Immunization with centrin-Deficient Leishmania braziliensis Does Not Protect against Homologous Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Culture
2.2. Bone Marrow-Derived Macrophage (BMDM) Culture and In Vitro Exposure to LbCen−/−
2.3. Nitric Oxide and Cytokine Quantification
2.4. Safety and Efficacy of LbCen−/− in BALB/c Mice
2.5. Leishmanization
2.6. Immunization with LbCen−/− and Challenge with LbWT
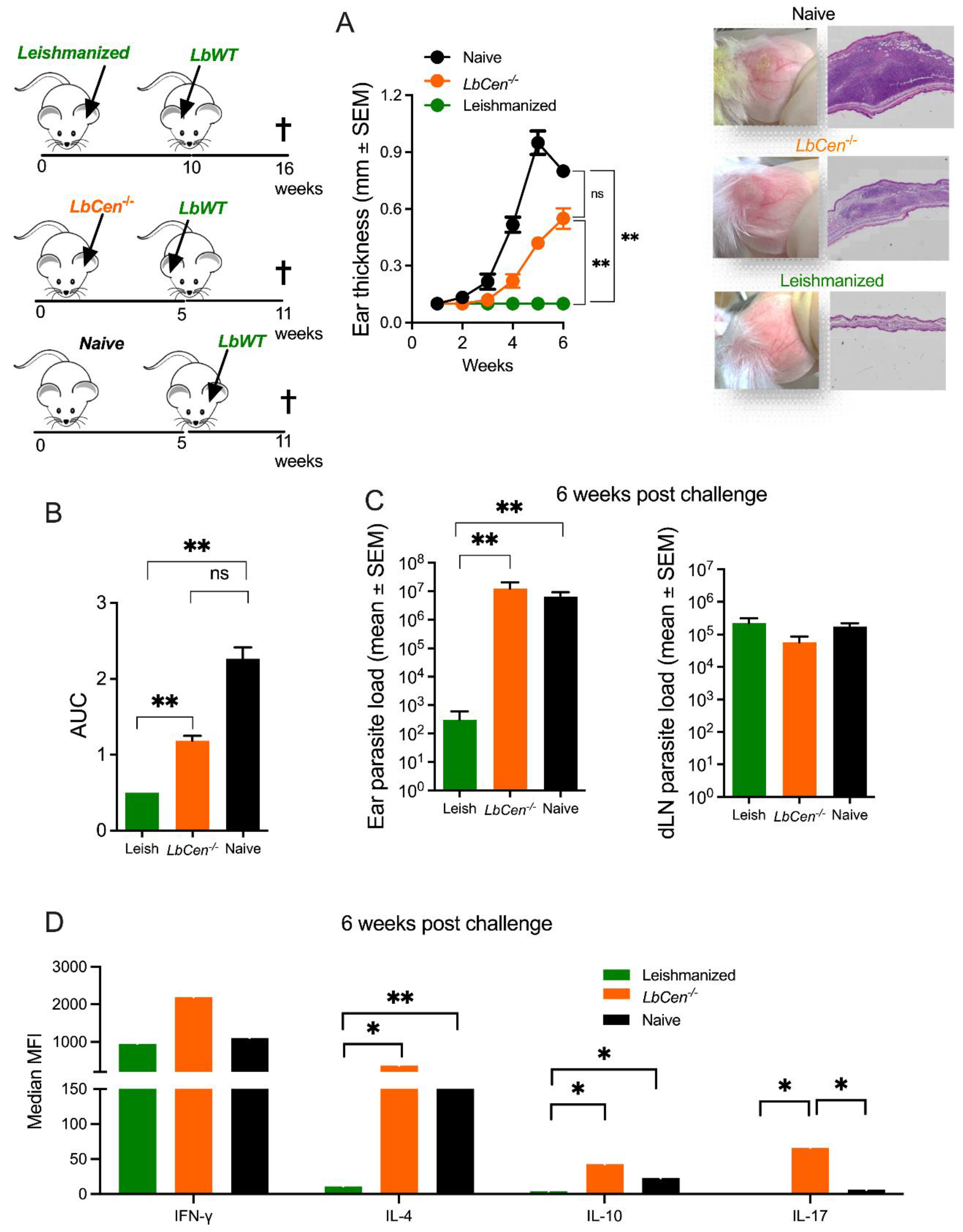
2.7. Efficacy of Immunization with LbCen−/− versus Leishmanization
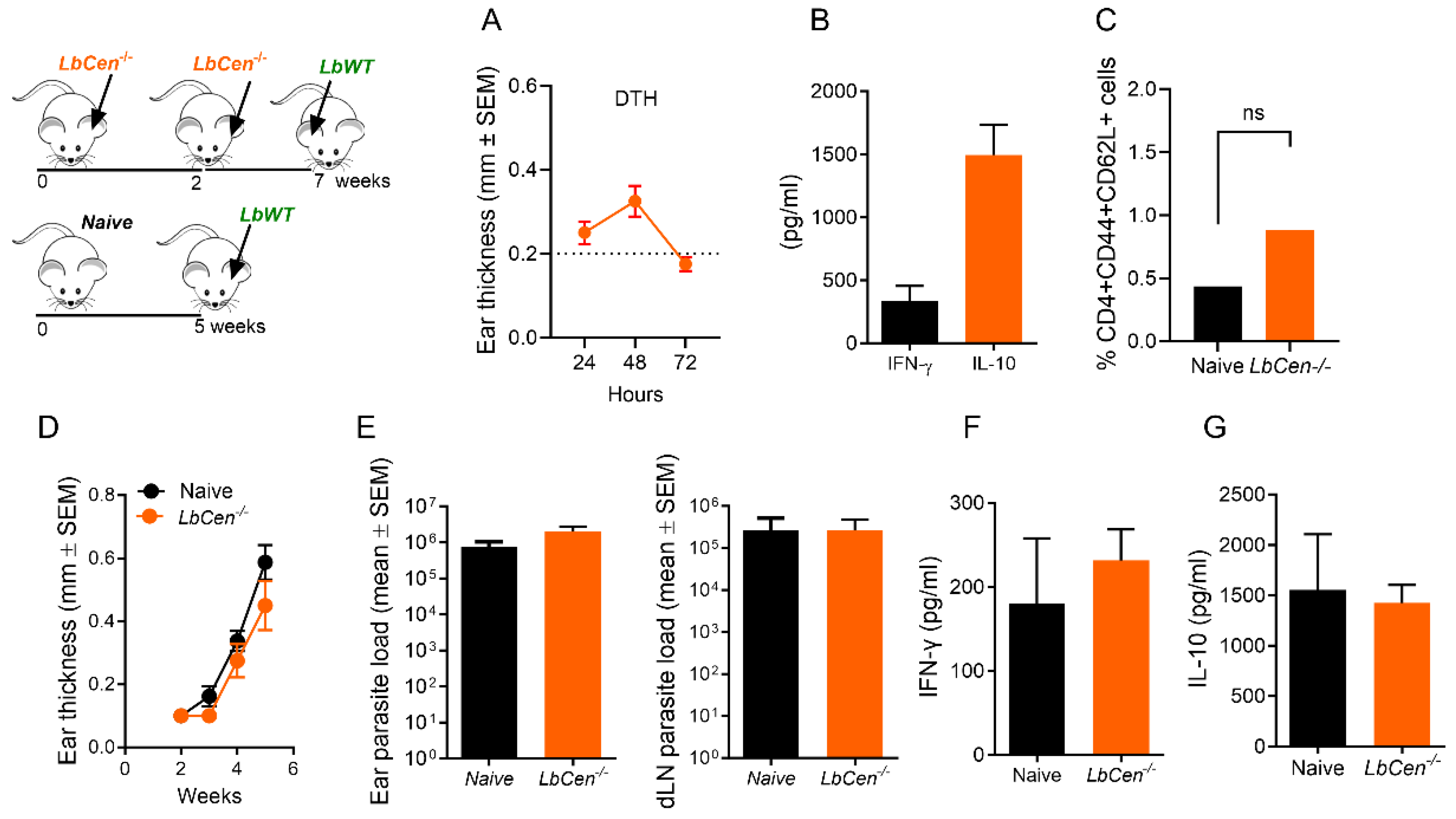
2.8. Primary and Booster Immunization
2.9. Cross-Protection: Immunization with LdonCen−/− and Challenge with LbWT
2.10. Statistical Analysis
3. Results
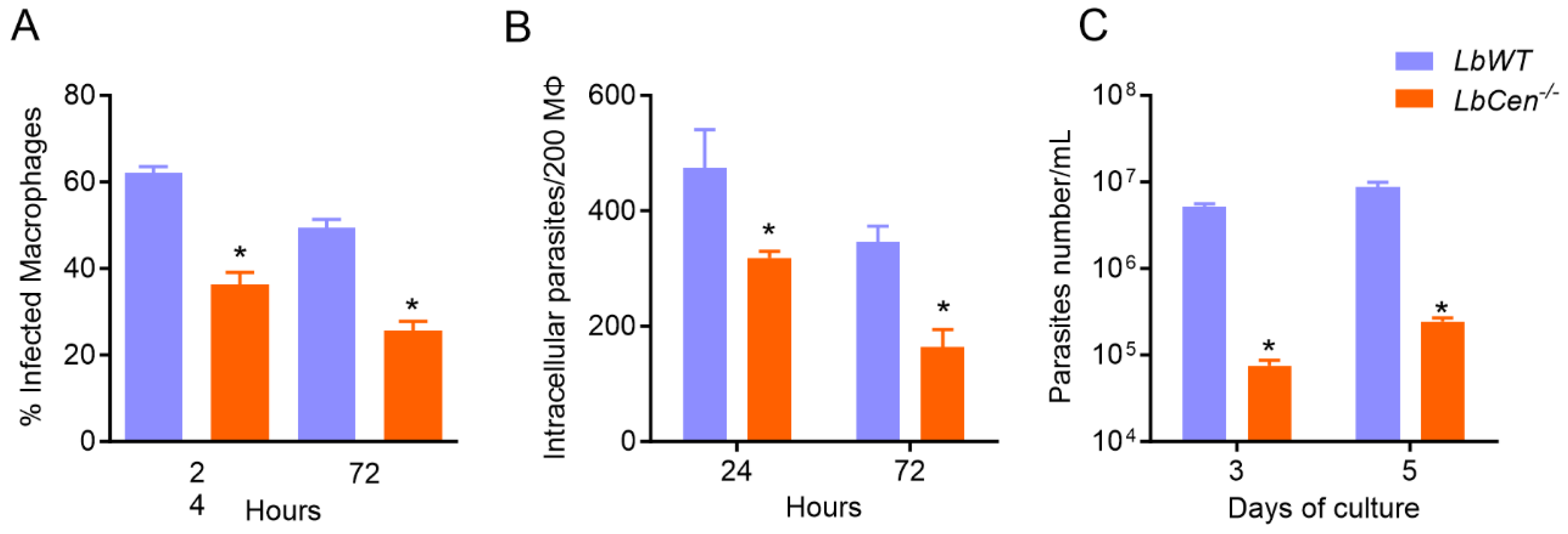
3.1. LbCen−/− Parasites Exhibit Impaired Growth and Survival in BMDMs
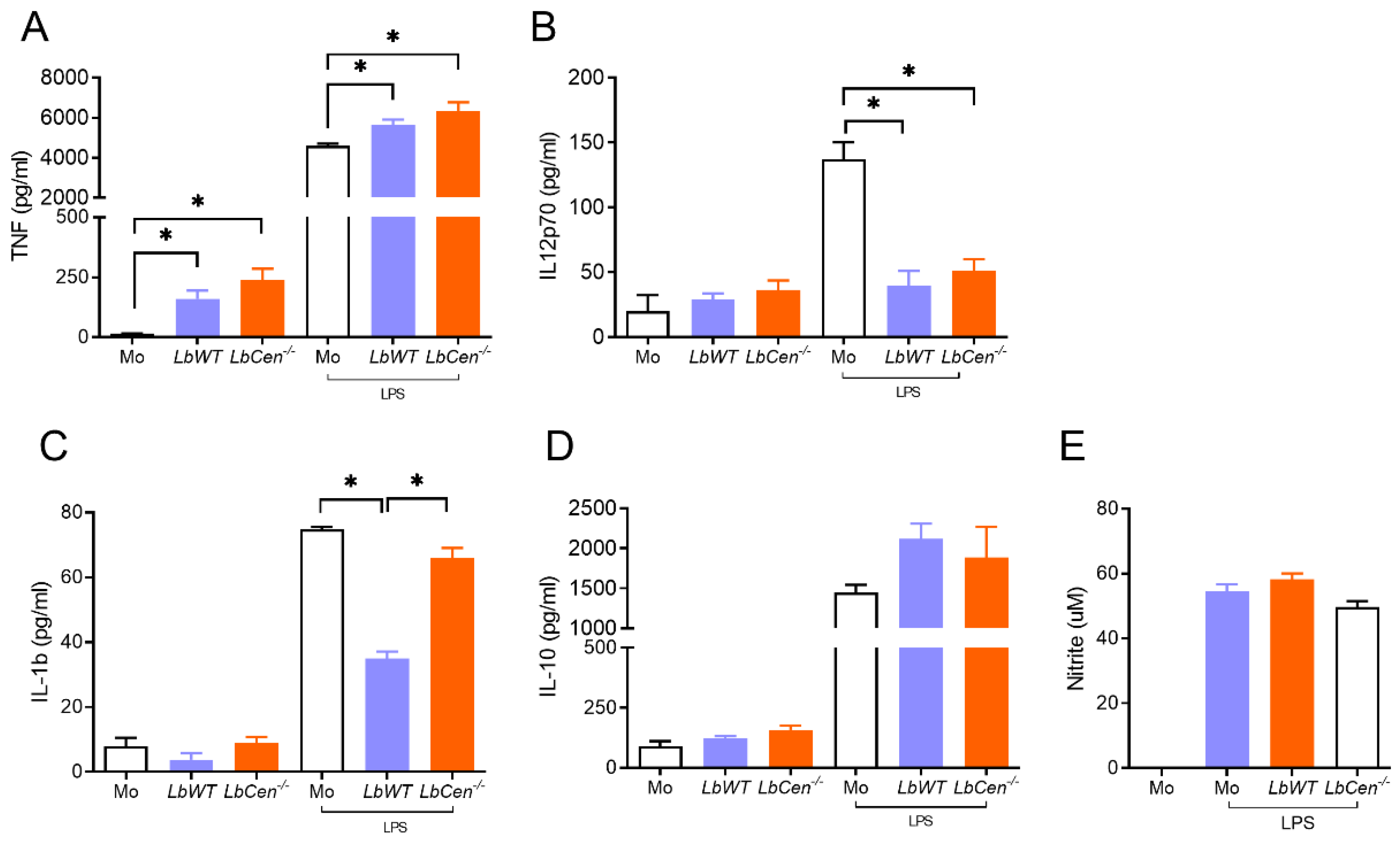
3.2. Macrophage Exposure to LbCen−/− or LbWT Induces Similar Cytokine Response
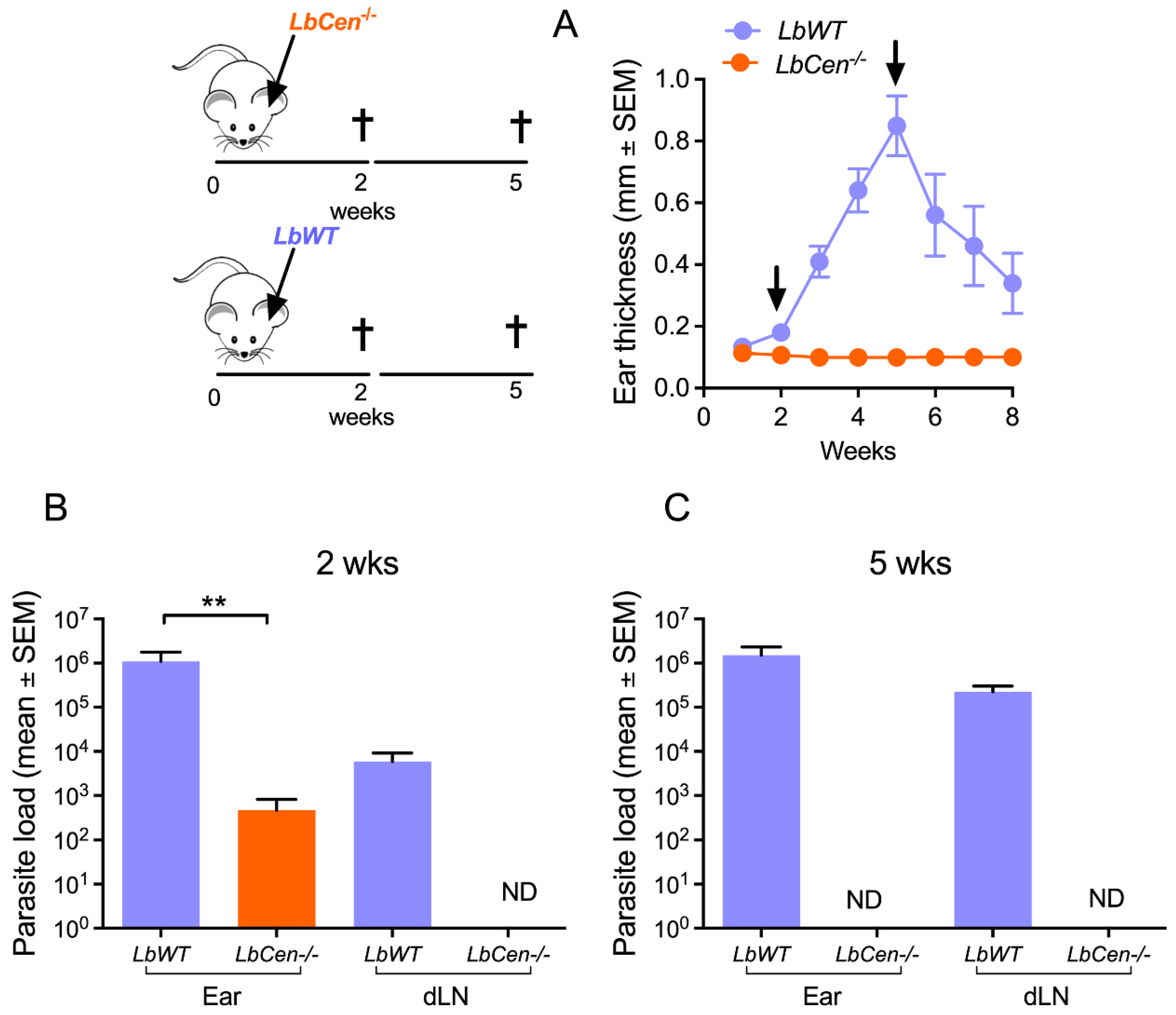
3.3. Immunization with LbCen−/− Does Not Cause Lesions and Is Safe
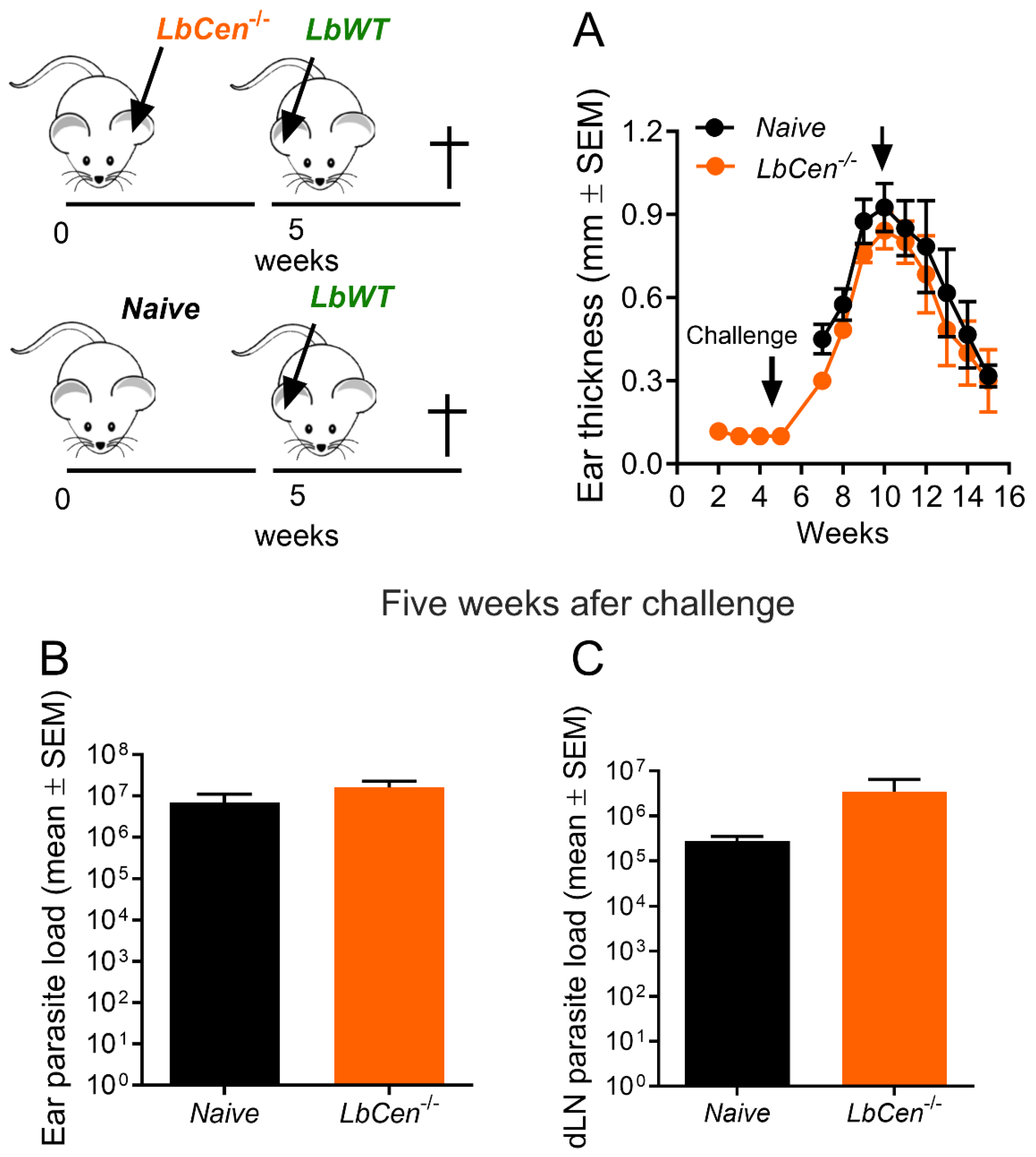
3.4. Immunization with LbCen−/− Does Not Protect against LbWT Infection
3.5. Immunization with LbCen−/− Cannot Recapitulate the Immune Response Induced by a Healed Primary Infection with LbWT
3.6. Priming/Boosting Induces Prolonged Parasite Persistence
3.7. Immunization with LdonCen−/− Cross-Protects against LbWT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 15, 951–970. [Google Scholar] [CrossRef]
- Pan American Health Organization. LEISHMANIASES Epidemiological Report on the Region of the Americas. Available online: https://iris.paho.org/bitstream/handle/10665.2/56831/PAHOCDEVT220021_eng.pdf?sequence=1&isAllowed=y (accessed on 2 February 2023).
- Sundar, S.; Singh, B. Identifying vaccine targets for anti-leishmanial vaccine development. Expert. Rev. Vaccines 2014, 13, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Mauël, J.; Ransijn, A.; Buchmüller-Rouiller, Y. Killing of Leishmania parasites in activated murine macrophages is based on an L-arginine-dependent process that produces nitrogen derivatives. J. Leukoc. Biol. 1991, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Cummings, H.E.; Tuladhar, R.; Satoskar, A.R. Cytokines and their STATs in cutaneous and visceral leishmaniasis. J. Biomed. Biotechnol. 2010, 2010, 294389. [Google Scholar] [CrossRef]
- Ikeogu, N.M.; Akaluka, G.N.; Edechi, C.A.; Salako, E.S.; Onyilagha, C.; Barazandeh, A.F.; Uzonna, J.E. Leishmania Immunity: Advancing Immunotherapy and Vaccine Development. Microorganisms 2020, 8, 1201. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.O.L.; Nogueira, P.M.; Monte-Neto, R.L. Next-generation Leishmanization: Revisiting Molecular Targets for Selecting Genetically Enigneered Live-Attenuated Leishmania. Microorganisms 2023, 11, 1043. [Google Scholar] [CrossRef]
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: A review of available data on their safety and efficacy. Trop. Med. Int. Health 2020, 25, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Matlashewski, G. CRISPR-Cas9-Mediated Genome Editing in Leishmania donovani. mBio 2015, 6, e00861. [Google Scholar] [CrossRef]
- Saljoughian, N.; Taheri, T.; Rafati, S. Live vaccination tactics: Possible approaches for controlling visceral leishmaniasis. Front. Immunol. 2014, 31, 134. [Google Scholar] [CrossRef]
- Wiech, H.; Geier, B.M.; Paschke, T.; Spang, A.; Grein, K.; Steinkötter, J.; Melkonian, M.; Schiebel, E. Characterization of green alga, yeast, and human centrins. Specific subdomain features determine functional diversity. J. Biol. Chem. 1996, 271, 22453–22461. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Dey, R.; Nylen, S.; Duncan, R.; Sacks, D.; Nakhasi, H.L. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J. Immunol. 2009, 183, 1813–1820. [Google Scholar] [CrossRef]
- de Moura, T.R.; Novais, F.O.; Oliveira, F.; Clarêncio, J.; Noronha, A.; Barral, A.; Brodskyn, C.; de Oliveira, C.I. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect. Immun. 2005, 73, 5827–5834. [Google Scholar] [CrossRef]
- Sharma, R.; Avendaño-Rangel, F.; Reis-Cunha, J.L.; Marques, L.P.; Figueira, C.P.; Borba, P.B.; Viana, S.M.; Beneke, T.; Bartholomeu, D.C.; de Oliveira, C.I. Targeted Deletion of Centrin in Leishmania braziliensis Using CRISPR-Cas9-Based Editing. Front. Cell Infect. Microbiol. 2022, 11, 790418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Karmakar, S.; Gannavaram, S.; Dey, R.; Lypaczewski, P.; Ismail, N.; Siddiqui, A.; Simonyan, V.; Oliveira, F.; Coutinho-Abreu, I.V.; et al. A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing. Nat. Commun. 2020, 11, 3461. [Google Scholar] [CrossRef] [PubMed]
- Volpedo, G.; Pacheco-Fernandez, T.; Holcomb, E.A.; Zhang, W.W.; Lypaczewski, P.; Cox, B.; Fultz, R.; Mishan, C.; Verma, C.; Huston, R.H.; et al. Centrin-deficient Leishmania mexicana confers protection against New World cutaneous leishmaniasis. NPJ Vaccines 2022, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.H.; Nathan, C.F.; Stuehr, D.J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Novais, F.O.; Carvalho, L.P.; Graff, J.W.; Beiting, D.P.; Ruthel, G.; Roos, D.S.; Betts, M.R.; Goldschmidt, M.H.; Wilson, M.E.; de Oliveira, C.I.; et al. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog. 2013, 9, e1003504. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.G.; Badaró, R.; Masur, H.; Carvalho, E.M.; Lorenco, R.; Lisboa, A.; Teixeira, R.; Johnson, W.D., Jr.; Jones, T.C. Selection of a skin test antigen for American visceral leishmaniasis. Am. J. Trop. Med. Hyg. 1986, 35, 79–85. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Debrabant, A.; Duncan, R.; Muller, J.; Salotra, P.; Sreenivas, G.; Salisbury, J.L.; Nakhasi, H.L. Centrin gene disruption impairs stage-specific basal body duplication and cell cycle progression in Leishmania. J. Biol. Chem. 2004, 279, 25703–25710. [Google Scholar] [CrossRef]
- Zaph, C.; Uzonna, J.; Beverley, S.M.; Scott, P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 2004, 10, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.C.; Kimblin, N.; Secundino, N.; Kamhawi, S.; Lawyer, P.; Sacks, D.L. Vector Transmission of Leishmania Abrogates Vaccine-Induced Protective Immunity. PLOS Pathog. 2009, 5, e1000484. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, M.; Nadim, A.; Khamesipour, A. An overview of leishmanization experience: A successful control measure and a tool to evaluate candidate vaccines. Acta Trop. 2019, 200, 105173. [Google Scholar] [CrossRef] [PubMed]
- Lu, S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef] [PubMed]
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical Syndromes and Treatment. QJM 2014, 107, 7–14. [Google Scholar] [CrossRef]
- Khamesipour, A.; Dowlati, Y.; Asilian, A.; Hashemi-Fesharki, R.; Javadi, A.; Noazin, S.; Modabber, F. Leishmanization: Use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine 2005, 23, 3642–3648. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Inchaustegui, D.A.; Xin, L.; Soong, L. Leishmania braziliensis infection induces dendritic cell activation, ISG15 transcription, and the generation of protective immune responses. J. Immunol. 2008, 180, 7537–7545. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.J.; Schleicher, U.; Mattner, J.; Alber, G.; Bogdan, C. Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice. Infect. Immun. 2007, 75, 3823–3832. [Google Scholar] [CrossRef]
- Lima-Junior, D.S.; Costa, D.L.; Carregaro, V.; Cunha, L.D.; Silva, A.L.N.; Mineo, T.W.P.; Gutierrez, F.R.S.; Bellio, M.; Bortoluci, K.R.; Flavell, R.A.; et al. Inflammasome-derived IL-1β production induces nitric oxide-mediated resistance to Leishmania. Nat. Med. 2013, 19, 909–915. [Google Scholar] [CrossRef]
- Uzonna, J.E.; Wei, G.; Yurkowski, D.; Bretscher, P. Immune elimination of Leishmania major in mice: Implications for immune memory, vaccination, and reactivation disease. J. Immunol. 2001, 167, 6967–6974. [Google Scholar] [CrossRef]
- Belkaid, Y.; Piccirillo, C.A.; Mendez, S.; Shevach, E.M.; Sacks, D.L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002, 420, 502–507. [Google Scholar] [CrossRef]
- Chatelain, R.; Varkila, K.; Coffman, R.L. IL-4 induces a Th2 response in Leishmania major-infected mice. J. Immunol. 1992, 148, 1182–1187. [Google Scholar] [CrossRef]
- Kopf, M.; Brombacher, F.; Köhler, G.; Kienzle, G.; Widmann, K.H.; Lefrang, K.; Humborg, C.; Ledermann, B.; Solbach, W. IL-4-deficient Balb/c mice resist infection with Leishmania major. J. Exp. Med. 1996, 184, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hoffmann, K.F.; Mendez, S.; Kamhawi, S.; Udey, M.C.; Wynn, T.A.; Sacks, D.L. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 2001, 194, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.S.; Carregaro, V.; Lima-Júnior, D.S.; Costa, D.L.; Ryffel, B.; Duthie, M.S.; de Jesus, A.; de Almeida, R.P.; da Silva, J.S. Interleukin 17A acts synergistically with interferon γ to promote protection against Leishmania infantum infection. J. Infect. Dis. 2015, 211, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.A.; Ronet, C.; Zangger, H.; Beverley, S.M.; Fasel, N. Leishmania RNA virus: When the host pays the toll. Front. Cell Infect. Microbiol. 2012, 2, 99. [Google Scholar] [CrossRef]
- Oljuskin, T.; Azodi, N.; Volpedo, G.; Bhattacharya, P.; Markle, H.L.; Hamano, S.; Matlashewski, G.; Satoskar, A.R.; Gannavaram, S.; Nakhasi, H.L. Leishmania major centrin knock-out parasites reprogram tryptophan metabolism to induce a pro-inflammatory response. iScience 2023, 26, 107593. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avendaño-Rangel, F.; Agra-Duarte, G.; Borba, P.B.; Moitinho, V.; Avila, L.T.; da Silva, L.O.; Viana, S.M.; Sharma, R.; Gannavaram, S.; Nakhasi, H.L.; et al. Immunization with centrin-Deficient Leishmania braziliensis Does Not Protect against Homologous Challenge. Vaccines 2024, 12, 310. https://doi.org/10.3390/vaccines12030310
Avendaño-Rangel F, Agra-Duarte G, Borba PB, Moitinho V, Avila LT, da Silva LO, Viana SM, Sharma R, Gannavaram S, Nakhasi HL, et al. Immunization with centrin-Deficient Leishmania braziliensis Does Not Protect against Homologous Challenge. Vaccines. 2024; 12(3):310. https://doi.org/10.3390/vaccines12030310
Chicago/Turabian StyleAvendaño-Rangel, Francys, Gabriela Agra-Duarte, Pedro B. Borba, Valdomiro Moitinho, Leslye T. Avila, Larissa O. da Silva, Sayonara M. Viana, Rohit Sharma, Sreenivas Gannavaram, Hira L. Nakhasi, and et al. 2024. "Immunization with centrin-Deficient Leishmania braziliensis Does Not Protect against Homologous Challenge" Vaccines 12, no. 3: 310. https://doi.org/10.3390/vaccines12030310
APA StyleAvendaño-Rangel, F., Agra-Duarte, G., Borba, P. B., Moitinho, V., Avila, L. T., da Silva, L. O., Viana, S. M., Sharma, R., Gannavaram, S., Nakhasi, H. L., & de Oliveira, C. I. (2024). Immunization with centrin-Deficient Leishmania braziliensis Does Not Protect against Homologous Challenge. Vaccines, 12(3), 310. https://doi.org/10.3390/vaccines12030310





