Exploratory Study of the Phase IV Immunization Schedule of Quadrivalent Influenza Split-Virion Vaccine in Children Aged 3–8 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Vaccine
2.4. Sample Size
2.5. Study Population, Grouping, and Vaccination
2.6. Evaluation of Immunogenicity
2.7. Evaluation of Safety
2.8. Statistical Analysis
2.9. Serological Methods
3. Results
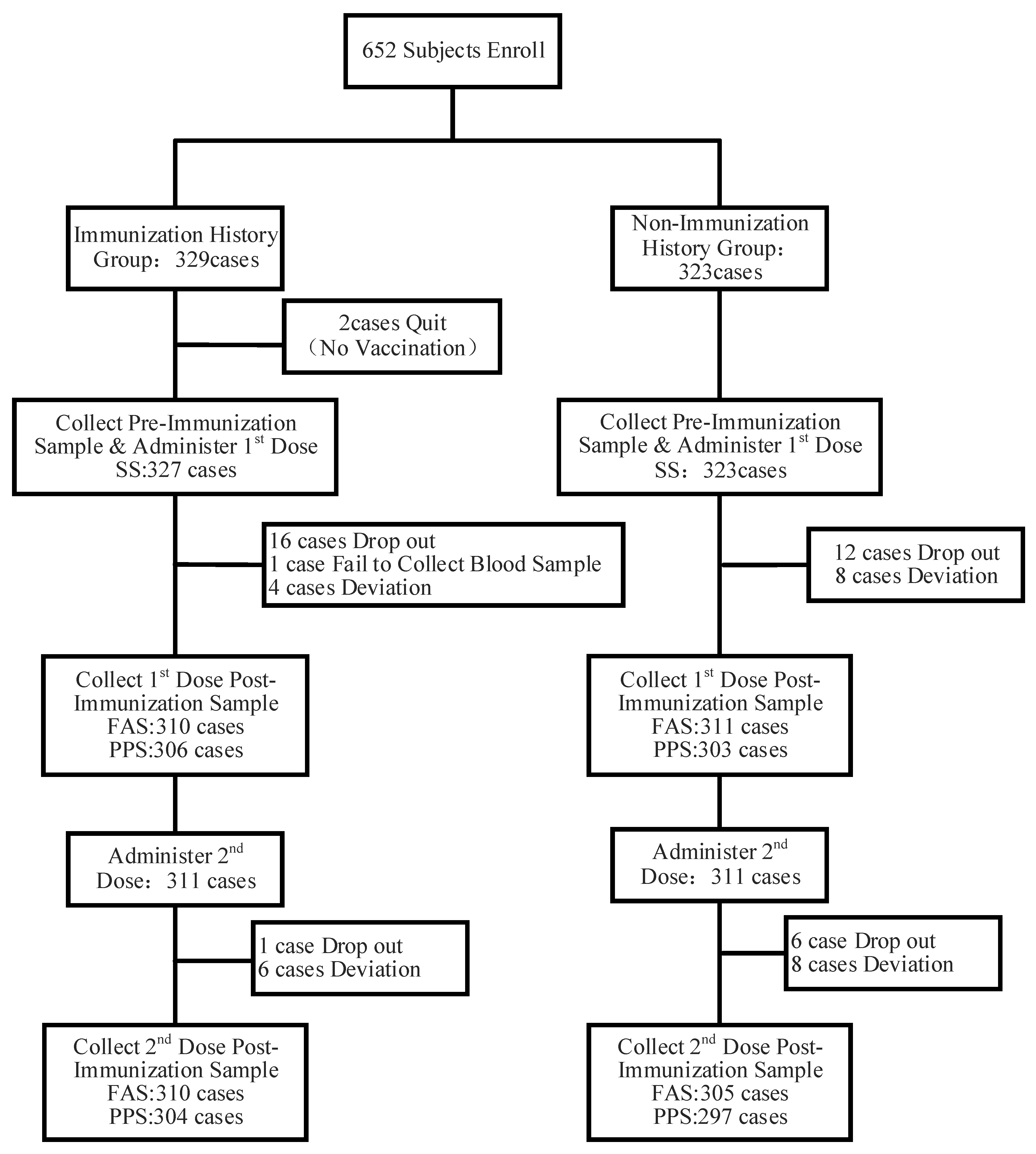
3.1. Basic Information on the Research Subjects
3.2. Immunogenicity
3.3. Safety Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Y.L.; Xue, S.; Xie, Y.R.; Zhang, Y.P.; Wang, D.Y.; Zhao, T.; Du, W.; Chen, T.; Miao, H.; Qin, Y.; et al. Characterization of influenza seasonality in China, 2010–2018: Implications for seasonal influenza vaccination timing. Influenza Other Respir. Viruses 2022, 16, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Chinese Center for Disease Control and Prevention. Technical Guidelines for Seasonal Influenza Vaccination in China (2022–2023); National Health Commission of the People’s Republic of China: Beijing, China. Available online: https://pubmed.ncbi.nlm.nih.gov/36274602/ (accessed on 25 August 2022).
- National Health Commission of the People’s Republic of China. Protocol for Diagnosis and Treatment of Influenza (2020 Version); National Health Commission of the People’s Republic of China: Beijing, China. Available online: http://www.nhc.gov.cn/yzygj/s7652m/202011/4669b15fd1f247b9bf977de7bad261eb.shtml (accessed on 1 November 2020).
- Chinese Center for Disease Control and Prevention. Echnical Guidelines for Seasonal Influenza Vaccination in China (2020–2021); National Health Commission of the People’s Republic of China: Beijing, China; Available online: https://www.chinacdc.cn/jkzt/crb/bl/lxxgm/jszl_2251/202009/t20200910_219046.html (accessed on 10 September 2020).
- Centers for Disease Control and Prevention. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices United States, 2020–21 Influenza Season; Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs: Washington, DC, USA. Available online: https://www.cdc.gov/mmwr/volumes/69/rr/rr6908a1.htm (accessed on 21 August 2021).
- Center for Drug Evaluation of National Medical Products Administration. Technical Guidelines for Clinical Research of Seasonal Influenza Virus Vaccines (Draft for Solicitation of Comments); Center for Drug Evaluation of National Medical Products Administration: Beijing, China. Available online: https://www.cde.org.cn/main/news/viewInfoCommon/237d5f7de6bcfcd08037dcce873794f3 (accessed on 19 November 2021).
- National Medical Products Administration. Guidelines for the Classification of Adverse Events in Clinical Trials of Preventive Vaccine; Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs: Washington, DC, USA. Available online: https://www.nmpa.gov.cn/yaopin/ypggtg/ypqtgg/20191231111901460.html (accessed on 31 December 2019).
- Wang, D.Y. Development and prospect of Influenza Surveillance Network in China. Zhonghua Liu Xing Bing Xue Za Zhi 2018, 39, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Agarkhedkar, S.; Chhatwal, J.; Kompithra, R.Z.; Lalwani, S.K.; Narayan, A.; Muninarayanaswam, V.; Gogtay, N.; Dotter, K.; Gresset-Bourgeois, V. Immunogenicity and safety of an intramuscular split-virion quadrivalent inactivated influenza vaccine in individuals aged ≥6 months in India. Hum. Vaccines Immunother. 2019, 15, 973–977. [Google Scholar] [CrossRef]
- Chen, J.M.; Jiang, F.; Zhao, C.Y.; Chai, I.J.; Li, L.S.; Guan, Q.H.; Li, X.Y.; Wang, F.Y.; Li, A.S.; Gao, H.X.; et al. Immunogenicity and safety of the quadrivalent inactivated split-virion influenza vaccine in populations aged ≥3 years: A phase 3, randomized, double-blind, non-inferiority clinical trial. Hum. Vaccines Immunother. 2023, 19, 2245721. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.X.; Greene, C.M.; He, Q.; Liao, Y.; Wan, Y.M.; Shen, J.C.; Rong, C.; Zhou, S.Z. Dose effect of influenza vaccine on protection against laboratory-confirmed influenza illness among children aged 6 months to 8 years of age in southern China, 2013/14–2015/16 seasons: A matched case-control study. Hum. Vaccines Immunother. 2020, 16, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Ritzwoller, D.P.; Bridges, C.B.; Shetterly, S.; Yamasaki, K.; Kolczak, M.; France, E. Effectiveness of the 2003–2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs. 2 doses. Pediatrics 2005, 116, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, K.M.; Jackson, L.A.; Nelson, J.; Klimov, A.; Cox, N.; Bridges, C.B.; Dunn, J.; DeStefano, F.; Shay, D. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5–8-year-old children. J. Infect. Dis. 2006, 194, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Wang, Y.X.; Jia, C.Y.; Li, G.F.; Zhang, W.; Li, Q.; Chen, X.F.; Leng, W.N.; Huang, L.L.; Xie, Z.Q.; et al. Immunogenicity and safety of an egg culture-based quadrivalent inactivated non-adjuvanted subunit influenza vaccine in subjects ≥3 years: A randomized, multicenter, double-blind, active-controlled phase III, non-inferiority trial. Vaccine 2022, 40, 4933–4941. [Google Scholar] [CrossRef] [PubMed]
- Thiem, V.D.; Chabanon, A.L.; Fournier, M.; Lavis, N.; Quang, N.D.; Ha, V.H.; Sanicas, M. Safety of a quadrivalent influenza vaccine in Vietnamese healthy subjects aged 6 months and older. Hum. Vaccines Immunother. 2021, 17, 690–693. [Google Scholar] [CrossRef] [PubMed]
| Antibody Strain | Group | N | Min, Max | M(P25, P75) | GMT(95% CI) | t | p |
|---|---|---|---|---|---|---|---|
| H1N1 | IH | 327 | 5, 160 | 10(5, 40) | 13.66(12.30~15.16) | 2.957 | 0.003 |
| NH | 323 | 5, 160 | 5(5, 20) | 10.94(9.87~12.14) | |||
| H3N2 | IH | 327 | 5, 320 | 20(5, 40) | 16.11(14.51~17.89) | 0.644 | 0.520 |
| NH | 323 | 5, 320 | 20(5, 40) | 15.36(13.88~17.00) | |||
| BV | IH | 327 | 5, 40 | 5(5, 5) | 5.68(5.47~5.89) | 1.470 | 0.142 |
| NH | 323 | 5, 40 | 5(5, 5) | 5.47(5.29~5.65) | |||
| BY | IH | 327 | 5, 80 | 5(5, 10) | 7.25(6.76~7.77) | 1.592 | 0.112 |
| NH | 323 | 5, 160 | 5(5, 5) | 6.71(6.29~7.16) |
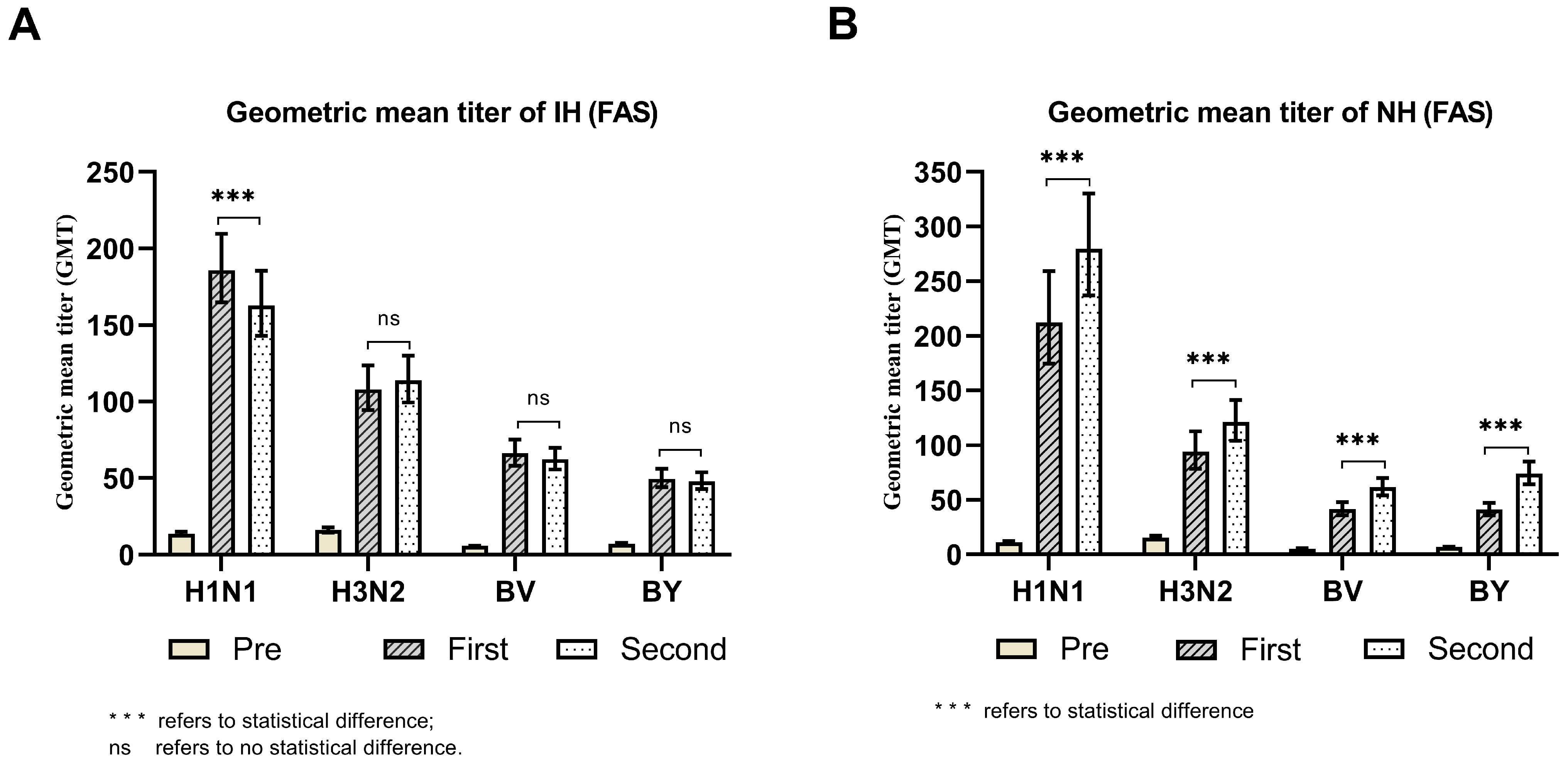
| Group | Antibody Strain | Dose | N * | Min, Max | M(P25, P75) | GMT(95% CI) | t | p |
|---|---|---|---|---|---|---|---|---|
| IH | H1N1 | 1st dose | 309 | 10, 2560 | 160(80, 320) | 185.95(164.82~209.79) | 2.397 | 0.017 |
| 2nd dose | 309 | 10, 2560 | 160(80, 320) | 162.90(142.96~185.61) | ||||
| H3N2 | 1st dose | 309 | 10, 5120 | 80(40, 320) | 108.05(94.40~123.68) | 0.793 | 0.429 | |
| 2nd dose | 309 | 10, 5120 | 80(40, 160) | 113.77(99.53~130.06) | ||||
| BV | 1st dose | 309 | 5, 1280 | 80(40, 160) | 66.26(58.27~75.35) | 1.079 | 0.282 | |
| 2nd dose | 309 | 5, 640 | 80(40, 160) | 62.37(55.70~69.83) | ||||
| BY | 1st dose | 309 | 5, 1280 | 40(40, 80) | 49.72(43.99~56.21) | 0.688 | 0.492 | |
| 2nd dose | 309 | 5, 1280 | 40(40, 80) | 48.08(42.97~53.80) | ||||
| NH | H1N1 | 1st dose | 305 | 5, 5120 | 320(40, 1280) | 212.57(174.44~259.03) | 3.357 | 0.001 |
| 2nd dose | 305 | 5, 5120 | 320(80, 1280) | 279.85(237.12~330.26) | ||||
| H3N2 | 1st dose | 305 | 5, 5120 | 80(20, 320) | 94.01(78.32~112.85) | 3.293 | 0.001 | |
| 2nd dose | 305 | 5, 5120 | 160(40, 320) | 121.26(104.08~141.27) | ||||
| BV | 1st dose | 305 | 5, 1280 | 40(20, 80) | 41.48(35.94~47.88) | 6.760 | <0.001 | |
| 2nd dose | 305 | 5, 1280 | 80(40, 160) | 61.46(54.01~69.94) | ||||
| BY | 1st dose | 305 | 5, 1280 | 40(20, 80) | 40.92(35.60~47.03) | 8.215 | <0.001 | |
| 2nd dose | 305 | 5, 1280 | 80(40, 160) | 73.88(64.30~84.89) |
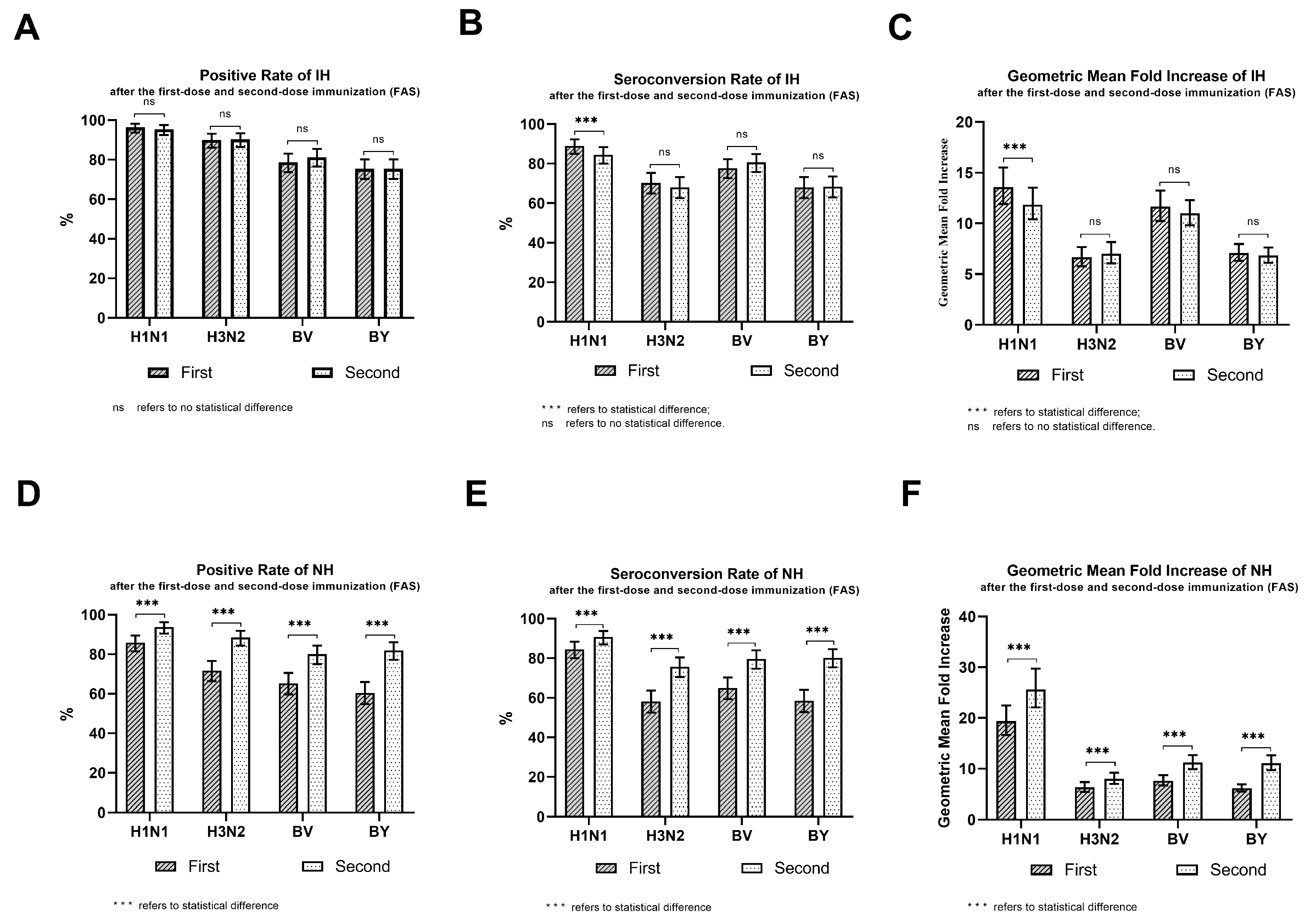
| Group | Strain | Dose | N | Positive Number | Positive Rate | Seroconversion Number | Seroconversion Rate | GMI * |
|---|---|---|---|---|---|---|---|---|
| IH | H1N1 | 1st | 310 | 299 | 96.45(93.74~98.22) | 276 | 89.03(85.01~92.28) | 13.59(11.91~15.50) |
| 2nd | 310 | 296 | 95.48(92.54~97.51) | 262 | 84.52(80.00~88.36) | 11.86(10.40~13.53) | ||
| H3N2 | 1st | 310 | 279 | 90.00(86.11~93.10) | 218 | 70.32(64.90~75.35) | 6.66(5.78~7.68) | |
| 2nd | 310 | 280 | 90.32(86.47~93.38) | 211 | 68.06(62.56~73.22) | 7.03(6.05~8.16) | ||
| BV | 1st | 310 | 244 | 78.71(73.73~83.13) | 241 | 77.74(72.70~82.25) | 11.65(10.24~13.24) | |
| 2nd | 310 | 252 | 81.29(76.50~85.48) | 250 | 80.65(75.80~84.89) | 10.99(9.82~12.29) | ||
| BY | 1st | 310 | 234 | 75.48(70.30~80.17) | 211 | 68.06(62.56~73.22) | 7.09(6.31~7.97) | |
| 2nd | 310 | 234 | 75.48(70.30~80.17) | 212 | 68.39(62.89~73.53) | 6.84(6.13~7.63) | ||
| NH | H1N1 | 1st | 311 | 267 | 85.85(81.48~89.53) | 263 | 84.57(80.06~88.40) | 19.34(16.65~22.46) |
| 2nd | 305 | 286 | 93.77(90.44~96.21) | 277 | 90.82(87.01~93.81) | 25.61(22.07~29.73) | ||
| H3N2 | 1st | 311 | 223 | 71.70(66.35~76.64) | 181 | 58.20(52.50~63.74) | 6.30(5.41~7.34) | |
| 2nd | 305 | 270 | 88.52(84.40~91.88) | 231 | 75.74(70.53~80.44) | 8.02(6.98~9.21) | ||
| BV | 1st | 311 | 203 | 65.27(59.70~70.56) | 202 | 64.95(59.37~70.25) | 7.62(6.67~8.70) | |
| 2nd | 305 | 244 | 80.00(75.06~84.34) | 243 | 79.67(74.71~84.04) | 11.20(9.89~12.68) | ||
| BY | 1st | 311 | 188 | 60.45(54.78~65.92) | 182 | 58.52(52.82~64.05) | 6.16(5.49~6.92) | |
| 2nd | 305 | 250 | 81.97(77.18~86.12) | 245 | 80.33(75.42~84.64) | 11.07(9.70~12.64) |
| AEs | Group | N | * Times | # Cases | Rate (95% CI) | X2 | p |
|---|---|---|---|---|---|---|---|
| Total Adverse Reaction | IH | 327 | 111 | 68 | 20.80(16.53~25.60) | 0.168 | 0.682 |
| NH | 323 | 102 | 63 | 19.50(15.33~24.25) | |||
| Total | 650 | 213 | 131 | 20.15(17.13~23.45) | |||
| Local Adverse Reaction | IH | 327 | 74 | 44 | 13.46(9.95~17.64) | 3.267 | 0.071 |
| NH | 323 | 49 | 29 | 8.98(6.10~12.64) | |||
| Total | 650 | 123 | 73 | 11.23(8.91~13.91) | |||
| Systemic Adverse Reaction | IH | 327 | 37 | 28 | 8.56(5.77~12.14) | 1.826 | 0.177 |
| NH | 323 | 53 | 38 | 11.76(8.46~15.79) | |||
| Total | 650 | 90 | 66 | 10.15(7.94~12.74) |
| AEs | Group | Times | Grade 1 | Grade 2 | Grade 3 | X2 | p |
|---|---|---|---|---|---|---|---|
| Total Adverse Reaction | IH | 111 | 75 | 34 | 2 | 0.518 a | |
| NH | 102 | 74 | 28 | 0 | |||
| Total | 213 | 149 | 62 | 2 | |||
| Local Adverse Reaction | IH | 74 | 58 | 15 | 1 | 0.003 a | |
| NH | 49 | 48 | 1 | 0 | |||
| Total | 123 | 106 | 16 | 1 | |||
| Systemic Adverse Reaction | IH | 37 | 17 | 19 | 1 | 0.644 a | |
| NH | 53 | 26 | 27 | 0 | |||
| Total | 90 | 43 | 46 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Kou, Z.; Liu, T.; An, W.; An, W.; Zhang, W.; Zhang, K.; Dong, J.; Yu, J.; Li, Y.; et al. Exploratory Study of the Phase IV Immunization Schedule of Quadrivalent Influenza Split-Virion Vaccine in Children Aged 3–8 Years. Vaccines 2024, 12, 321. https://doi.org/10.3390/vaccines12030321
Li X, Kou Z, Liu T, An W, An W, Zhang W, Zhang K, Dong J, Yu J, Li Y, et al. Exploratory Study of the Phase IV Immunization Schedule of Quadrivalent Influenza Split-Virion Vaccine in Children Aged 3–8 Years. Vaccines. 2024; 12(3):321. https://doi.org/10.3390/vaccines12030321
Chicago/Turabian StyleLi, Xiaoyu, Zengqiang Kou, Ti Liu, Wenjue An, Wenqi An, Wei Zhang, Ke Zhang, Jie Dong, Jiangxuan Yu, Yaqi Li, and et al. 2024. "Exploratory Study of the Phase IV Immunization Schedule of Quadrivalent Influenza Split-Virion Vaccine in Children Aged 3–8 Years" Vaccines 12, no. 3: 321. https://doi.org/10.3390/vaccines12030321
APA StyleLi, X., Kou, Z., Liu, T., An, W., An, W., Zhang, W., Zhang, K., Dong, J., Yu, J., Li, Y., & Zhao, C. (2024). Exploratory Study of the Phase IV Immunization Schedule of Quadrivalent Influenza Split-Virion Vaccine in Children Aged 3–8 Years. Vaccines, 12(3), 321. https://doi.org/10.3390/vaccines12030321






