Estimating the Impact of Vaccination Campaigns on Measles Transmission in Somalia
Abstract
1. Introduction
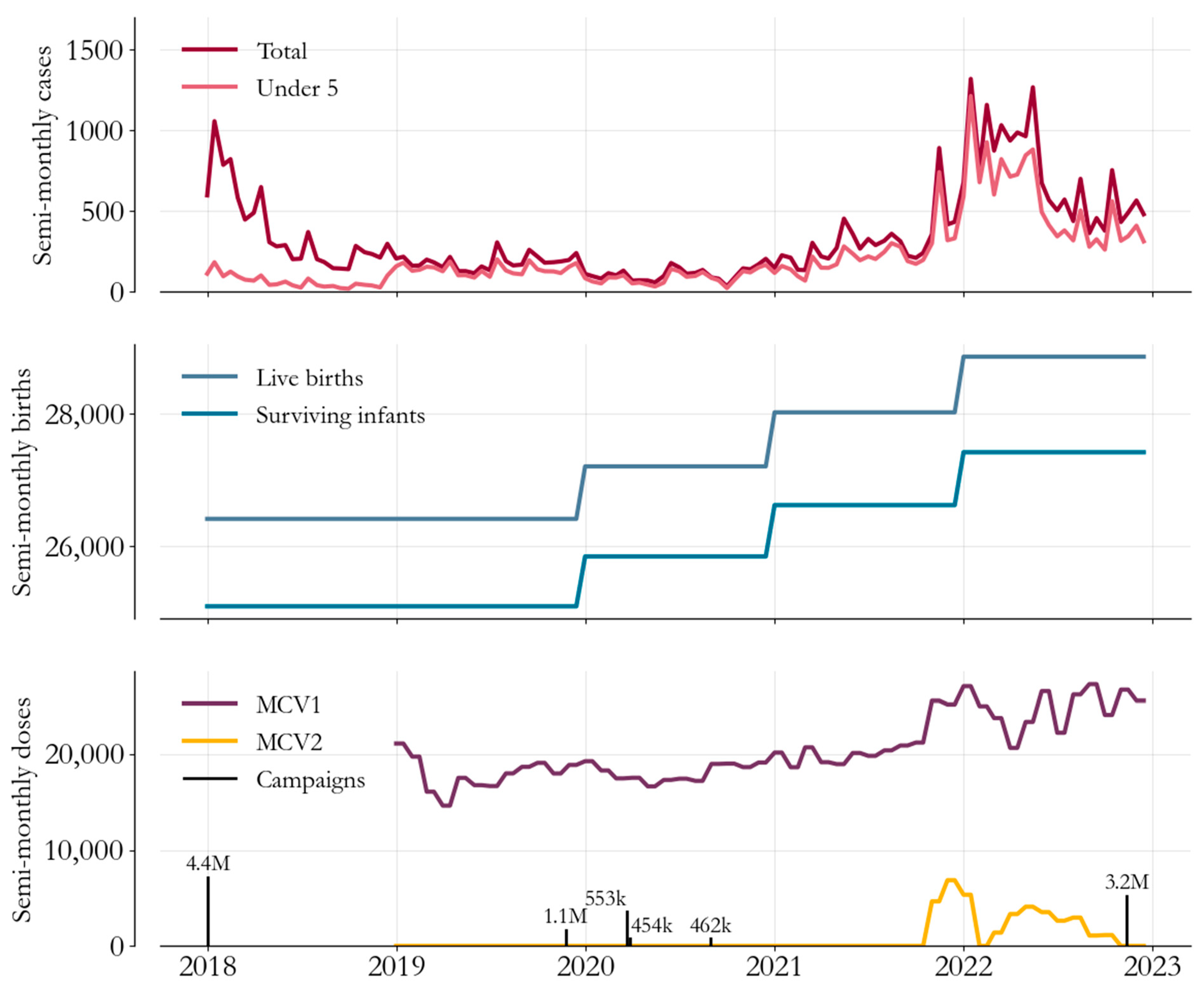
2. Measles Epidemiology and Immunization in Somalia
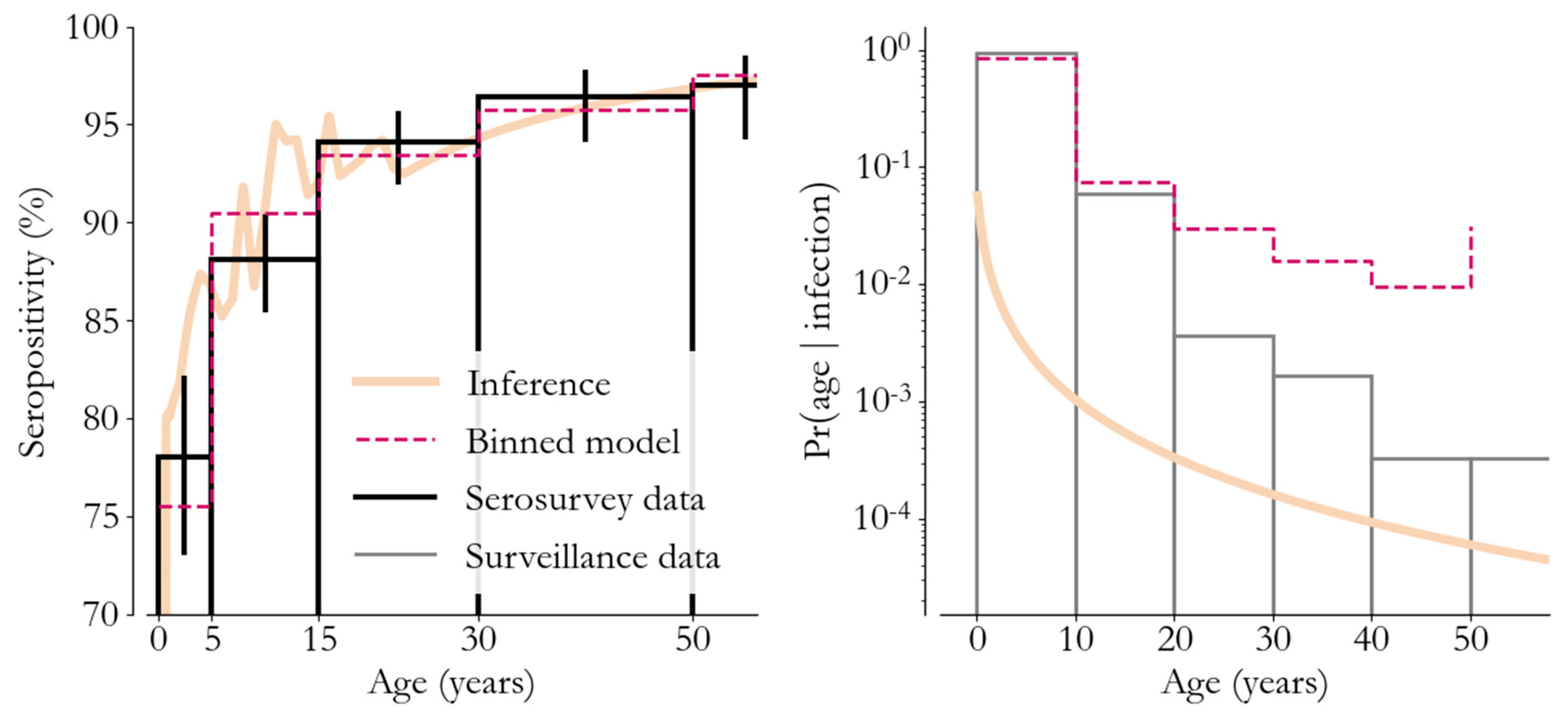
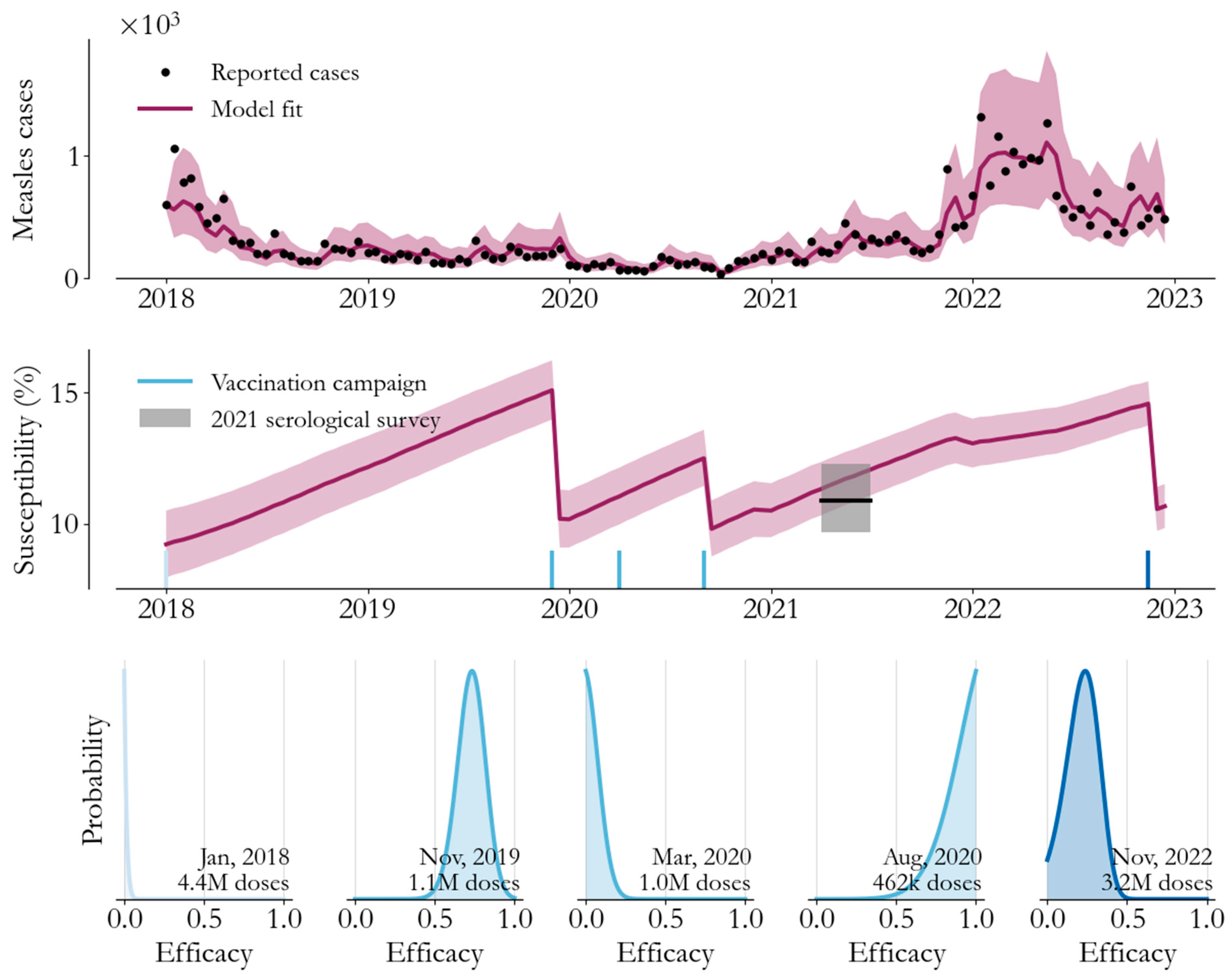
3. Transmission Modeling
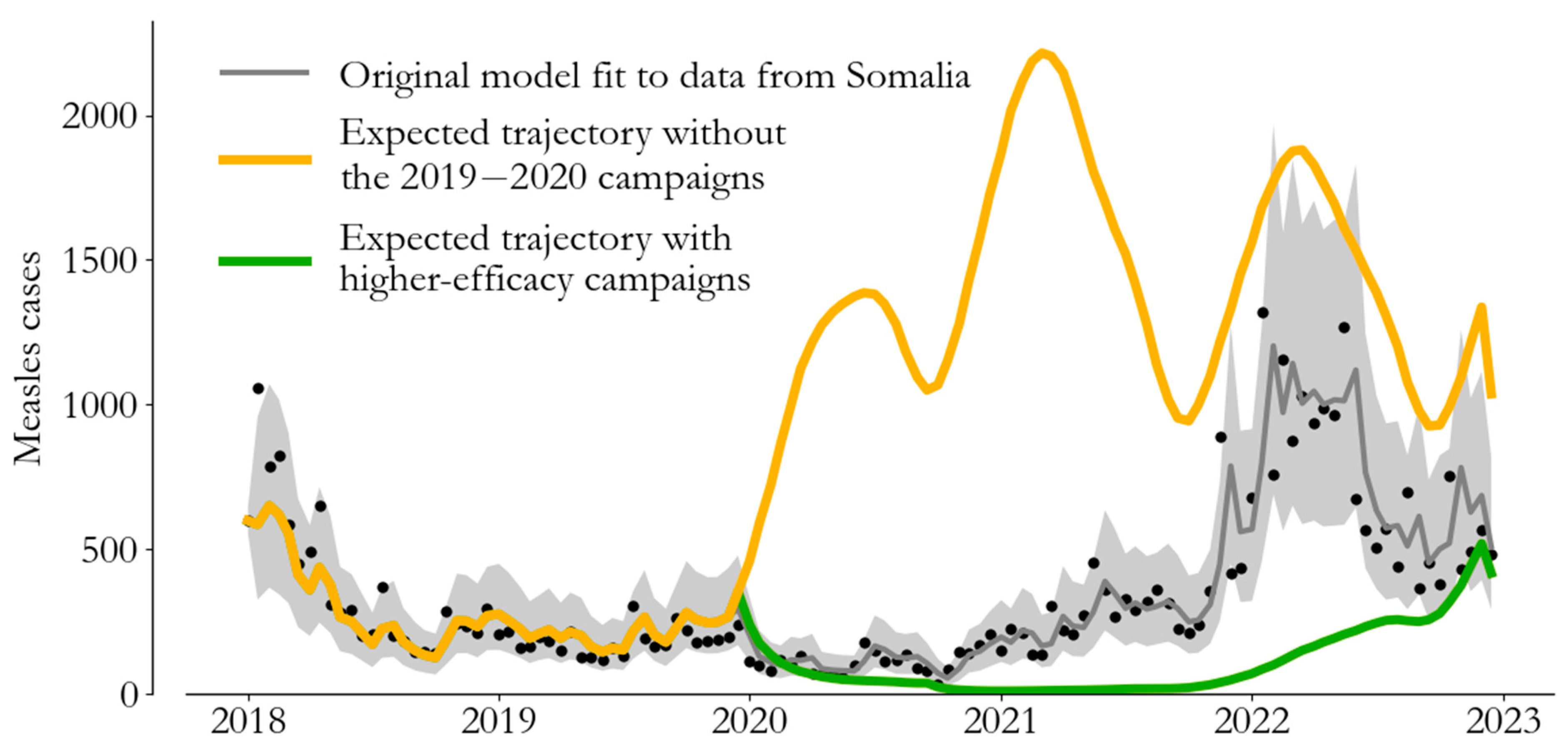
4. From Campaign Immunity to Averted Burden
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Measles vaccines: WHO position paper—April 2017. Wkly Epidemiol Rec. 2017, 92, 205–228.
- World Health Organization. Immunization Agenda 2030: A Global Strategy to Leave No One Behind. Available online: https://cdn.who.int/media/docs/default-source/immunization/strategy/ia2030/ia2030-draft-4-wha_b8850379-1fce-4847-bfd1-5d2c9d9e32f8.pdf?sfvrsn=5389656e_69&download=true (accessed on 26 January 2023).
- McLean, A.R.; Anderson, R.M. Measles in developing countries Part I. Epidemiological parameters and patterns. Epidemiol. Infect. 1988, 100, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, L.J.; Grais, R.F.; Luquero, F.J.; Birmingham, M.E.; Strebel, P.M. Estimates of measles case fatality ratios: A comprehensive review of community-based studies. Int. J. Epidemiol. 2009, 38, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Integrated Food Security Phase Classification. Somalia: Acute Malnutrition Situation July–September 2022 and Projection for October–December 2022. Available online: https://www.ipcinfo.org/ipc-country-analysis/details-map/en/c/1155886/?iso3=SOM (accessed on 26 January 2023).
- Mohamud, A.K.; Ahmed, O.A.; Ali, I.A.; Dirie, N.I. Demographical, clinical, and complication differences between vaccinated and unvaccinated hospitalized children with measles in Mogadishu Somalia: A hospital-based retrospective cohort study. Ann. Med. Surg. 2023, 85, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Nchasi, G.; Paul, I.K.; Sospeter, S.B.; Mallya, M.R.; Ruaichi, J.; Malunga, J. Measles outbreak in sub-Saharan Africa amidst COVID-19: A rising concern, efforts, challenges, and future recommendations. Ann. Med. Surg. 2022, 81, 104264. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.populationpyramid.net/somalia/2018/ (accessed on 11 May 2023).
- Carcelen, A.C.; Winter, A.K.; Moss, W.J.; Chilumba, I.; Mutale, I.; Chongwe, G.; Monze, M.; Mulundu, G.; Nkamba, H.; Mwansa, F.D.; et al. Leveraging a national biorepository in Zambia to assess measles and rubella immunity gaps across age and space. Sci. Rep. 2022, 12, 10217. [Google Scholar] [CrossRef] [PubMed]
- Prada, J.M.; Metcalf, C.J.E.; Ferrari, M.J. Improving measles incidence inference using age-structured serological data. Epidemiol. Infect. 2018, 146, 1699–1706. [Google Scholar] [CrossRef]
- Thakkar, N.; Gilani, S.S.A.; Hasan, Q.; McCarthy, K.A. Decreasing measles burden by optimizing campaign timing. Proc. Natl. Acad. Sci. USA 2019, 116, 11069–11073. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, N. A modeling approach for estimating dynamic measles case detection rates. arXiv 2022, arXiv:2202.11222. [Google Scholar]
- Finkenstädt, B.F.; Grenfell, B.T. Time series modelling of childhood diseases: A dynamical systems approach. J. R. Stat. Soc. Ser. C Appl. Stat. 2000, 49, 187–205. [Google Scholar] [CrossRef]
- Ferrari, M.J.; Grais, R.F.; Bharti, N.; Conlan, A.J.K.; Bjørnstad, O.N.; Wolfson, L.J.; Guerin, P.J.; Djibo, A.; Grenfell, B.T. The dynamics of measles in sub-Saharan Africa. Nature 2008, 451, 679–684. [Google Scholar] [CrossRef]
- Portnoy, A.; Jit, M.; Ferrari, M.; Hanson, M.; Brenzel, L.; Verguet, S. Estimates of case-fatality ratios of measles in low-income and middle-income countries: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e472–e481. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Frey, K.; Hagedorn, B.; Oteri, A.; Yahya, A.; Hamisu, M.; Mogekwu, F.; Shuaib, F.; McCarthy, K.A.; Chabot-Couture, G. Optimization of frequency and targeting of measles supplemental immunization activities in Nigeria: A cost-effectiveness analysis. Vaccine 2019, 37, 6039–6047. [Google Scholar] [CrossRef] [PubMed]
- Grais, R.; Conlan, A.; Ferrari, M.; Djibo, A.; Le Menach, A.; Bjørnstad, O.; Grenfell, B.T. Time is of the essence: Exploring a measles outbreak response vaccination in Niamey, Niger. J. R. Soc. Interface 2008, 5, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Msemburi, W.; Sim, S.Y.; Gaythorpe, K.A.; Lambach, P.; Lindstrand, A.; Hutubessy, R. Modeling the impact of vaccination for the immunization Agenda 2030: Deaths averted due to vaccination against 14 pathogens in 194 countries from 2021 to 2030. Vaccine. Available online: https://www.sciencedirect.com/science/article/pii/S0264410X2300854X (accessed on 1 August 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakkar, N.; Abubakar, A.H.A.; Shube, M.; Jama, M.A.; Derow, M.; Lambach, P.; Ashmony, H.; Farid, M.; Sim, S.Y.; O’Connor, P.; et al. Estimating the Impact of Vaccination Campaigns on Measles Transmission in Somalia. Vaccines 2024, 12, 314. https://doi.org/10.3390/vaccines12030314
Thakkar N, Abubakar AHA, Shube M, Jama MA, Derow M, Lambach P, Ashmony H, Farid M, Sim SY, O’Connor P, et al. Estimating the Impact of Vaccination Campaigns on Measles Transmission in Somalia. Vaccines. 2024; 12(3):314. https://doi.org/10.3390/vaccines12030314
Chicago/Turabian StyleThakkar, Niket, Ali Haji Adam Abubakar, Mukhtar Shube, Mustafe Awil Jama, Mohamed Derow, Philipp Lambach, Hossam Ashmony, Muhammad Farid, So Yoon Sim, Patrick O’Connor, and et al. 2024. "Estimating the Impact of Vaccination Campaigns on Measles Transmission in Somalia" Vaccines 12, no. 3: 314. https://doi.org/10.3390/vaccines12030314
APA StyleThakkar, N., Abubakar, A. H. A., Shube, M., Jama, M. A., Derow, M., Lambach, P., Ashmony, H., Farid, M., Sim, S. Y., O’Connor, P., Minta, A., Bose, A. S., Musanhu, P., Hasan, Q., Bar-Zeev, N., & Malik, S. M. M. R. (2024). Estimating the Impact of Vaccination Campaigns on Measles Transmission in Somalia. Vaccines, 12(3), 314. https://doi.org/10.3390/vaccines12030314







