Treatment of Cancer. Adv. Exp. Med. Biol. 2016, 909, 241–283. [Google Scholar]
- Ma, J.; Ramachandran, M.; Jin, C.; Quijano-Rubio, C.; Martikainen, M.; Yu, D.; Essand, M. Characterization of virus-mediated immunogenic cancer cell death and the consequences for oncolytic virus-based immunotherapy of cancer. Cell Death Dis. 2020, 11, 48. [Google Scholar] [CrossRef]
- Tekant, Y.; Davydova, J.; Ramirez, P.J.; Curiel, D.T.; Vickers, S.M.; Yamamoto, M. Oncolytic adenoviral therapy in gallbladder carcinoma. Surgery 2005, 137, 527–535. [Google Scholar] [CrossRef]
- Eissa, I.R.; Bustos-Villalobos, I.; Ichinose, T.; Matsumura, S.; Naoe, Y.; Miyajima, N.; Morimoto, D.; Mukoyama, N.; Zhiwen, W.; Tanaka, M.; et al. The Current Status and Future Prospects of Oncolytic Viruses in Clinical Trials against Melanoma, Glioma, Pancreatic, and Breast Cancers. Cancers 2018, 10, 356. [Google Scholar] [CrossRef]
- Bauerschmitz, G.J.; Lam, J.T.; Kanerva, A.; Suzuki, K.; Nettelbeck, D.M.; Dmitriev, I.; Krasnykh, V.; Mikheeva, G.V.; Barnes, M.N.; Alvarez, R.D.; et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res. 2002, 62, 1266–1270. [Google Scholar]
- Koski, A.; Kangasniemi, L.; Escutenaire, S.; Pesonen, S.; Cerullo, V.; Diaconu, I.; Nokisalmi, P.; Raki, M.; Rajecki, M.; Guse, K.; et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 2010, 18, 1874–1884. [Google Scholar] [CrossRef]
- Cerullo, V.; Pesonen, S.; Diaconu, I.; Escutenaire, S.; Arstila, P.T.; Ugolini, M.; Nokisalmi, P.; Raki, M.; Laasonen, L.; Sarkioja, M.; et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010, 70, 4297–4309. [Google Scholar] [CrossRef]
- Pesonen, S.; Diaconu, I.; Kangasniemi, L.; Ranki, T.; Kanerva, A.; Pesonen, S.K.; Gerdemann, U.; Leen, A.M.; Kairemo, K.; Oksanen, M.; et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: Assessment of safety and immunologic responses in patients. Cancer Res. 2012, 72, 1621–1631. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, H.L.; Zhang, X.R.; Xu, J.P.; Hu, W.K.; Liang, M.; Chen, S.Y.; Hu, F.; Chu, D.T. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009, 16, 376–382. [Google Scholar] [CrossRef]
- Kloos, A.; Woller, N.; Gurlevik, E.; Ureche, C.I.; Niemann, J.; Armbrecht, N.; Martin, N.T.; Geffers, R.; Manns, M.P.; Gerardy-Schahn, R.; et al. PolySia-Specific Retargeting of Oncolytic Viruses Triggers Tumor-Specific Immune Responses and Facilitates Therapy of Disseminated Lung Cancer. Cancer Immunol. Res. 2015, 3, 751–763. [Google Scholar] [CrossRef]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.X.; Levin, V.A.; Yung, W.K.; Kyritsis, A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000, 19, 2–12. [Google Scholar] [CrossRef]
- Whyte, P.; Williamson, N.M.; Harlow, E. Cellular targets for transformation by the adenovirus E1A proteins. Cell 1989, 56, 67–75. [Google Scholar] [CrossRef]
- Kraus, V.B.; Moran, E.; Nevins, J.R. Promoter-specific trans-activation by the adenovirus E1A12S product involves separate E1A domains. Mol. Cell. Biol. 1992, 12, 4391–4399. [Google Scholar] [CrossRef][Green Version]
- Elpek, K.G.; Yolcu, E.S.; Franke, D.D.; Lacelle, C.; Schabowsky, R.H.; Shirwan, H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J. Immunol. 2007, 179, 7295–7304. [Google Scholar] [CrossRef]
- Schabowsky, R.H.; Elpek, K.G.; Madireddi, S.; Sharma, R.K.; Yolcu, E.S.; Bandura-Morgan, L.; Miller, R.; MacLeod, K.J.; Mittler, R.S.; Shirwan, H. A novel form of 4-1BBL has better immunomodulatory activity than an agonistic anti-4-1BB Ab without Ab-associated severe toxicity. Vaccine 2009, 28, 512–522. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Tuve, S.; Ni, S.; Hellström, K.E.; Hellström, I.; Lieber, A. Effect of Adenovirus-Mediated Heat Shock Protein Expression and Oncolysis in Combination with Low-Dose Cyclophosphamide Treatment on Antitumor Immune Responses. Cancer Res. 2006, 66, 960–969. [Google Scholar] [CrossRef][Green Version]
- Halldén, G.; Hill, R.; Wang, Y.; Anand, A.; Liu, T.C.; Lemoine, N.R.; Francis, J.; Hawkins, L.; Kirn, D. Novel immunocompetent murine tumor models for the assessment of replication-competent oncolytic adenovirus efficacy. Mol. Ther. 2003, 8, 412–424. [Google Scholar] [CrossRef]
- Radko, S.; Jung, R.; Olanubi, O.; Pelka, P. Effects of Adenovirus Type 5 E1A Isoforms on Viral Replication in Arrested Human Cells. PLoS ONE 2015, 10, e0140124. [Google Scholar] [CrossRef]
- Tazawa, H.; Kuroda, S.; Hasei, J.; Kagawa, S.; Fujiwara, T. Impact of Autophagy in Oncolytic Adenoviral Therapy for Cancer. Int. J. Mol. Sci. 2017, 18, 1479. [Google Scholar] [CrossRef]
- Giménez-Xavier, P.; Francisco, R.; Platini, F.; Pérez, R.; Ambrosio, S. LC3-I conversion to LC3-II does not necessarily result in complete autophagy. Int. J. Mol. Med. 2008, 22, 781–785. [Google Scholar]
- Bjorkoy, G.; Lamark, T.; Pankiv, S.; Overvatn, A.; Brech, A.; Johansen, T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009, 452, 181–197. [Google Scholar]
- Liikanen, I.; Ahtiainen, L.; Hirvinen, M.L.; Bramante, S.; Cerullo, V.; Nokisalmi, P.; Hemminki, O.; Diaconu, I.; Pesonen, S.; Koski, A.; et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol. Ther. 2013, 21, 1212–1223. [Google Scholar] [CrossRef]
- Siurala, M.; Bramante, S.; Vassilev, L.; Hirvinen, M.; Parviainen, S.; Tahtinen, S.; Guse, K.; Cerullo, V.; Kanerva, A.; Kipar, A.; et al. Oncolytic adenovirus and doxorubicin-based chemotherapy results in synergistic antitumor activity against soft-tissue sarcoma. Int. J. Cancer 2015, 136, 945–954. [Google Scholar] [CrossRef]
- Lillie, J.W.; Green, M.R. Transcription activation by the adenovirus E1a protein. Nature 1989, 338, 39–44. [Google Scholar] [CrossRef]
- Mardiana, S.; John, L.B.; Henderson, M.A.; Slaney, C.Y.; von Scheidt, B.; Giuffrida, L.; Davenport, A.J.; Trapani, J.A.; Neeson, P.J.; Loi, S.; et al. A Multifunctional Role for Adjuvant Anti-4-1BB Therapy in Augmenting Antitumor Response by Chimeric Antigen Receptor T Cells. Cancer Res. 2017, 77, 1296–1309. [Google Scholar] [CrossRef]
- Bartkowiak, T.; Singh, S.; Yang, G.; Galvan, G.; Haria, D.; Ai, M.; Allison, J.P.; Sastry, K.J.; Curran, M.A. Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc. Natl. Acad. Sci. USA 2015, 112, E5290–E5299. [Google Scholar] [CrossRef]
- Uno, T.; Takeda, K.; Kojima, Y.; Yoshizawa, H.; Akiba, H.; Mittler, R.S.; Gejyo, F.; Okumura, K.; Yagita, H.; Smyth, M.J. Eradication of established tumors in mice by a combination antibody-based therapy. Nat. Med. 2006, 12, 693–698. [Google Scholar] [CrossRef]
- Palazon, A.; Teijeira, A.; Martinez-Forero, I.; Hervas-Stubbs, S.; Roncal, C.; Penuelas, I.; Dubrot, J.; Morales-Kastresana, A.; Perez-Gracia, J.L.; Ochoa, M.C.; et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 2011, 71, 801–811. [Google Scholar] [CrossRef]
- Compte, M.; Harwood, S.L.; Munoz, I.G.; Navarro, R.; Zonca, M.; Perez-Chacon, G.; Erce-Llamazares, A.; Merino, N.; Tapia-Galisteo, A.; Cuesta, A.M.; et al. A tumor-targeted trimeric 4-1BB-agonistic antibody induces potent anti-tumor immunity without systemic toxicity. Nat. Commun. 2018, 9, 4809. [Google Scholar] [CrossRef]
- Mantwill, K.; Klein, F.G.; Wang, D.; Hindupur, S.V.; Ehrenfeld, M.; Holm, P.S.; Nawroth, R. Concepts in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2021, 22, 10522. [Google Scholar] [CrossRef]
- Davola, M.E.; Mossman, K.L. Oncolytic viruses: How “lytic” must they be for therapeutic efficacy? Oncoimmunology 2019, 8, e1581528. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim-Schulze, S.; Kim, D.W.; Kaufman, H.L. Host lymphodepletion enhances the therapeutic activity of an oncolytic vaccinia virus expressing 4-1BB ligand. Cancer Res. 2009, 69, 8516–8525. [Google Scholar] [CrossRef]
- Huang, J.H.; Zhang, S.N.; Choi, K.J.; Choi, I.K.; Kim, J.H.; Lee, M.G.; Kim, H.; Yun, C.O. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol. Ther. 2010, 18, 264–274. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Andersson, A.M.; Pedersen, A.E.; Laursen, H.; Holst, P.J. An adenoviral cancer vaccine co-encoding a tumor associated antigen together with secreted 4-1BBL leads to delayed tumor progression. Vaccine 2016, 34, 2147–2156. [Google Scholar] [CrossRef]
- Eriksson, E.; Milenova, I.; Wenthe, J.; Stahle, M.; Leja-Jarblad, J.; Ullenhag, G.; Dimberg, A.; Moreno, R.; Alemany, R.; Loskog, A. Shaping the Tumor Stroma and Sparking Immune Activation by CD40 and 4-1BB Signaling Induced by an Armed Oncolytic Virus. Clin. Cancer Res. 2017, 23, 5846–5857. [Google Scholar] [CrossRef]
- Lee-Chang, C.; Bodogai, M.; Moritoh, K.; Olkhanud, P.B.; Chan, A.C.; Croft, M.; Mattison, J.A.; Holst, P.J.; Gress, R.E.; Ferrucci, L.; et al. Accumulation of 4-1BBL+ B cells in the elderly induces the generation of granzyme-B+ CD8+ T cells with potential antitumor activity. Blood 2014, 124, 1450–1459. [Google Scholar] [CrossRef]
- Martinez-Perez, A.G.; Perez-Trujillo, J.J.; Garza-Morales, R.; Ramirez-Avila, N.E.; Loera-Arias, M.J.; Gomez-Gutierrez, J.G.; Saucedo-Cardenas, O.; Garcia-Garcia, A.; Rodriguez-Rocha, H.; Montes-de-Oca-Luna, R. An Oncolytic Adenovirus Encoding SA-4-1BBL Adjuvant Fused to HPV-16 E7 Antigen Produces a Specific Antitumor Effect in a Cancer Mouse Model. Vaccines 2021, 9, 149. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yolcu, E.S.; Srivastava, A.K.; Shirwan, H. CD4+ T cells play a critical role in the generation of primary and memory antitumor immune responses elicited by SA-4-1BBL and TAA-based vaccines in mouse tumor models. PLoS ONE 2013, 8, e73145. [Google Scholar] [CrossRef]
- Samadi, M.; Mokhtari-Azad, T.; Nejati, A.; Norooz-Babaei, Z.; Foroushani, A.R.; Haghshenas, M.R.; Adjaminejad, F.; Zargaran, H.; Salimi, V.; Ghaemi, A. The antitumor effect of oncolytic respiratory syncytial virus via the tumor necrosis factor-alpha induction and ROS-bax-mediated mechanisms. BMC Cancer 2023, 23, 803. [Google Scholar] [CrossRef]
- Wilcox, R.A.; Chapoval, A.I.; Gorski, K.S.; Otsuji, M.; Shin, T.; Flies, D.B.; Tamada, K.; Mittler, R.S.; Tsuchiya, H.; Pardoll, D.M.; et al. Cutting Edge: Expression of Functional CD137 Receptor by Dendritic Cells. J. Immunol. 2002, 168, 4262–4267. [Google Scholar] [CrossRef]
- Futagawa, T.; Akiba, H.; Kodama, T.; Takeda, K.; Hosoda, Y.; Yagita, H.; Okumura, K. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 2002, 14, 275–286. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Gonzalez, M.R.; Tarique, M.; Batra, L.; Arguc, F.; Garza-Morales, R.; Shirwan, H.; Yolcu, E.S.; Gomez-Gutierrez, J.G. Oncolytic Adenovirus Armed with a Novel Agonist of the CD137 Immune Checkpoint Stimulator Suppresses Tumor Growth. Vaccines 2024, 12, 340. https://doi.org/10.3390/vaccines12030340
Ramos-Gonzalez MR, Tarique M, Batra L, Arguc F, Garza-Morales R, Shirwan H, Yolcu ES, Gomez-Gutierrez JG. Oncolytic Adenovirus Armed with a Novel Agonist of the CD137 Immune Checkpoint Stimulator Suppresses Tumor Growth. Vaccines. 2024; 12(3):340. https://doi.org/10.3390/vaccines12030340
Chicago/Turabian StyleRamos-Gonzalez, Martin R., Mohammad Tarique, Lalit Batra, Feyza Arguc, Rodolfo Garza-Morales, Haval Shirwan, Esma S. Yolcu, and Jorge G. Gomez-Gutierrez. 2024. "Oncolytic Adenovirus Armed with a Novel Agonist of the CD137 Immune Checkpoint Stimulator Suppresses Tumor Growth" Vaccines 12, no. 3: 340. https://doi.org/10.3390/vaccines12030340
APA StyleRamos-Gonzalez, M. R., Tarique, M., Batra, L., Arguc, F., Garza-Morales, R., Shirwan, H., Yolcu, E. S., & Gomez-Gutierrez, J. G. (2024). Oncolytic Adenovirus Armed with a Novel Agonist of the CD137 Immune Checkpoint Stimulator Suppresses Tumor Growth. Vaccines, 12(3), 340. https://doi.org/10.3390/vaccines12030340









