Towards a Safer Future: Enhancing Vaccine Development to Combat Animal Coronaviruses
Abstract
1. Introduction
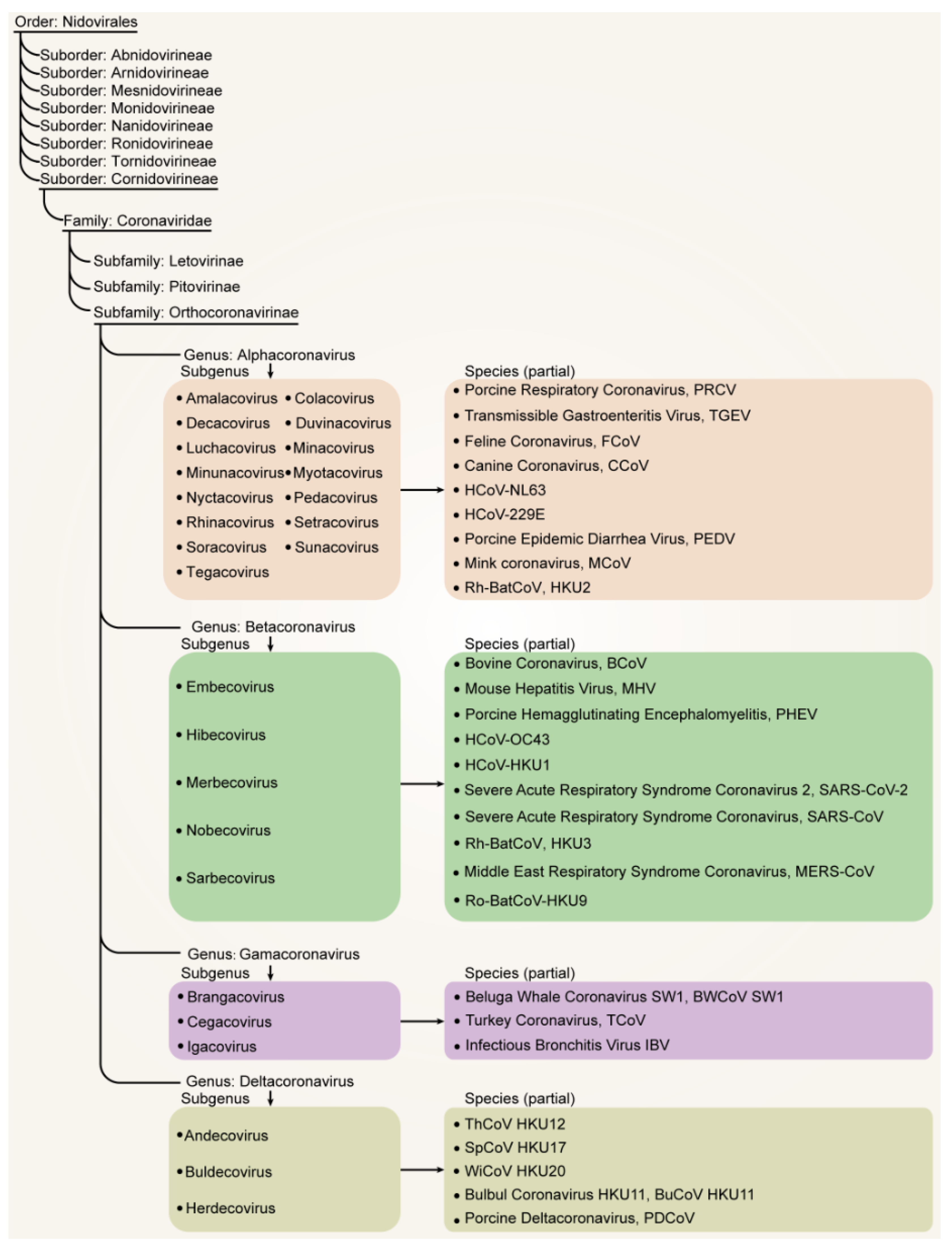
2. Main Animal Coronaviruses and Their Taxonomic Perspectives
2.1. Porcine Coronaviruses
2.2. Canine Coronavirus (CCoV)
2.3. Equine Coronavirus (ECoV)
2.4. Feline coronavirus (FCoV) and Feline Infectious Peritonitis Virus (FIPV)
2.5. Bovine CoV
2.6. Avian CoVs
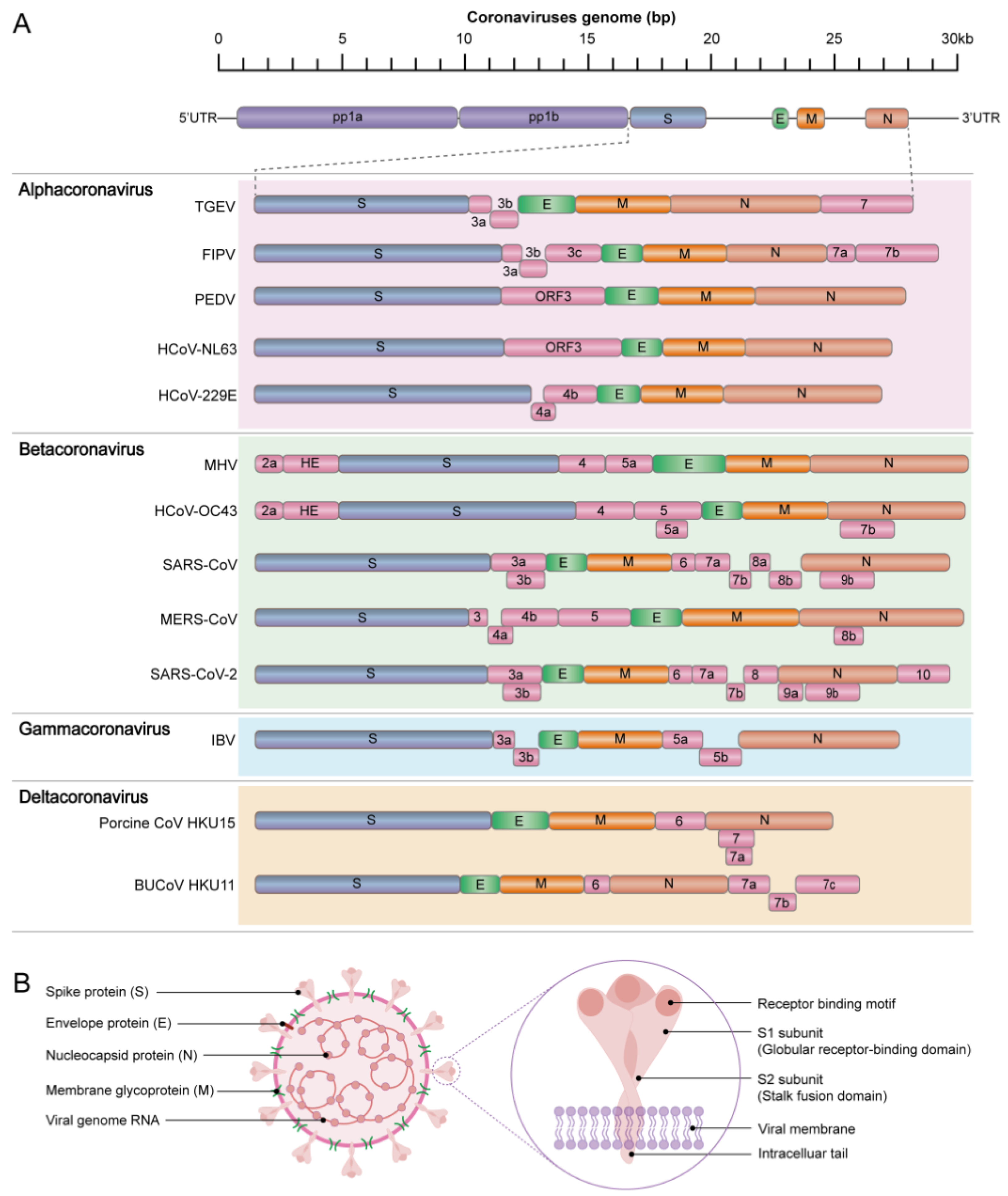
3. Genomic Structure of Animal CoVs and Function of Their Related Proteins
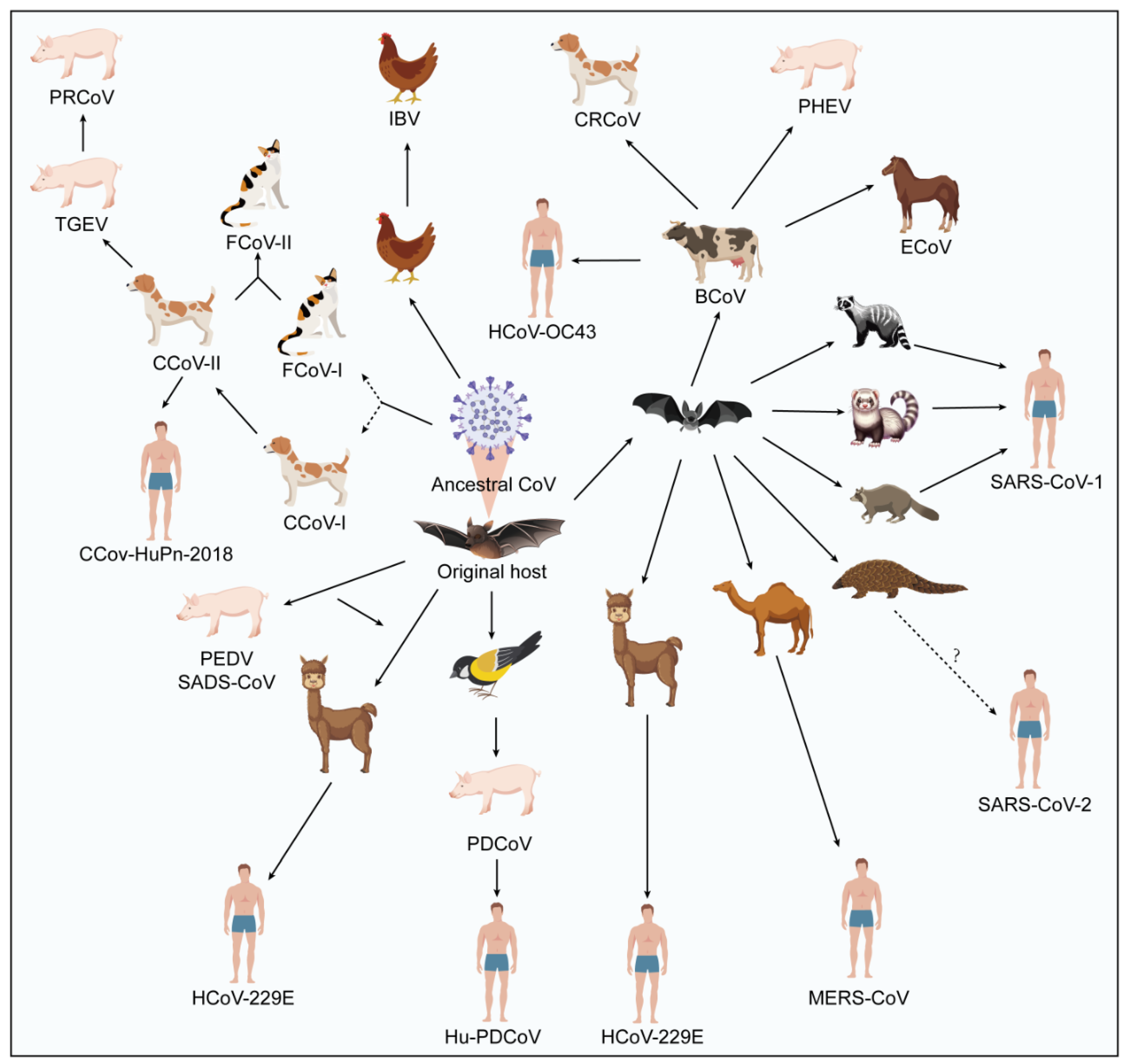
4. Spillover Event and Cross-Species Potential of Animal Coronavirus
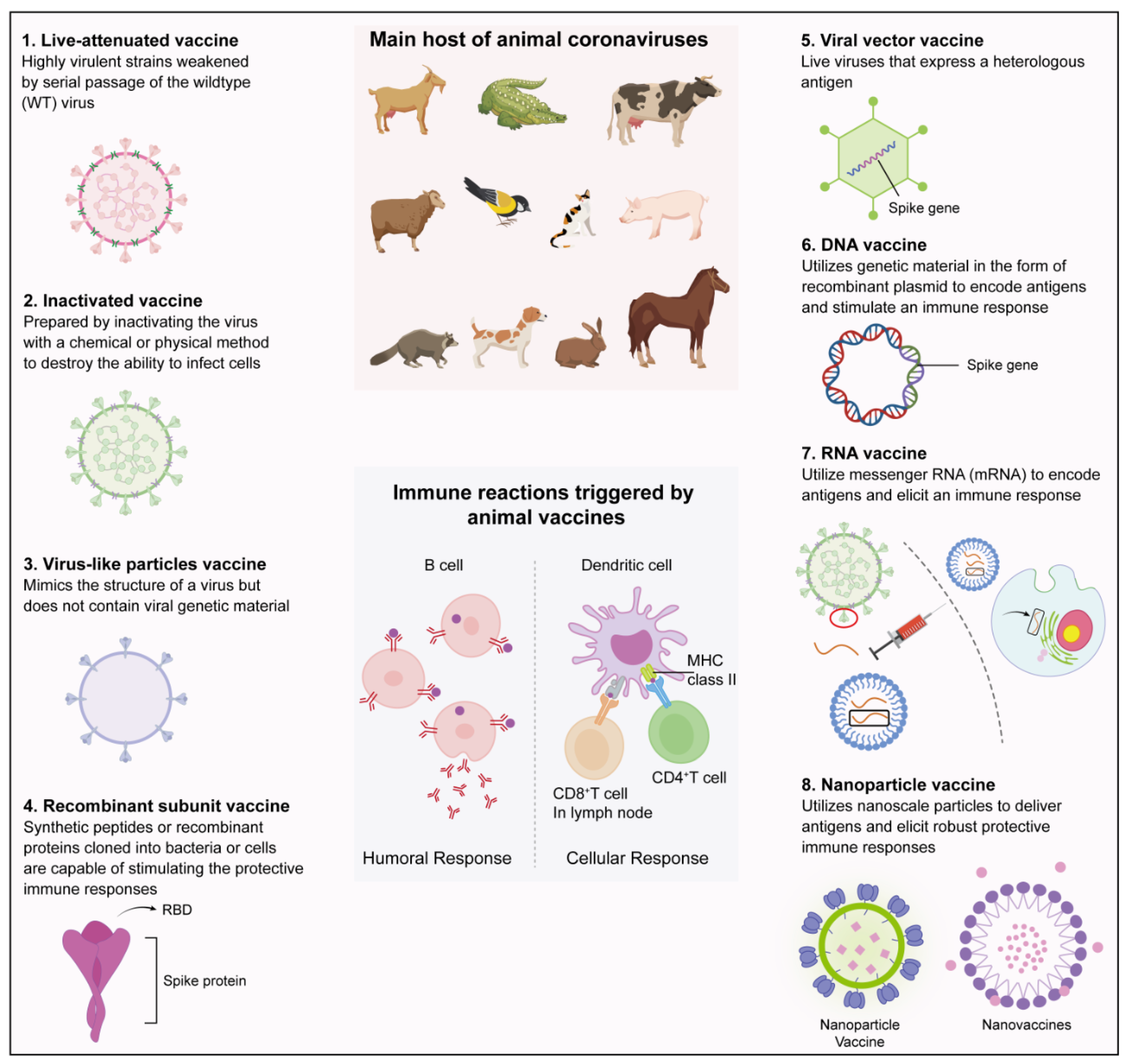
5. Types of Vaccine Development Platforms and the Trialed or Generated Animal Coronavirus Vaccines
6. Status of Vaccine Development for Animal Coronaviruses
7. Main Targets for Animal Coronavirus Vaccine Development
7.1. S protein
7.2. N Protein
7.3. M Protein
7.4. E Protein
7.5. Non-Structural Proteins (NSPs)
7.6. The Entire Virus as a Target
8. Challenges in the Development of Vaccines against Animal Coronavirus
8.1. High Mutation Rates and Viral RNA Quasispecies
8.2. Lack of Suitable Cell Lines Capable of High Yield Production
8.3. “Off-Target” Antibody Responses
8.4. Antibody-Dependent Enhancement (ADE)
8.5. Vaccine-Associated Enhanced Diseases (VAED)
8.6. Recombination Events between Human and Animal CoV Strains
9. Urgent Need to Develop a “Dual-Effect” Vaccine Capable of Inducing Both Cellular and Humoral Immune Responses
10. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vlasova, A.N.; Diaz, A.; Damtie, D.; Xiu, L.; Toh, T.-H.; Lee, J.S.-Y.; Saif, L.J.; Gray, G.C. Novel Canine Coronavirus Isolated from a Hospitalized Patient With Pneumonia in East Malaysia. Clin. Infect. Dis. 2021, 74, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Lednicky, J.A.; Tagliamonte, M.S.; White, S.K.; Elbadry, M.A.; Alam, M.M.; Stephenson, C.J.; Bonny, T.S.; Loeb, J.C.; Telisma, T.; Chavannes, S. Independent infections of porcine deltacoronavirus among Haitian children. Nature 2021, 600, 133–137. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Chen, B.; Tian, E.-K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar]
- Ge, X.-Y.; Li, J.-L.; Yang, X.-L.; Chmura, A.A.; Zhu, G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013, 503, 535–538. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Yang, W.-H.; Zhou, J.-H.; Li, B.; Zhang, W.; Shi, Z.-L.; Zhang, Y.-Z. Detection of alpha-and betacoronaviruses in rodents from Yunnan, China. Virol. J. 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zeng, L.-P.; Yang, X.-L.; Ge, X.-Y.; Zhang, W.; Li, B.; Xie, J.-Z.; Shen, X.-R.; Zhang, Y.-Z.; Wang, N. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017, 13, e1006698. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef]
- Hu, B.; Ge, X.; Wang, L.-F.; Shi, Z. Bat origin of human coronaviruses. Virol. J. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Cherry, J.D.; Krogstad, P. SARS: The first pandemic of the 21st century. Pediatr. Res. 2004, 56, 1–5. [Google Scholar] [CrossRef]
- Corman, V.M.; Ithete, N.L.; Richards, L.R.; Schoeman, M.C.; Preiser, W.; Drosten, C.; Drexler, J.F. Rooting the phylogenetic tree of middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J. Virol. 2014, 88, 11297–11303. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Wacharapluesadee, S.; Tan, C.W.; Maneeorn, P.; Duengkae, P.; Zhu, F.; Joyjinda, Y.; Kaewpom, T.; Chia, W.N.; Ampoot, W.; Lim, B.L.; et al. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat. Commun. 2021, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- Worobey, M.; Levy, J.I.; Malpica Serrano, L.; Crits-Christoph, A.; Pekar, J.E.; Goldstein, S.A.; Rasmussen, A.L.; Kraemer, M.U.G.; Newman, C.; Koopmans, M.P.G.; et al. The Huanan Seafood Wholesale Market in Wuhan was the early epicenter of the COVID-19 pandemic. Science 2022, 377, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Vijgen, L.; Keyaerts, E.; Moës, E.; Thoelen, I.; Wollants, E.; Lemey, P.; Vandamme, A.-M.; Van Ranst, M. Complete genomic sequence of human coronavirus OC43: Molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005, 79, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Baldwin, H.J.; Tateno, A.F.; Zerbinati, R.M.; Annan, A.; Owusu, M.; Nkrumah, E.E.; Maganga, G.D.; Oppong, S.; Adu-Sarkodie, Y.; et al. Evidence for an Ancestral Association of Human Coronavirus 229E with Bats. J. Virol. 2015, 89, 11858–11870. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Shi, M.; Chommanard, C.; Queen, K.; Zhang, J.; Markotter, W.; Kuzmin, I.V.; Holmes, E.C.; Tong, S. Surveillance of Bat Coronaviruses in Kenya Identifies Relatives of Human Coronaviruses NL63 and 229E and Their Recombination History. J. Virol. 2017, 91, e01953-16. [Google Scholar] [CrossRef]
- Graham, R.L.; Donaldson, E.F.; Baric, R.S. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013, 11, 836–848. [Google Scholar] [CrossRef]
- Cankat, S.; Demael, M.U.; Swadling, L. In search of a pan-coronavirus vaccine: Next-generation vaccine design and immune mechanisms. Cell. Mol. Immunol. 2024, 21, 103–118. [Google Scholar] [CrossRef]
- Tan, C.W.; Valkenburg, S.A.; Poon, L.L.; Wang, L.-F. Broad-spectrum pan-genus and pan-family virus vaccines. Cell Host Microbe 2023, 31, 902–916. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; de Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; van der Hoek, L.; Wong, A.C.P.; Yeh, S.-H. ICTV Virus Taxonomy Profile: Coronaviridae 2023. J. Gen. Virol. 2023, 104, 001843. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, Y.; Ge, X. The taxonomy, host range and pathogenicity of coronaviruses and other viruses in the Nidovirales order. Anim. Dis. 2021, 1, 5. [Google Scholar] [CrossRef]
- Hussein, H.A.; Hassan, R.Y.; Chino, M.; Febbraio, F. Point-of-care diagnostics of COVID-19: From current work to future perspectives. Sensors 2020, 20, 4289. [Google Scholar] [CrossRef]
- Bukhari, K.; Mulley, G.; Gulyaeva, A.A.; Zhao, L.; Shu, G.; Jiang, J.; Neuman, B.W. Description and initial characterization of metatranscriptomic nidovirus-like genomes from the proposed new family Abyssoviridae, and from a sister group to the Coronavirinae, the proposed genus Alphaletovirus. Virology 2018, 524, 160–171. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, N.J.; Dubovi, E.J. Chapter 24—Coronaviridae. In Fenner’s Veterinary Virology, 5th ed.; Academic Press: Boston, MA, USA, 2017; pp. 435–461. [Google Scholar]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef]
- Regan, A.D.; Millet, J.K.; Tse, L.P.V.; Chillag, Z.; Rinaldi, V.D.; Licitra, B.N.; Dubovi, E.J.; Town, C.D.; Whittaker, G.R. Characterization of a recombinant canine coronavirus with a distinct receptor-binding (S1) domain. Virology 2012, 430, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, A.; Owczarek, K.; Bzowska, M.; Gula, K.; Drebot, I.; Ochman, M.; Maksym, B.; Rajfur, Z.; Mitchell, J.A.; Pyrc, K. Canine respiratory coronavirus, bovine coronavirus, and human coronavirus OC43: Receptors and attachment factors. Viruses 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Lednicky, J.A.; Tagliamonte, M.S.; White, S.K.; Blohm, G.M.; Alam, M.M.; Iovine, N.M.; Salemi, M.; Mavian, C.; Morris, J.G., Jr. Isolation of a novel recombinant canine coronavirus from a visitor to Haiti: Further evidence of transmission of coronaviruses of zoonotic origin to humans. Clin. Infect. Dis. 2022, 75, e1184–e1187. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, M.A.; Walls, A.C.; Joshi, A.; Park, Y.-J.; Eguia, R.T.; Miranda, M.C.; Kepl, E.; Dosey, A.; Stevens-Ayers, T.; Boeckh, M.J. Structure, receptor recognition, and antigenicity of the human coronavirus CCoV-HuPn-2018 spike glycoprotein. Cell 2022, 185, 2279–2291.e2217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, D.; Wang, Y.; Li, X.; Qiu, Y.; Zheng, M.; Song, Y.; Li, G.; Song, C.; Liu, T. Characterization of CCoV-HuPn-2018 spike protein-mediated viral entry. J. Virol. 2023, 97, e00601–e00623. [Google Scholar] [CrossRef] [PubMed]
- Hulswit, R.J.; Lang, Y.; Bakkers, M.J.; Li, W.; Li, Z.; Schouten, A.; Ophorst, B.; Van Kuppeveld, F.J.; Boons, G.-J.; Bosch, B.-J. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc. Natl. Acad. Sci. USA 2019, 116, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Delmas, B.; Gelfi, J.; L’Haridon, R.; Sjöström, H.; Laude, H. Aminopeptidase N is a major receptor for the enteropathogenic coronavirus TGEV. Nature 1992, 357, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Wang, X.; Zhou, J.; Ma, L.; Li, J.; Yang, L.; Ouyang, H.; Yuan, H.; Pang, D. Transmissible Gastroenteritis Virus: An Update Review and Perspective. Viruses 2023, 15, 359. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.-Y. Porcine enteric coronaviruses: An updated overview of the pathogenesis, prevalence, and diagnosis. Vet. Res. Commun. 2021, 45, 75–86. [Google Scholar] [CrossRef]
- Islam, A.; Ferdous, J.; Islam, S.; Sayeed, M.; Dutta Choudhury, S.; Saha, O.; Hassan, M.M.; Shirin, T. Evolutionary dynamics and epidemiology of endemic and emerging coronaviruses in humans, domestic animals, and wildlife. Viruses 2021, 13, 1908. [Google Scholar] [CrossRef]
- Turlewicz-Podbielska, H.; Pomorska-Mól, M. Porcine coronaviruses: Overview of the state of the art. Virol. Sin. 2021, 36, 833–851. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, H.; Li, L.; Yang, Y.; Zou, X.; Chen, J.; Tang, X. PEDV: Insights and Advances into Types, Function, Structure, and Receptor Recognition. Viruses 2022, 14, 1744. [Google Scholar] [CrossRef]
- Liu, C.; Tang, J.; Ma, Y.; Liang, X.; Yang, Y.; Peng, G.; Qi, Q.; Jiang, S.; Li, J.; Du, L. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 2015, 89, 6121–6125. [Google Scholar] [CrossRef]
- Yeager, C.L.; Ashmun, R.A.; Williams, R.K.; Cardellichio, C.B.; Shapiro, L.H.; Look, A.T.; Holmes, K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 1992, 357, 420–422. [Google Scholar] [CrossRef]
- Liu, D.; Chen, C.; Chen, D.; Zhu, A.; Li, F.; Zhuang, Z.; Mok, C.K.P.; Dai, J.; Li, X.; Jin, Y.; et al. Mouse models susceptible to HCoV-229E and HCoV-NL63 and cross protection from challenge with SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2023, 120, e2202820120. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020, 94, e00127-00120. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Liu, W.; Gan, M.; Zhang, L.; Wang, J.; Zhang, Z.; Zhu, A.; Li, F.; Sun, J. Discovery of a subgenotype of human coronavirus NL63 associated with severe lower respiratory tract infection in China, 2018. Emerg. Microbes Infect. 2020, 9, 246–255. [Google Scholar] [CrossRef]
- Hofmann, H.; Pyrc, K.; Van Der Hoek, L.; Geier, M.; Berkhout, B.; Pöhlmann, S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Yu, J.-Q.; Huang, Y.-W. Swine enteric alphacoronavirus (swine acute diarrhea syndrome coronavirus): An update three years after its discovery. Virus Res. 2020, 285, 198024. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, Z.-Y.; Yang, Y.-L.; Qin, Y.; Pan, D.; Yuan, L.-X.; Huang, Y.-W.; Wang, B. The origin and evolution of emerged swine acute diarrhea syndrome coronavirus with zoonotic potential. J. Med. Virol. 2023, 95, e28672. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hu, B.; Si, H.-R.; Zhu, Y.; Zhang, W.; Li, B.; Li, A.; Geng, R.; Lin, H.-F.; Yang, X.-L. Identification of a novel lineage bat SARS-related coronaviruses that use bat ACE2 receptor. Emerg. Microbes Infect. 2021, 10, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Hemmila, E.; Turbide, C.; Olson, M.; Jothy, S.; Holmes, K.V.; Beauchemin, N. Ceacam1a−/− Mice Are Completely Resistant to Infection by Murine Coronavirus Mouse Hepatitis Virus A59. J. Virol. 2004, 78, 10156–10165. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shah, T.; Wang, B.; Qu, L.; Wang, R.; Hou, Y.; Baloch, Z.; Xia, X. Cross-species transmission, evolution and zoonotic potential of coronaviruses. Front. Cell. Infect. Microbiol. 2023, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J.; Jung, K. Comparative pathogenesis of bovine and porcine respiratory coronaviruses in the animal host species and SARS-CoV-2 in humans. J. Clin. Microbiol. 2020, 58, e01355-01320. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Wong, S.K.-M.; Huang, Z.; Lam, C.S.-C.; Chow, A.K.-M.; Foo, D.C.-C.; Lo, O.S.-H.; Pang, R.W.-C.; Law, W.-L. CD26 induces colorectal cancer angiogenesis and metastasis through CAV1/MMP1 signaling. Int. J. Mol. Sci. 2022, 23, 1181. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Pöhlmann, S. Novel SARS-CoV-2 receptors: Asgr1 and Kremen1. Cell Res. 2022, 32, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Zamorano Cuervo, N.; Grandvaux, N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. eLife 2020, 9, e61390. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Cao, J.; Zhang, X.; Gao, H.; Wang, Y.; Wang, J.; He, J.; Jiang, X.; Zhang, J.; Shen, G.; et al. Receptome profiling identifies KREMEN1 and ASGR1 as alternative functional receptors of SARS-CoV-2. Cell Res. 2022, 32, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Vin, R.; Leutenegger, C.M.; Mittel, L.D.; Divers, T.J. Enteric coronavirus infection in adult horses. Vet. J. 2018, 231, 13–18. [Google Scholar] [CrossRef]
- Winter, C.; Schwegmann-Weßels, C.; Cavanagh, D.; Neumann, U.; Herrler, G. Sialic acid is a receptor determinant for infection of cells by avian Infectious bronchitis virus. J. Gen. Virol. 2006, 87, 1209–1216. [Google Scholar] [CrossRef]
- You, R.; Liu, K.; Huang, M.; Tang, L.; Zhang, X.; Huang, Y.; Zhao, J.; Zhao, Y.; Ye, L.; Zhang, G. Identification and Comparison of the Sialic Acid-Binding Domain Characteristics of Avian Coronavirus Infectious Bronchitis Virus Spike Protein. J. Virol. 2023, 97, e00489-23. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Liu, Y.; Da, S.; Shi, H.; Zhang, X.; Liu, J.; Cao, L.; Zhu, X.; Wang, X. Pathogenicity of porcine deltacoronavirus (PDCoV) strain NH and immunization of pregnant sows with an inactivated PDCoV vaccine protects 5-day-old neonatal piglets from virulent challenge. Transbound. Emerg. Dis. 2020, 67, 572–583. [Google Scholar] [CrossRef]
- Wang, Q.; Vlasova, A.N.; Kenney, S.P.; Saif, L.J. Emerging and re-emerging coronaviruses in pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kenney, S.P.; Wang, Q.; Vlasova, A.; Jung, K.; Saif, L. Naturally occurring animal coronaviruses as models for studying highly pathogenic human coronaviral disease. Vet. Pathol. 2021, 58, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Khamassi Khbou, M.; Daaloul Jedidi, M.; Bouaicha Zaafouri, F.; Benzarti, M.h. Coronaviruses in farm animals: Epidemiology and public health implications. Vet. Med. Sci. 2021, 7, 322–347. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V.; Zakhartchouk, A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet. Microbiol. 2017, 206, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef]
- Li, W.; van Kuppeveld, F.J.; He, Q.; Rottier, P.J.; Bosch, B.-J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016, 226, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Si, F.; Chen, B.; Hu, X.; Yu, R.; Dong, S.; Wang, R.; Li, Z. Porcine Epidemic Diarrhea Virus ORF3 Protein Is Transported through the Exocytic Pathway. J. Virol. 2020, 94, 00808-20. [Google Scholar] [CrossRef]
- Si, F.; Song, S.; Yu, R.; Li, Z.; Wei, W.; Wu, C. Coronavirus accessory protein ORF3 biology and its contribution to viral behavior and pathogenesis. iScience 2023, 26, 106280. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Dong, W.; Gan, S.; Du, J.; Zhou, X.; Fang, W.; Wang, X.; Song, H. Porcine epidemic diarrhea virus activates PERK-ROS axis to benefit its replication in Vero E6 cells. Vet. Res. 2023, 54, 9. [Google Scholar] [CrossRef]
- Wang, H.; Kong, N.; Jiao, Y.; Dong, S.; Sun, D.; Chen, X.; Zheng, H.; Tong, W.; Yu, H.; Yu, L.; et al. EGR1 Suppresses Porcine Epidemic Diarrhea Virus Replication by Regulating IRAV To Degrade Viral Nucleocapsid Protein. J. Virol. 2021, 95, 00645-21. [Google Scholar] [CrossRef]
- Doyle, L.; Hutchings, L. A transmissible gastroenteritis in pigs. J. Am. Vet. Med. Assoc. 1946, 108, 257–259. [Google Scholar] [PubMed]
- Lamphear, B.J.; Jilka, J.M.; Kesl, L.; Welter, M.; Howard, J.A.; Streatfield, S.J. A corn-based delivery system for animal vaccines: An oral transmissible gastroenteritis virus vaccine boosts lactogenic immunity in swine. Vaccine 2004, 22, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Chattha, K.S.; Roth, J.A.; Saif, L.J. Strategies for Design and Application of Enteric Viral Vaccines. Annu. Rev. Anim. Biosci. 2015, 3, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Moxley, R.; Olson, L. Clinical evaluation of transmissible gastroenteritis virus vaccines and vaccination procedures for inducing lactogenic immunity in sows. Am. J. Vet. Res. 1989, 50, 111–118. [Google Scholar]
- Bohl, E.H.; Gupta, R.K.P.; Olquin, M.V.F.; Saif, L.J. Antibody Responses in Serum, Colostrum, and Milk of Swine After Infection or Vaccination with Transmissible Gastroenteritis Virus. Infect. Immun. 1972, 6, 289–301. [Google Scholar] [CrossRef]
- Mora-Díaz, J.C.; Piñeyro, P.E.; Houston, E.; Zimmerman, J.; Giménez-Lirola, L.G. Porcine hemagglutinating encephalomyelitis virus: A review. Front. Vet. Sci. 2019, 6, 53. [Google Scholar] [CrossRef]
- Li, Z.; He, W.; Lan, Y.; Zhao, K.; Lv, X.; Lu, H.; Ding, N.; Zhang, J.; Shi, J.; Shan, C. The evidence of porcine hemagglutinating encephalomyelitis virus induced nonsuppurative encephalitis as the cause of death in piglets. PeerJ 2016, 4, e2443. [Google Scholar] [CrossRef]
- Li, W.; Hulswit, R.J.; Kenney, S.P.; Widjaja, I.; Jung, K.; Alhamo, M.A.; van Dieren, B.; van Kuppeveld, F.J.; Saif, L.J.; Bosch, B.-J. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc. Natl. Acad. Sci. USA 2018, 115, E5135–E5143. [Google Scholar] [CrossRef]
- Ji, W.; Peng, Q.; Fang, X.; Li, Z.; Li, Y.; Xu, C.; Zhao, S.; Li, J.; Chen, R.; Mo, G.; et al. Structures of a deltacoronavirus spike protein bound to porcine and human receptors. Nat. Commun. 2022, 13, 1467. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Zhang, S.; Xu, S.; Wang, J.; Wang, S.; Hu, X.; Zhang, L.; Ren, L.; Zhang, J.; Liu, X.; et al. Porcine Epidemic Diarrhea Virus Replication in Human Intestinal Cells Reveals Potential Susceptibility to Cross-Species Infection. Viruses 2023, 15, 956. [Google Scholar] [CrossRef] [PubMed]
- Alluwaimi, A.M.; Alshubaith, I.H.; Al-Ali, A.M.; Abohelaika, S. The coronaviruses of animals and birds: Their zoonosis, vaccines, and models for SARS-CoV and SARS-CoV2. Front. Vet. Sci. 2020, 7, 582287. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, A.; Martella, V.; Decaro, N.; Tinelli, A.; Camero, M.; Cirone, F.; Elia, G.; Cavalli, A.; Corrente, M.; Greco, G. Genetic diversity of a canine coronavirus detected in pups with diarrhoea in Italy. J. Virol. Methods 2003, 110, 9–17. [Google Scholar] [CrossRef]
- Binn, L.; Lazar, E.; Keenan, K.; Huxsoll, D.; Marchwicki, R.; Strano, A. Recovery and characterization of a coronavirus from military dogs with diarrhea. Proc. Annu. Meet. U. S. Anim. Health Assoc. 1974, 78, 359–366. [Google Scholar]
- Decaro, N.; Buonavoglia, C. Canine coronavirus: Not only an enteric pathogen. Vet. Clin. Small Anim. Pract. 2011, 41, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, C.; Decaro, N.; Martella, V.; Elia, G.; Campolo, M.; Desario, C.; Castagnaro, M.; Tempesta, M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006, 12, 492. [Google Scholar] [CrossRef]
- Decaro, N.; Mari, V.; Campolo, M.; Lorusso, A.; Camero, M.; Elia, G.; Martella, V.; Cordioli, P.; Enjuanes, L.; Buonavoglia, C. Recombinant canine coronaviruses related to transmissible gastroenteritis virus of swine are circulating in dogs. J. Virol. 2009, 83, 1532–1537. [Google Scholar] [CrossRef]
- Lorusso, A.; Desario, C.; Mari, V.; Campolo, M.; Lorusso, E.; Elia, G.; Martella, V.; Buonavoglia, C.; Decaro, N. Molecular characterization of a canine respiratory coronavirus strain detected in Italy. Virus Res. 2009, 141, 96–100. [Google Scholar] [CrossRef]
- Lu, S.; Chen, Y.; Qin, K.; Zhou, J.; Lou, Y.; Tan, W. Genetic and antigenic characterization of recombinant nucleocapsid proteins derived from canine coronavirus and canine respiratory coronavirus in China. Sci. China Life Sci. 2016, 59, 615–621. [Google Scholar] [CrossRef]
- Guy, J.S.; Breslin, J.J.; Breuhaus, B.; Vivrette, S.; Smith, L.G. Characterization of a coronavirus isolated from a diarrheic foal. J. Clin. Microbiol. 2000, 38, 4523–4526. [Google Scholar] [CrossRef] [PubMed]
- Mattei, D.N.; Kopper, J.J.; Sanz, M.G. Equine coronavirus-associated colitis in horses: A retrospective study. J. Equine Vet. Sci. 2020, 87, 102906. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M. Morphogenesis of a virus in cats with experimental feline infectious peritonitis. Virology 1970, 41, 191. [Google Scholar] [CrossRef] [PubMed]
- Tresnan, D.B.; Levis, R.; Holmes, K.V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 1996, 70, 8669–8674. [Google Scholar] [CrossRef] [PubMed]
- Vogel, L.; Van der Lubben, M.; Te Lintelo, E.G.; Bekker, C.P.; Geerts, T.; Schuijff, L.S.; Grinwis, G.C.; Egberink, H.F.; Rottier, P.J. Pathogenic characteristics of persistent feline enteric coronavirus infection in cats. Vet. Res. 2010, 41, 71. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Su, B.-L.; Hsieh, L.-E.; Chueh, L.-L. An outbreak of feline infectious peritonitis in a Taiwanese shelter: Epidemiologic and molecular evidence for horizontal transmission of a novel type II feline coronavirus. Vet. Res. 2013, 44, 57. [Google Scholar] [CrossRef]
- Kipar, A.; Meli, M. Feline infectious peritonitis: Still an enigma? Vet. Pathol. 2014, 51, 505–526. [Google Scholar] [CrossRef]
- Felten, S.; Hartmann, K. Diagnosis of feline infectious peritonitis: A review of the current literature. Viruses 2019, 11, 1068. [Google Scholar] [CrossRef]
- Day, M.J.; Horzinek, M.; Schultz, R.; Squires, R. WSAVA Guidelines for the vaccination of dogs and cats. J. Small Anim. Pract. 2016, 57, E1–E45. [Google Scholar] [CrossRef]
- Benfield, D.; Saif, L. Cell culture propagation of a coronavirus isolated from cows with winter dysentery. J. Clin. Microbiol. 1990, 28, 1454–1457. [Google Scholar] [CrossRef]
- Saif, L.J. Bovine respiratory coronavirus. Vet. Clin. Food Anim. Pract. 2010, 26, 349–364. [Google Scholar] [CrossRef]
- Hasoksuz, M.; Alekseev, K.; Vlasova, A.; Zhang, X.; Spiro, D.; Halpin, R.; Wang, S.; Ghedin, E.; Saif, L.J. Biologic, antigenic, and full-length genomic characterization of a bovine-like coronavirus isolated from a giraffe. J. Virol. 2007, 81, 4981–4990. [Google Scholar] [CrossRef]
- Ismail, M.; Cho, K.; Ward, L.; Saif, L.; Saif, Y. Experimental bovine coronavirus in turkey poults and young chickens. Avian Dis. 2001, 45, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kaneshima, T.; Hohdatsu, T.; Hagino, R.; Hosoya, S.; Nojiri, Y.; Murata, M.; Takano, T.; Tanabe, M.; Tsunemitsu, H.; Koyama, H. The infectivity and pathogenicity of a group 2 bovine coronavirus in pups. J. Vet. Med. Sci. 2007, 69, 301–303. [Google Scholar] [CrossRef][Green Version]
- Nemoto, M.; Kanno, T.; Bannai, H.; Tsujimura, K.; Yamanaka, T.; Kokado, H. Antibody response to equine coronavirus in horses inoculated with a bovine coronavirus vaccine. J. Vet. Med. Sci. 2017, 79, 1889–1891. [Google Scholar] [CrossRef]
- Franzo, G.; Legnardi, M.; Tucciarone, C.M.; Drigo, M.; Martini, M.; Cecchinato, M. Evolution of infectious bronchitis virus in the field after homologous vaccination introduction. Vet. Res. 2019, 50, 92. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Zhao, J.; Zhong, Q.; Jin, J.-h.; Zhang, G.-z. Evolution of infectious bronchitis virus in China over the past two decades. J. Gen. Virol. 2016, 97, 1566. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Chen, T.C.; Lin, S.Y.; Mase, M.; Murakami, S.; Horimoto, T.; Chen, H.W. Emerging lethal infectious bronchitis coronavirus variants with multiorgan tropism. Transbound. Emerg. Dis. 2020, 67, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef]
- Shahwan, K.; Hesse, M.; Mork, A.-K.; Herrler, G.; Winter, C. Sialic acid binding properties of soluble coronavirus spike (S1) proteins: Differences between infectious bronchitis virus and transmissible gastroenteritis virus. Viruses 2013, 5, 1924–1933. [Google Scholar] [CrossRef]
- De Wit, J.; Cook, J.K. Spotlight on avian coronaviruses. Avian Pathol. 2020, 49, 313–316. [Google Scholar] [CrossRef]
- Liais, E.; Croville, G.; Mariette, J.; Delverdier, M.; Lucas, M.-N.; Klopp, C.; Lluch, J.; Donnadieu, C.; Guy, J.; Corrand, L.; et al. Novel Avian Coronavirus and Fulminating Disease in Guinea Fowl, France. Emerg. Infect. Dis. J. 2014, 20, 105–108. [Google Scholar] [CrossRef]
- Baron, M.D.; Iqbal, M.; Nair, V. Recent advances in viral vectors in veterinary vaccinology. Curr. Opin. Virol. 2018, 29, 1–7. [Google Scholar] [CrossRef]
- Laconi, A.; Weerts, E.; Bloodgood, J.; Marrero, J.D.; Berends, A.; Cocciolo, G.; de Wit, J.; Verheije, M. Attenuated live infectious bronchitis virus QX vaccine disseminates slowly to target organs distant from the site of inoculation. Vaccine 2020, 38, 1486–1493. [Google Scholar] [CrossRef]
- Masoudi, S.; Pishraft-Sabet, L.; Shahsavandi, S. Immunogenicity and efficacy of live infectious bronchitis 793/B. 08IR vaccine in SPF chickens. Arch. Razi Inst. 2020, 75, 23. [Google Scholar]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses: Methods and Protocols; Humana Press: New York, NY, USA, 2015; pp. 1–23. [Google Scholar]
- Jaimes, J.A.; Whittaker, G.R. Feline coronavirus: Insights into viral pathogenesis based on the spike protein structure and function. Virology 2018, 517, 108–121. [Google Scholar] [CrossRef]
- Bredenbeek, P.J.; Pachuk, C.J.; Noten, A.F.; Charité, J.; Luytjes, W.; Weiss, S.R.; Spaan, W.J. The primary structure and expression of the second open reading frame of the polymerase gene of the coronavirus MHV-A59; a highly conserved polymerase is expressed by an efficient ribosomal frameshifting mechanism. Nucleic Acids Res. 1990, 18, 1825–1832. [Google Scholar] [CrossRef]
- Lang, Y.; Li, W.; Li, Z.; Koerhuis, D.; Van Den Burg, A.C.; Rozemuller, E.; Bosch, B.-J.; Van Kuppeveld, F.J.; Boons, G.-J.; Huizinga, E.G. Coronavirus hemagglutinin-esterase and spike proteins coevolve for functional balance and optimal virion avidity. Proc. Natl. Acad. Sci. USA 2020, 117, 25759–25770. [Google Scholar] [CrossRef]
- Yan, W.; Zheng, Y.; Zeng, X.; He, B.; Cheng, W. Structural biology of SARS-CoV-2: Open the door for novel therapies. Signal Transduct. Target. Ther. 2022, 7, 26. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Wang, X.; Pascal, K.E.; Tomkins-Tinch, C.; Nyalile, T.P.; Wang, Y.; Baum, A.; Diehl, W.E.; Dauphin, A.; Carbone, C. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell 2020, 183, 739–751.e738. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.; De Marco, A.; Lempp, F.A.; Tortorici, M.A.; Pinto, D.; Walls, A.C.; Beltramello, M.; Chen, A.; Liu, Z.; Zatta, F. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell 2021, 184, 2332–2347.e2316. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Gorkhali, R.; Koirala, P.; Rijal, S.; Mainali, A.; Baral, A.; Bhattarai, H.K. Structure and function of major SARS-CoV-2 and SARS-CoV proteins. Bioinform. Biol. Insights 2021, 15, 11779322211025876. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, Y.; Zhou, H.; Sun, C.; Zhang, S. The SARS-CoV-2 nucleocapsid protein: Its role in the viral life cycle, structure and functions, and use as a potential target in the development of vaccines and diagnostics. Virol. J. 2023, 20, 6. [Google Scholar] [CrossRef]
- Pan, P.; Ge, W.; Lei, Z.; Luo, W.; Liu, Y.; Guan, Z.; Chen, L.; Yu, Z.; Shen, M.; Hu, D.; et al. SARS-CoV-2 N protein enhances the anti-apoptotic activity of MCL-1 to promote viral replication. Signal Transduct. Target. Ther. 2023, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nomura, N.; Muramoto, Y.; Ekimoto, T.; Uemura, T.; Liu, K.; Yui, M.; Kono, N.; Aoki, J.; Ikeguchi, M. Structure of SARS-CoV-2 membrane protein essential for virus assembly. Nat. Commun. 2022, 13, 4399. [Google Scholar] [CrossRef]
- Mahtarin, R.; Islam, S.; Islam, M.J.; Ullah, M.O.; Ali, M.A.; Halim, M.A. Structure and dynamics of membrane protein in SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 4725–4738. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, R.; Lee, I.; Zhang, W.; Sun, J.; Wang, W.; Meng, X. Characterization of the SARS-CoV-2 E protein: Sequence, structure, viroporin, and inhibitors. Protein Sci. 2021, 30, 1114–1130. [Google Scholar] [CrossRef]
- Tomar, P.P.S.; Arkin, I.T. SARS-CoV-2 E protein is a potential ion channel that can be inhibited by Gliclazide and Memantine. Biochem. Biophys. Res. Commun. 2020, 530, 10–14. [Google Scholar] [CrossRef]
- Fang, P.; Fang, L.; Zhang, H.; Xia, S.; Xiao, S. Functions of Coronavirus Accessory Proteins: Overview of the State of the Art. Viruses 2021, 13, 1139. [Google Scholar] [CrossRef]
- Hassan, S.S.; Choudhury, P.P.; Dayhoff, G.W.; Aljabali, A.A.A.; Uhal, B.D.; Lundstrom, K.; Rezaei, N.; Pizzol, D.; Adadi, P.; Lal, A.; et al. The importance of accessory protein variants in the pathogenicity of SARS-CoV-2. Arch. Biochem. Biophys. 2022, 717, 109124. [Google Scholar] [CrossRef] [PubMed]
- Redondo, N.; Zaldívar-López, S.; Garrido, J.J.; Montoya, M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns. Front. Immunol. 2021, 12, 708264. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, X.; Zhu, Y.; Wang, W.; Wang, Y.; Hu, G.; Liu, C.; Li, J.; Ren, S.; Xiao, M.Z.X.; et al. ORF3a-Mediated Incomplete Autophagy Facilitates Severe Acute Respiratory Syndrome Coronavirus-2 Replication. Front. Cell Dev. Biol. 2021, 9, 716208. [Google Scholar] [CrossRef]
- Si, F.; Hu, X.; Wang, C.; Chen, B.; Wang, R.; Dong, S.; Yu, R.; Li, Z. Porcine Epidemic Diarrhea Virus (PEDV) ORF3 Enhances Viral Proliferation by Inhibiting Apoptosis of Infected Cells. Viruses 2020, 12, 214. [Google Scholar] [CrossRef]
- Piñeyro, P.E.; Lozada, M.I.; Alarcón, L.V.; Sanguinetti, R.; Cappuccio, J.A.; Pérez, E.M.; Vannucci, F.; Armocida, A.; Madson, D.M.; Perfumo, C.J. First retrospective studies with etiological confirmation of porcine transmissible gastroenteritis virus infection in Argentina. BMC Vet. Res. 2018, 14, 292. [Google Scholar] [CrossRef]
- Huang, Y.W.; Dickerman, A.W.; Pineyro, P.; Li, L.; Fang, L.; Kiehne, R.; Opriessnig, T.; Meng, X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. MBio 2013, 4, e00737-00713. [Google Scholar] [CrossRef]
- Gong, L.; Li, J.; Zhou, Q.; Xu, Z.; Chen, L.; Zhang, Y.; Xue, C.; Wen, Z.; Cao, Y. A new bat-HKU2–like coronavirus in swine, China, 2017. Emerg. Infect. Dis. 2017, 23, 1607. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tian, X.; Qin, P.; Wang, B.; Zhao, P.; Yang, Y.-L.; Wang, L.; Wang, D.; Song, Y.; Zhang, X. Discovery of a novel swine enteric alphacoronavirus (SeACoV) in southern China. Vet. Microbiol. 2017, 211, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Fan, H.; Lan, T.; Yang, X.-L.; Shi, W.-F.; Zhang, W.; Zhu, Y.; Zhang, Y.-W.; Xie, Q.-M.; Mani, S. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 2018, 556, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Sparrer McKenzie, N.; Hodges Natasha, F.; Sherman, T.; VandeWoude, S.; Bosco-Lauth Angela, M.; Mayo Christie, E. Role of Spillover and Spillback in SARS-CoV-2 Transmission and the Importance of One Health in Understanding the Dynamics of the COVID-19 Pandemic. J. Clin. Microbiol. 2023, 61, e01610–e01622. [Google Scholar]
- Pratelli, A.; Tinelli, A.; Decaro, N.; Martella, V.; Camero, M.; Tempesta, M.; Martini, M.; Carmichael, L.E.; Buonavoglia, C. Safety and efficacy of a modified-live canine coronavirus vaccine in dogs. Vet. Microbiol. 2004, 99, 43–49. [Google Scholar] [CrossRef]
- Fehr, D.; Holznagel, E.; Bolla, S.; Hauser, B.; Herrewegh, A.A.; Horzinek, M.C.; Lutz, H. Placebo-controlled evaluation of a modified life virus vaccine against feline infectious peritonitis: Safety and efficacy under field conditions. Vaccine 1997, 15, 1101–1109. [Google Scholar] [CrossRef]
- Haijema, B.J.; Volders, H.; Rottier, P.J. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis. J. Virol. 2004, 78, 3863–3871. [Google Scholar] [CrossRef]
- Glansbeek, H.L.; Haagmans, B.L.; te Lintelo, E.G.; Egberink, H.F.; Duquesne, V.; Aubert, A.; Horzinek, M.C.; Rottier, P.J.M. Adverse effects of feline IL-12 during DNA vaccination against feline infectious peritonitis virus. J. Gen. Virol. 2002, 83, 1–10. [Google Scholar] [CrossRef]
- De Arriba, M.; Carvajal, A.; Pozo, J.; Rubio, P. Mucosal and systemic isotype-specific antibody responses and protection in conventional pigs exposed to virulent or attenuated porcine epidemic diarrhoea virus. Vet. Immunol. Immunopathol. 2002, 85, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takeyama, N.; Katsumata, A.; Tuchiya, K.; Kodama, T.; Kusanagi, K.-i. Mutations in the spike gene of porcine epidemic diarrhea virus associated with growth adaptation in vitro and attenuation of virulence in vivo. Virus Genes 2011, 43, 72–78. [Google Scholar] [CrossRef]
- Kweon, C.H.; Kwon, B.J.; Lee, J.G.; Kwon, G.O.; Kang, Y.B. Derivation of attenuated porcine epidemic diarrhea virus (PEDV) as vaccine candidate. Vaccine 1999, 17, 2546–2553. [Google Scholar] [CrossRef]
- Song, D.S.; Oh, J.S.; Kang, B.K.; Yang, J.S.; Moon, H.J.; Yoo, H.S.; Jang, Y.S.; Park, B.K. Oral efficacy of Vero cell attenuated porcine epidemic diarrhea virus DR13 strain. Res. Vet. Sci. 2007, 82, 134–140. [Google Scholar] [CrossRef]
- Baek, P.-S.; Choi, H.-W.; Lee, S.; Yoon, I.-J.; Lee, Y.J.; Lee, D.S.; Lee, S.; Lee, C. Efficacy of an inactivated genotype 2b porcine epidemic diarrhea virus vaccine in neonatal piglets. Vet. Immunol. Immunopathol. 2016, 174, 45–49. [Google Scholar] [CrossRef]
- Singh, G.; Singh, P.; Pillatzki, A.; Nelson, E.; Webb, B.; Dillberger-Lawson, S.; Ramamoorthy, S. A minimally replicative vaccine protects vaccinated piglets against challenge with the porcine epidemic diarrhea virus. Front. Vet. Sci. 2019, 6, 347. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.D.; Kim, Y.; Yang, M.; Vannucci, F.; Molitor, T.; Torremorell, M.; Cheeran, M.C.-J. Immune responses to porcine epidemic diarrhea virus (PEDV) in swine and protection against subsequent infection. PLoS ONE 2020, 15, e0231723. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, L.; Fan, B.; Sun, B.; Zhou, J.; Guo, R.; Yu, Z.; Shi, D.; He, K.; Li, B. A flagellin-adjuvanted inactivated porcine epidemic diarrhea virus (PEDV) vaccine provides enhanced immune protection against PEDV challenge in piglets. Arch. Virol. 2020, 165, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Oroku, K.; Ohshima, Y.; Furuya, Y.; Sasakawa, C. Efficacy of genogroup 1 based porcine epidemic diarrhea live vaccine against genogroup 2 field strain in Japan. Virol. J. 2018, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Yugo, D.M.; Heffron, C.L.; Rogers, A.J.; Sooryanarain, H.; LeRoith, T.; Overend, C.; Cao, D.; Meng, X.J. Vaccination of sows with a dendritic cell-targeted porcine epidemic diarrhea virus S1 protein-based candidate vaccine reduced viral shedding but exacerbated gross pathological lesions in suckling neonatal piglets. J. Gen. Virol. 2018, 99, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Hain, K.S.; Joshi, L.R.; Okda, F.; Nelson, J.; Singrey, A.; Lawson, S.; Martins, M.; Pillatzki, A.; Kutish, G.F.; Nelson, E.A.; et al. Immunogenicity of a recombinant parapoxvirus expressing the spike protein of Porcine epidemic diarrhea virus. J. Gen. Virol. 2016, 97, 2719–2731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fan, B.; Song, X.; Gao, J.; Guo, R.; Yi, C.; He, Z.; Hu, H.; Jiang, J.; Zhao, L.; et al. PEDV-spike-protein-expressing mRNA vaccine protects piglets against PEDV challenge. mBio 2024, 15, e02958-02923. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Hsu, W.-T.; Chao, Y.-C.; Chang, H.-W. Display of porcine epidemic diarrhea virus spike protein on baculovirus to improve immunogenicity and protective efficacy. Viruses 2018, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, Z.; Wu, T.; Peng, O.; Huang, L.; Zhang, Y.; Xue, C.; Wen, Z.; Zhou, Q.; Cao, Y. A flagellin-adjuvanted PED subunit vaccine improved protective efficiency against PEDV variant challenge in pigs. Vaccine 2018, 36, 4228–4235. [Google Scholar] [CrossRef]
- Pascual-Iglesias, A.; Sanchez, C.M.; Penzes, Z.; Sola, I.; Enjuanes, L.; Zuñiga, S. Recombinant Chimeric Transmissible Gastroenteritis Virus (TGEV)—Porcine Epidemic Diarrhea Virus (PEDV) Virus Provides Protection against Virulent PEDV. Viruses 2019, 11, 682. [Google Scholar] [CrossRef]
- Decaro, N.; Campolo, M.; Mari, V.; Desario, C.; Colaianni, M.L.; Di Trani, L.; Cordioli, P.; Buonavoglia, C. A candidate modified-live bovine coronavirus vaccine: Safety and immunogenicity evaluation. New Microbiol. 2009, 32, 109. [Google Scholar]
- Koo, M.; Bendahmane, M.; Lettieri, G.A.; Paoletti, A.D.; Lane, T.E.; Fitchen, J.H.; Buchmeier, M.J.; Beachy, R.N. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc. Natl. Acad. Sci. USA 1999, 96, 7774–7779. [Google Scholar] [CrossRef]
- Lee, H.J.; Youn, H.N.; Kwon, J.S.; Lee, Y.J.; Kim, J.H.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Characterization of a novel live attenuated infectious bronchitis virus vaccine candidate derived from a Korean nephropathogenic strain. Vaccine 2010, 28, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, S.S.; Kingstad-Bakke, B.A.; Wu, C.-W.; Phanse, Y.; Osorio, J.E.; Talaat, A.M. A DNA Prime and MVA Boost Strategy Provides a Robust Immunity against Infectious Bronchitis Virus in Chickens. Vaccines 2023, 11, 302. [Google Scholar] [CrossRef]
- Zhai, K.; Zhang, Z.; Liu, X.; Lv, J.; Zhang, L.; Li, J.; Ma, Z.; Wang, Y.; Guo, H.; Zhang, Y.; et al. Mucosal immune responses induced by oral administration of recombinant Lactococcus lactis expressing the S1 protein of PDCoV. Virology 2023, 578, 180–189. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Peng, Q.; Li, J.; Huang, J.; Cai, X.; Li, S.; Zhang, B.; Xiao, L.; Gao, J.; Wang, C.; et al. Attenuation of a Highly Pathogenic Porcine Deltacoronavirus Strain CZ2020 by a Serial Passage In Vitro. Transbound. Emerg. Dis. 2023, 2023, 2830485. [Google Scholar] [CrossRef]
- Simões, R.S.d.Q.; Rodríguez-Lázaro, D. Classical and next-generation vaccine platforms to SARS-CoV-2: Biotechnological strategies and genomic variants. Int. J. Environ. Res. Public. Health 2022, 19, 2392. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Huang, Z.; Elankumaran, S.; Yunus, A.S.; Samal, S.K. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J. Virol. 2004, 78, 10054–10063. [Google Scholar] [CrossRef]
- Park, M.-S.; Steel, J.; García-Sastre, A.; Swayne, D.; Palese, P. Engineered viral vaccine constructs with dual specificity: Avian influenza and Newcastle disease. Proc. Natl. Acad. Sci. USA 2006, 103, 8203–8208. [Google Scholar] [CrossRef]
- Tang, N.; Zhang, Y.; Sadigh, Y.; Moffat, K.; Shen, Z.; Nair, V.; Yao, Y. Generation of a triple insert live avian herpesvirus vectored vaccine using CRISPR/Cas9-based gene editing. Vaccines 2020, 8, 97. [Google Scholar] [CrossRef]
- Kim, S.-H.; Samal, S.K. Reverse Genetics for Newcastle Disease Virus as a Vaccine Vector. Curr. Protoc. Microbiol. 2018, 48, 18.15.11–18.15.12. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Review nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yang, Z.-y.; Kong, W.-p.; Huang, Y.; Roberts, A.; Murphy, B.R.; Subbarao, K.; Nabel, G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 2004, 428, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Xia, F.; Chen, H.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J.; Luo, M. A guide to nucleic acid vaccines in the prevention and treatment of infectious diseases and cancers: From basic principles to current applications. Front. Cell Dev. Biol. 2021, 9, 830. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, F.; Yang, D.; Li, Q.; Yan, L.; Ou, J.; Zhang, L.; Liu, Y.; Zhan, Q.; Li, R. Rice-produced classical swine fever virus glycoprotein E2 with herringbone-dimer design to enhance immune responses. Plant Biotechnol. J. 2023, 21, 2546–2559. [Google Scholar] [CrossRef]
- Ma, F.; Xu, Q.; Wang, A.; Yang, D.; Li, Q.; Guo, J.; Zhang, L.; Ou, J.; Li, R.; Yin, H. A universal design of restructured dimer antigens: Development of a superior vaccine against the paramyxovirus in transgenic rice. Proc. Natl. Acad. Sci. USA 2024, 121, e2305745121. [Google Scholar] [CrossRef]
- Thakor, J.C.; Dinesh, M.; Manikandan, R.; Bindu, S.; Sahoo, M.; Sahoo, D.; Dhawan, M.; Pandey, M.K.; Tiwari, R.; Emran, T.B. Swine coronaviruses (SCoVs) and their emerging threats to swine population, inter-species transmission, exploring the susceptibility of pigs for SARS-CoV-2 and zoonotic concerns. Vet. Q. 2022, 42, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, M.; Zhou, J.; Feng, L. Adaptation of porcine epidemic diarrhea virus to Vero cells and evaluation of the inactivated vaccine against porcine epidemic diarrhea virus. Chin. Anim. Infect. Dis 1994, 2, 15–18. [Google Scholar]
- Ma, S.; Wang, M.; Feng, L.; Li, W. Development of bi-combined inactivated vaccine against transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Chin. Anim. Infect. Dis 1995, 17, 23–27. [Google Scholar]
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012, 18, 161–163. [Google Scholar] [CrossRef]
- Wang, D.; Fang, L.; Xiao, S. Porcine epidemic diarrhea in China. Virus Res. 2016, 226, 7–13. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Q. Prevention and Control of Porcine Epidemic Diarrhea: The Development of Recombination-Resistant Live Attenuated Vaccines. Viruses 2022, 14, 1317. [Google Scholar] [CrossRef]
- Hosseini, Z.S.; Amani, J.; Baghbani Arani, F.; Nazarian, S.; Motamedi, M.J.; Shafighian, F.; Lee, S.H.; Yang, D.-K.; Kim, H.-H.; Cho, I.-S. Efficacy of inactivated variant porcine epidemic diarrhea virus vaccines in growing pigs. Clin. Exp. Vaccine Res. 2018, 7, 61–69. [Google Scholar]
- Opriessnig, T.; Gerber, P.F.; Shen, H.; de Castro, A.M.M.G.; Zhang, J.; Chen, Q.; Halbur, P. Evaluation of the efficacy of a commercial inactivated genogroup 2b-based porcine epidemic diarrhea virus (PEDV) vaccine and experimental live genogroup 1b exposure against 2b challenge. Vet. Res. 2017, 48, 69. [Google Scholar] [CrossRef]
- Hou, Y.; Ke, H.; Kim, J.; Yoo, D.; Su, Y.; Boley, P.; Chepngeno, J.; Vlasova, A.N.; Saif, L.J.; Wang, Q. Engineering a live attenuated porcine epidemic diarrhea virus vaccine candidate via inactivation of the viral 2’-O-methyltransferase and the endocytosis signal of the spike protein. J. Virol. 2019, 93, e00406–e00419. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Ghimire, S.; Hou, Y.; Boley, P.; Langel, S.N.; Vlasova, A.N.; Saif, L.J.; Wang, Q. Pathogenicity and immunogenicity of attenuated porcine epidemic diarrhea virus PC22A strain in conventional weaned pigs. BMC Vet. Res. 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Q. Emerging highly virulent porcine epidemic diarrhea virus: Molecular mechanisms of attenuation and rational design of live attenuated vaccines. Int. J. Mol. Sci. 2019, 20, 5478. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Paim, F.C.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus Res. 2016, 226, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Lee, D.; Lee, C. Development of a Next-Generation Vaccine Platform for Porcine Epidemic Diarrhea Virus Using a Reverse Genetics System. Viruses 2022, 14, 2319. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Liu, M.; Yang, S.; Xu, J.; Hou, Y.J.; Liu, D.; Tang, Q.; Zhu, H.; Wang, Q. A recombination-resistant genome for live attenuated and stable PEDV vaccines by engineering the transcriptional regulatory sequences. J. Virol. 2023, e01193-01123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lin, S.; Li, J.; Deng, S.; Zhang, J.; Wang, S. Modulation of Innate Antiviral Immune Response by Porcine Enteric Coronavirus. Front. Microbiol. 2022, 13, 845137. [Google Scholar] [CrossRef] [PubMed]
- Bijlenga, G.; Cook, J.K.; Gelb, J., Jr.; Wit, J.D. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: A review. Avian Pathol. 2004, 33, 550–557. [Google Scholar] [CrossRef]
- Guzmán, M.; Hidalgo, H. Live Attenuated Infectious Bronchitis Virus Vaccines in Poultry: Modifying Local Viral Populations Dynamics. Animals 2020, 10, 2058. [Google Scholar] [CrossRef]
- van Beurden, S.J.; Berends, A.J.; Krämer-Kühl, A.; Spekreijse, D.; Chenard, G.; Philipp, H.-C.; Mundt, E.; Rottier, P.J.; Verheije, M.H. Recombinant live attenuated avian coronavirus vaccines with deletions in the accessory genes 3ab and/or 5ab protect against infectious bronchitis in chickens. Vaccine 2018, 36, 1085–1092. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Clark, R.; Cheng, S.; Jordan, B.J. Protection following simultaneous vaccination with three or four different attenuated live vaccine types against infectious bronchitis virus. Avian Pathol. 2020, 49, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J. Overview of the development of a modified live temperature-sensitive FIP virus vaccine. Feline Pract. 1995, 23, 62–66. [Google Scholar]
- Balint, A.; Farsang, A.; Szeredi, L.; Zadori, Z.; Belak, S. Recombinant feline coronaviruses as vaccine candidates confer protection in SPF but not in conventional cats. Vet. Microbiol. 2014, 169, 154–162. [Google Scholar] [CrossRef]
- Tizard, I.R. Vaccination against coronaviruses in domestic animals. Vaccine 2020, 38, 5123–5130. [Google Scholar] [CrossRef]
- Addie, D.D. Feline infectious peritonitis: Answers to frequently asked questions concerning FIP and coronavirus. Vet. Nurs. J. 2019, 34, 201–206. [Google Scholar] [CrossRef]
- Scott, F.W. Evaluation of risks and benefits associated with vaccination against coronavirus infections in cats. Adv. Vet. Med. 1999, 41, 347. [Google Scholar]
- Ithinji, D.G.; Buchholz, D.W.; Ezzatpour, S.; Monreal, I.A.; Cong, Y.; Sahler, J.; Bangar, A.S.; Imbiakha, B.; Upadhye, V.; Liang, J. Multivalent viral particles elicit safe and efficient immunoprotection against Nipah Hendra and Ebola viruses. npj Vaccines 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, M.H.; Schmitz, M.; Meessen, J.M.H.; Wouters, P.A.W.M.; Vrijenhoek, M.P.; Makoschey, B. Vaccination of calves at day of birth with attenuated vaccines against bovine respiratory syncytial virus, bovine parainfluenza type 3 virus and respiratory bovine coronavirus. Vet. Vaccine 2023, 2, 100014. [Google Scholar] [CrossRef]
- Pratelli, A.; Tinelli, A.; Decaro, N.; Cirone, F.; Elia, G.; Roperto, S.; Tempesta, M.; Buonavoglia, C. Efficacy of an inactivated canine coronavirus vaccine in pups. New Microbiol. 2003, 26, 151–155. [Google Scholar]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.-J.; Jiang, S. The spike protein of SARS-CoV—A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Promkuntod, N.; Van Eijndhoven, R.; De Vrieze, G.; Gröne, A.; Verheije, M. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology 2014, 448, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhu, Y.; Qiao, W.-T.; Lu, W.-H.; Zhang, Y.-Q.; Li, J.-L. Based on the Results of PEDV Phylogenetic Analysis of the Most Recent Isolates in China, the Occurrence of Further Mutations in the Antigenic Site S1° and COE of the S Protein Which Is the Target Protein of the Vaccine. Transbound. Emerg. Dis. 2023, 2023, 1227110. [Google Scholar] [CrossRef]
- McBride, R.; Van Zyl, M.; Fielding, B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity 2020, 53, 248–263. [Google Scholar] [CrossRef]
- Feng, W.; Xiang, Y.; Wu, L.; Chen, Z.; Li, Q.; Chen, J.; Guo, Y.; Xia, D.; Chen, N.; Zhang, L. Nucleocapsid protein of SARS-CoV-2 is a potential target for developing new generation of vaccine. J. Clin. Lab. Anal. 2022, 36, e24479. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, J.H.; Hung, C.-F.; Peng, S.; Roden, R.; Wang, M.-C.; Viscidi, R.; Tsai, Y.-C.; He, L.; Chen, P.-J. Generation and characterization of DNA vaccines targeting the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J. Virol. 2004, 78, 4638–4645. [Google Scholar] [CrossRef]
- Collisson, E.W.; Pei, J.; Dzielawa, J.; Seo, S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000, 24, 187–200. [Google Scholar] [CrossRef]
- Seo, S.H.; Pei, J.; Briles, W.E.; Dzielawa, J.; Collisson, E.W. Adoptive transfer of infectious bronchitis virus primed αβ T cells bearing CD8 antigen protects chicks from acute infection. Virology 2000, 269, 183–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakanaga, K.; Yamanouchi, K.; Fujiwara, K. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J. Virol. 1986, 59, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Neuman, B.W.; Kiss, G.; Kunding, A.H.; Bhella, D.; Baksh, M.F.; Connelly, S.; Droese, B.; Klaus, J.P.; Makino, S.; Sawicki, S.G. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011, 174, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y.; Qi, J.; Chu, F.; Wu, H.; Gao, F.; Li, T.; Yan, J.; Gao, G.F. The membrane protein of severe acute respiratory syndrome coronavirus acts as a dominant immunogen revealed by a clustering region of novel functionally and structurally defined cytotoxic T-lymphocyte epitopes. J. Infect. Dis. 2010, 202, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Liu, Y.; Han, X.; Xu, Y.; Jiang, F.; Wu, D.; Kong, X.; Bartlam, M.; Rao, Z. Protective humoral responses to severe acute respiratory syndrome-associated coronavirus: Implications for the design of an effective protein-based vaccine. J. Gen. Virol. 2004, 85, 3109–3113. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ruch, T.R.; Machamer, C.E. The coronavirus E protein: Assembly and beyond. Viruses 2012, 4, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Banerjee, A.; Ray, S. Development of new vaccine target against SARS-CoV2 using envelope (E) protein: An evolutionary, molecular modeling and docking based study. Int. J. Biol. Macromol. 2021, 172, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fang, Y.; Xu, T.; Ni, W.J.; Shen, A.Z.; Meng, X.M. Potential therapeutic targets and promising drugs for combating SARS-CoV-2. Br. J. Pharmacol. 2020, 177, 3147–3161. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, M.; Chen, C.Z.; Guo, H.; Shen, M.; Hu, X.; Shinn, P.; Klumpp-Thomas, C.; Michael, S.G.; Zheng, W. Identification of SARS-CoV-2 3CL Protease Inhibitors by a Quantitative High-Throughput Screening. ACS Pharmacol. Transl. Sci. 2020, 3, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.; Ho, J.; Wills, S.; Mawri, A.; Lawson, L.; Ebert, M.C.C.J.C.; Fortin, G.M.; Rayalam, S.; Taval, S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 2021, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Saha, A.; Alshammari, A.; Alharbi, M.; Saikumar, G.; Pal, S.; Dhama, K.; Lee, S.-S. Revealing the structural and molecular interaction landscape of the favipiravir-RTP and SARS-CoV-2 RdRp complex through integrative bioinformatics: Insights for developing potent drugs targeting SARS-CoV-2 and other viruses. J. Infect. Public Health 2023, 16, 1048–1056. [Google Scholar] [CrossRef]
- Muhammed, Y.; Yusuf Nadabo, A.; Pius, M.; Sani, B.; Usman, J.; Anka Garba, N.; Mohammed Sani, J.; Opeyemi Olayanju, B.; Zeal Bala, S.; Garba Abdullahi, M.; et al. SARS-CoV-2 spike protein and RNA dependent RNA polymerase as targets for drug and vaccine development: A review. Biosaf. Health 2021, 3, 249–263. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, C.Z.; Gorshkov, K.; Xu, M.; Lo, D.C.; Zheng, W. RNA-Dependent RNA Polymerase as a Target for COVID-19 Drug Discovery. Slas Discov. Adv. Sci. Drug Discov. 2020, 25, 1141–1151. [Google Scholar] [CrossRef]
- Rajpoot, S.; Alagumuthu, M.; Baig, M.S. Dual targeting of 3CLpro and PLpro of SARS-CoV-2: A novel structure-based design approach to treat COVID-19. Curr. Res. Struct. Biol. 2021, 3, 9–18. [Google Scholar] [CrossRef]
- Mouffouk, C.; Mouffouk, S.; Mouffouk, S.; Hambaba, L.; Haba, H. Flavonols as potential antiviral drugs targeting SARS-CoV-2 proteases (3CLpro and PLpro), spike protein, RNA-dependent RNA polymerase (RdRp) and angiotensin-converting enzyme II receptor (ACE2). Eur. J. Pharmacol. 2021, 891, 173759. [Google Scholar] [CrossRef]
- Ali, Z.; Cardoza, J.V.; Basak, S.; Narsaria, U.; Singh, V.P.; Isaac, S.P.; França, T.C.C.; LaPlante, S.R.; George, S.S. Computational design of candidate multi-epitope vaccine against SARS-CoV-2 targeting structural (S and N) and non-structural (NSP3 and NSP12) proteins. J. Biomol. Struct. Dyn. 2023, 41, 13348–13367. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 5, 237. [Google Scholar] [CrossRef]
- Martinez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Resendiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 vaccines based on the spike glycoprotein and implications of new viral variants. Front. Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef]
- Ong, E.; Wong, M.U.; Huffman, A.; He, Y. COVID-19 coronavirus vaccine design using reverse vaccinology and machine learning. Front. Immunol. 2020, 11, 1581. [Google Scholar] [CrossRef]
- Singh, P.K.; Kulsum, U.; Rufai, S.B.; Mudliar, S.R.; Singh, S. Mutations in SARS-CoV-2 leading to antigenic variations in spike protein: A challenge in vaccine development. J. Lab. Physicians 2020, 12, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Mehta, D.; Dumka, S.; Chauhan, A.S.; Kumar, S. Quasispecies Nature of RNA Viruses: Lessons from the Past. Vaccines 2023, 11, 308. [Google Scholar] [CrossRef]
- Novella, I.S.; Domingo, E.; Holland, J.J. Rapid viral quasispecies evolution: Implications for vaccine and drug strategies. Mol. Med. Today 1995, 1, 248–253. [Google Scholar] [CrossRef]
- Andino, R.; Domingo, E. Viral quasispecies. Virology 2015, 479, 46–51. [Google Scholar] [CrossRef]
- Stevenson-Leggett, P.; Keep, S.; Bickerton, E. Treatment with exogenous trypsin expands in vitro cellular tropism of the avian coronavirus infectious bronchitis virus. Viruses 2020, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.M.; Kari, C.; Fragoso, R.C.; Rodeck, U.; Williams, J.C. Design and development of masked therapeutic antibodies to limit off-target effects: Application to anti-EGFR antibodies. Cancer Biol. Ther. 2009, 8, 2147–2152. [Google Scholar] [CrossRef]
- Tirado, S.M.C.; Yoon, K.-J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003, 16, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Zhao, X.; Yuan, M.; Zhang, K.; Dai, J.; Guan, X.; Qiu, H.-J.; Li, Y. Antibody-Dependent Enhancement:″Evil ″Antibodies Favorable for Viral Infections. Viruses 2022, 14, 1739. [Google Scholar] [CrossRef] [PubMed]
- Arvin, A.M.; Fink, K.; Schmid, M.A.; Cathcart, A.; Spreafico, R.; Havenar-Daughton, C.; Lanzavecchia, A.; Corti, D.; Virgin, H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020, 584, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020, 5, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 2020, 94, e02015–e02019. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-F.; Tseng, S.-P.; Yen, C.-H.; Yang, J.-Y.; Tsao, C.-H.; Shen, C.-W.; Chen, K.-H.; Liu, F.-T.; Liu, W.-T.; Chen, Y.-M.A. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 2014, 451, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Furukawa, T.; Kotake, M.; Takano, T.; Motokawa, K.; Gemma, T.; Watanabe, R.; Arai, S.; Hohdatsu, T. Screening and identification of T helper 1 and linear immunodominant antibody-binding epitopes in the spike 2 domain and the nucleocapsid protein of feline infectious peritonitis virus. Vaccine 2011, 29, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Gartlan, C.; Tipton, T.; Salguero, F.J.; Sattentau, Q.; Gorringe, A.; Carroll, M.W. Vaccine-associated enhanced disease and pathogenic human coronaviruses. Front. Immunol. 2022, 13, 882972. [Google Scholar] [CrossRef]
- Wang, C.-y.; Luo, Z.-b.; Shao, G.-q.; Hou, B. Genetic and pathogenic characteristics of a novel infectious bronchitis virus strain in genogroup VI (CK/CH/FJ/202005). Vet. Microbiol. 2022, 266, 109352. [Google Scholar] [CrossRef]
- Ren, M.; Han, Z.; Zhao, Y.; Sun, J.; Liu, S.; Ma, D. Multiple recombination events between field and vaccine strains resulted in the emergence of a novel infectious bronchitis virus with decreased pathogenicity and altered replication capacity. Poult. Sci. 2020, 99, 1928–1938. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Z.; Xie, L.; An, D.; Fan, Y.; Wang, X.; Li, Y.; Liu, X.; Wu, J.; Li, G. Research progress and challenges to coronavirus vaccine development. J. Med. Virol. 2021, 93, 741–754. [Google Scholar] [CrossRef]
- Bashor, L.; Gagne, R.B.; Bosco-Lauth, A.M.; Bowen, R.A.; Stenglein, M.; VandeWoude, S. SARS-CoV-2 evolution in animals suggests mechanisms for rapid variant selection. Proc. Natl. Acad. Sci. USA 2021, 118, e2105253118. [Google Scholar] [CrossRef]
- Graham, R.L.; Deming, D.J.; Deming, M.E.; Yount, B.L.; Baric, R.S. Evaluation of a recombination-resistant coronavirus as a broadly applicable, rapidly implementable vaccine platform. Commun. Biol. 2018, 1, 179. [Google Scholar] [CrossRef]
- Feng, C.; Shi, J.; Fan, Q.; Wang, Y.; Huang, H.; Chen, F.; Tang, G.; Li, Y.; Li, P.; Li, J. Protective humoral and cellular immune responses to SARS-CoV-2 persist up to 1 year after recovery. Nat. Commun. 2021, 12, 4984. [Google Scholar] [CrossRef] [PubMed]
- Stankov, M.V.; Hoffmann, M.; Jauregui, R.G.; Cossmann, A.; Ramos, G.M.; Graalmann, T.; Winter, E.J.; Friedrichsen, M.; Ravens, I.; Ilievska, T. Humoral and cellular immune responses following BNT162b2 XBB. 1.5 vaccination. Lancet Infect. Dis. 2024, 24, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fontela, C.; Dowling, W.E.; Funnell, S.G.; Gsell, P.-S.; Riveros-Balta, A.X.; Albrecht, R.A.; Andersen, H.; Baric, R.S.; Carroll, M.W.; Cavaleri, M. Animal models for COVID-19. Nature 2020, 586, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-D.; Chi, W.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.-C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Keusch, G.T.; Amuasi, J.H.; Anderson, D.E.; Daszak, P.; Eckerle, I.; Field, H.; Koopmans, M.; Lam, S.K.; Das Neves, C.G.; Peiris, M. Pandemic origins and a One Health approach to preparedness and prevention: Solutions based on SARS-CoV-2 and other RNA viruses. Proc. Natl. Acad. Sci. USA 2022, 119, e2202871119. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Feliciano, C.; Chapman, R.; Hooper, J.W.; Elma, K.; Zehrung, D.; Brennan, M.B.; Spiegel, E.K. Improved DNA vaccine delivery with needle-free injection systems. Vaccines 2023, 11, 280. [Google Scholar] [CrossRef]
- Lei, Z.; Zhu, L.; Pan, P.; Ruan, Z.; Gu, Y.; Xia, X.; Wang, S.; Ge, W.; Yao, Y.; Luo, F. A vaccine delivery system promotes strong immune responses against SARS-CoV-2 variants. J. Med. Virol. 2023, 95, e28475. [Google Scholar] [CrossRef]
| Genus | Virus Name | Host | Tissue Tropism | Cellular Receptor | Clinical Illness | Reference |
|---|---|---|---|---|---|---|
| Alpha | Canine coronavirus (CCoV) | Dog, Human | Intestines, respiratory tract, lungs | APN, Sialic Acid | Diarrhea, vomiting, drowsiness, mild fever, pneumonia | [1,28,29,30,31,32] |
| Feline coronavirus (FCoV) | Cat | Intestines, monocytes | APN, Sialic Acid | Peritonitis, enteritis | [33] | |
| Transmissible gastroenteritis virus (TGEV) | Pig | Intestines | APN, Sialic Acid | Gastroenteritis | [34,35] | |
| Porcine respiratory coronavirus (PRCV) | Pig | Respiratory tract, lungs, tonsils | APN | Fever, dyspnea, coughing | [36,37,38] | |
| Porcine epidemic diarrhea virus (PEDV) | Pig | Intestines | ND | Gastroenteritis | [39,40] | |
| Human coronavirus 229E (HCoV-229E) | Human | Intestines, respiratory tract | APN | Colds, pneumonia | [41,42] | |
| Human coronavirus NL63 (HCoV-NL63) | Human | Intestines, respiratory tract, duodenum, heart, kidney | ACE2 | Colds, pneumonia | [42,43,44,45] | |
| Swine acute diarrhea syndrome-coronavirus (SADS-CoV) | Pig | Intestines | ND | Diarrhea, vomiting | [36,37,46,47] | |
| Bat coronaviruses (Bat CoV) | Bat | Intestines | ACE2 | Diarrhea | [48] | |
| Beta | Bovine coronavirus (BCoV) | Cattle | Respiratory tract, intestines, trachea, lungs | Sialic Acid | Gastroenteritis, pneumonia | [29] |
| Mouse hepatitis virus (MHV) | Murine | Respiratory tract, intestines, CNS | CEACAM | Hepatitis, encephalitis | [49,50] | |
| Porcine hemagglutinating encephalomyelitis (PHEV) | Pig | CNS | ND | Neurological and/or enteric disease | [51] | |
| Human coronavirus OC43 (HCoV-OC43) | Human | Intestines, respiratory tract | Sialic Acid | Colds, pneumonia | [29,33] | |
| Middle east respiratory syndrome coronavirus (MERS-CoV) | Camel, Human | Intestines, kidney, placenta | DPP4 (CD26) | Respiratory disease | [50,52] | |
| Severe acute respiratory syndrome coronavirus (SARS-CoV) | Human | Intestines, duodenum, heart, kidney | ACE2 | Pneumonia, gastroenteritis | [43,53] | |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | Human | Intestines, duodenum, heart, kidney | ACE2, KREMEN1, ASGR1 | Pneumonia, gastroenteritis | [43,54,55,56,57] | |
| Equine coronavirus (ECoV) | Horse | Respiratory tract, CNS | ND | Diarrhea, fever, lethargy, and anorexia | [36,58] | |
| Gamma | Infectious bronchitis virus (IBV) | Avian | Respiratory tract, intestines, kidney | Sialic Acid | Respiratory, kidney, oviduct, and intestinal tract disease | [36,59,60] |
| Turkey coronavirus (TCoV) | Turkey | Intestines | Sialic Acid | Enteric disease | [37] | |
| Delta | Porcine deltacoronavirus (PDCoV) | Pig, Human | Intestines | APN | Enteric disease, fever, cough, abdominal pain | [2,37,61] |
| Genus | Targeted Viruses | Vaccine Name/ Candidate Strains | Vaccine Platform | Immunization Route | Reference |
|---|---|---|---|---|---|
| Alpha | CCoV | Strain 257/98-3c | Whole virus | Intramuscular (IM) | [142] |
| FCoV | FIPV-DF2 | Live-attenuated virus | Feline kidney (NLFK) cell/ intranasal | [143] | |
| FIPV 79-1146 | Live-attenuated virus | FCWF/inoculated oronasally | [144] | ||
| FIPV-M, FIPV-M (VR1012-M, VR1012-N) | DNA vaccine | Plasmid injection | [145] | ||
| PEDV | CV777, 83P-5 | Live-attenuated virus | Intramuscular (IM) | [146,147] | |
| SM98-1 | Live-attenuated virus | Intramuscular (IM) | [148] | ||
| DR13 | Live-attenuated virus | Oral | [149] | ||
| KNU-141112, | Inactivated virus | Intramuscular (IM) | [150] | ||
| PEDV CO2013 | Live-attenuated virus | Intramuscular (IM) and oral | [151] | ||
| USA/Colorado/2013 | Live-attenuated virus | Intragastric route | [152] | ||
| AH2012/12 | Inactivated virus | Intranasal | [153] | ||
| MZ0116-2/2013 | Live-attenuated virus | Intramuscular (IM) | [154] | ||
| 3B3scFv-pFc-PEDVsAg | DNA vaccine | Intramuscular (IM) | [155] | ||
| ORFV-PEDV-S | Recombinant virus | Intramuscular (IM) | [156] | ||
| S mRNA-LNP vaccine/ Sm mRNA-LNP | mRNA vaccine | Intramuscular (IM) | [157] | ||
| pTriEx-S (S1) | DNA vaccine | Intramuscular (IM) | [158] | ||
| rSF-COE-3D | Recombinant virus | Intramuscular (IM) | [159] | ||
| rTGEV-RS-SPEDV | Recombinant virus | Oral | [160] | ||
| TGEV | TGE/Rota | Recombinant virus | Intramuscular (IM), oral | [38] | |
| Beta | BCoV | 438/06-TN | Live-attenuated virus | Intramuscular (IM) | [161] |
| MHV | TMV-5B19 | DNA vaccine | Intranasal or subcutaneous | [162] | |
| Gamma | IBV | K2/01 | Live-attenuated virus | Eye drop | [163] |
| pCAG-N | DNA vaccine | Intranasal (IN) | [164] | ||
| Delta | PDCoV | L. lactis NZ9000-S1 | DNA vaccine | Oral | [165] |
| CZ2020 | Live-attenuated virus | Oral | [166] | ||
| PDCoV-NH | Inactivated virus | Oral | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, F.; Yu, R.; Dong, S.; Chen, B.; Li, C.; Song, S. Towards a Safer Future: Enhancing Vaccine Development to Combat Animal Coronaviruses. Vaccines 2024, 12, 330. https://doi.org/10.3390/vaccines12030330
Si F, Yu R, Dong S, Chen B, Li C, Song S. Towards a Safer Future: Enhancing Vaccine Development to Combat Animal Coronaviruses. Vaccines. 2024; 12(3):330. https://doi.org/10.3390/vaccines12030330
Chicago/Turabian StyleSi, Fusheng, Ruisong Yu, Shijuan Dong, Bingqing Chen, Chunhua Li, and Shuai Song. 2024. "Towards a Safer Future: Enhancing Vaccine Development to Combat Animal Coronaviruses" Vaccines 12, no. 3: 330. https://doi.org/10.3390/vaccines12030330
APA StyleSi, F., Yu, R., Dong, S., Chen, B., Li, C., & Song, S. (2024). Towards a Safer Future: Enhancing Vaccine Development to Combat Animal Coronaviruses. Vaccines, 12(3), 330. https://doi.org/10.3390/vaccines12030330







