SARS-CoV-2 Neutralization Capacity in Hemodialysis Patients with and without a Fifth Vaccination with the Updated Comirnaty Original/Omicron BA.4-5 Vaccine
Abstract
1. Introduction
2. Material and Methods
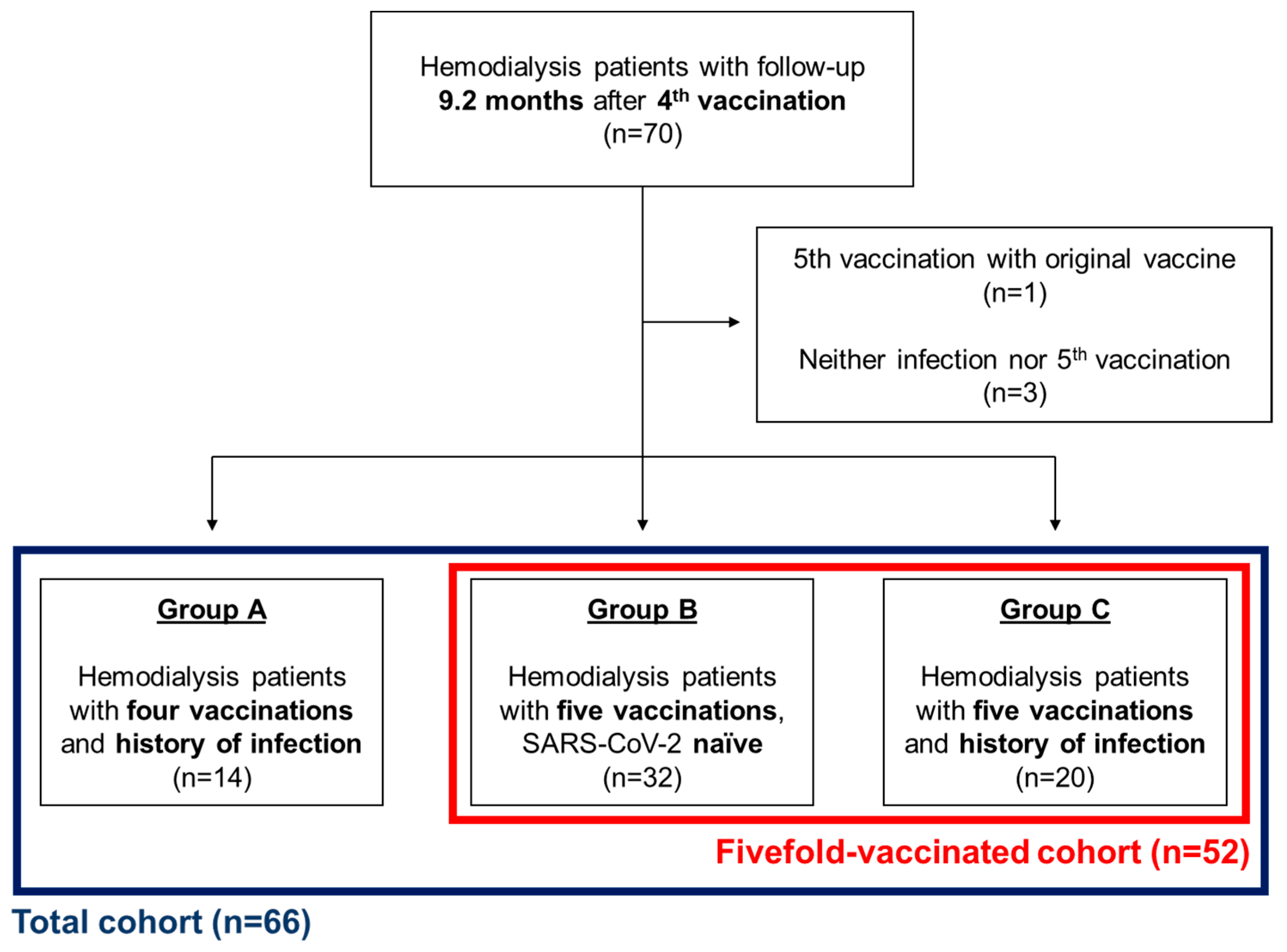
2.1. Study Design
2.2. Participants
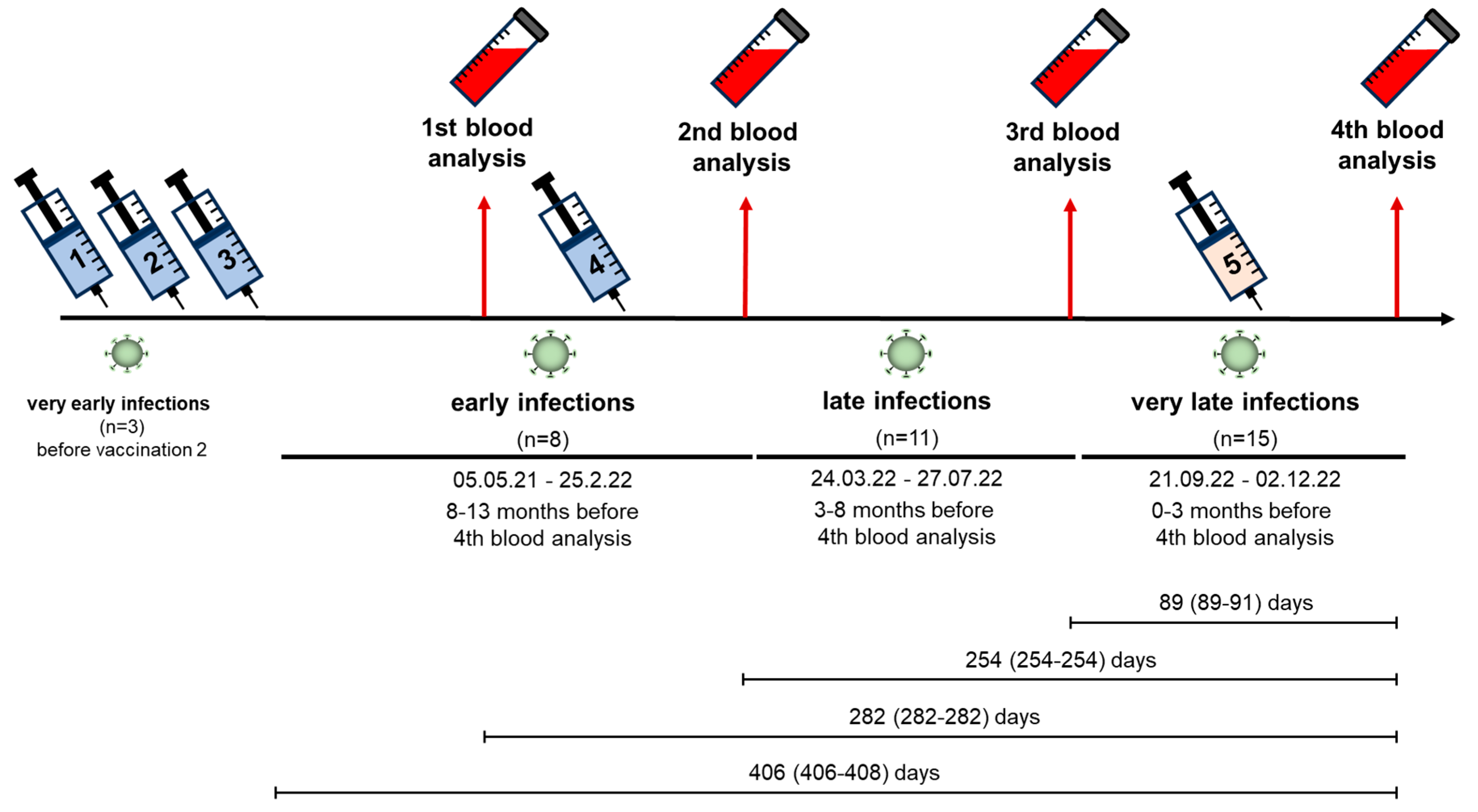
2.3. Infections
2.4. Serological Methods
2.5. Antibody Assays
2.6. NAb and Anti-n IgG
2.7. Anti-Spike (S) IgG
2.8. Live-Virus Neutralization Assays
2.9. Statistics
3. Results
3.1. Patient Characteristics
3.2. Vaccinations
3.3. SARS-CoV-2 Infections
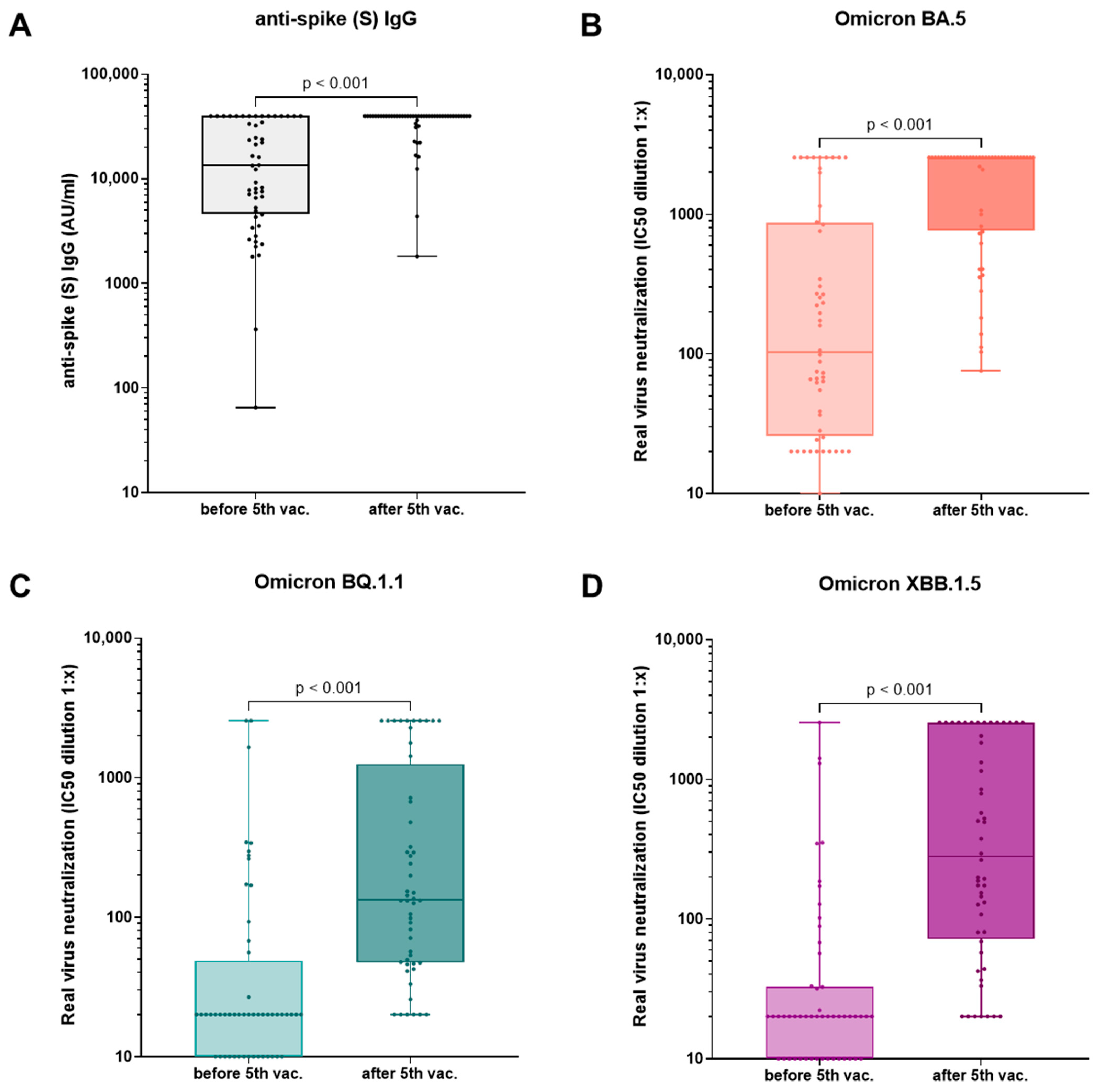
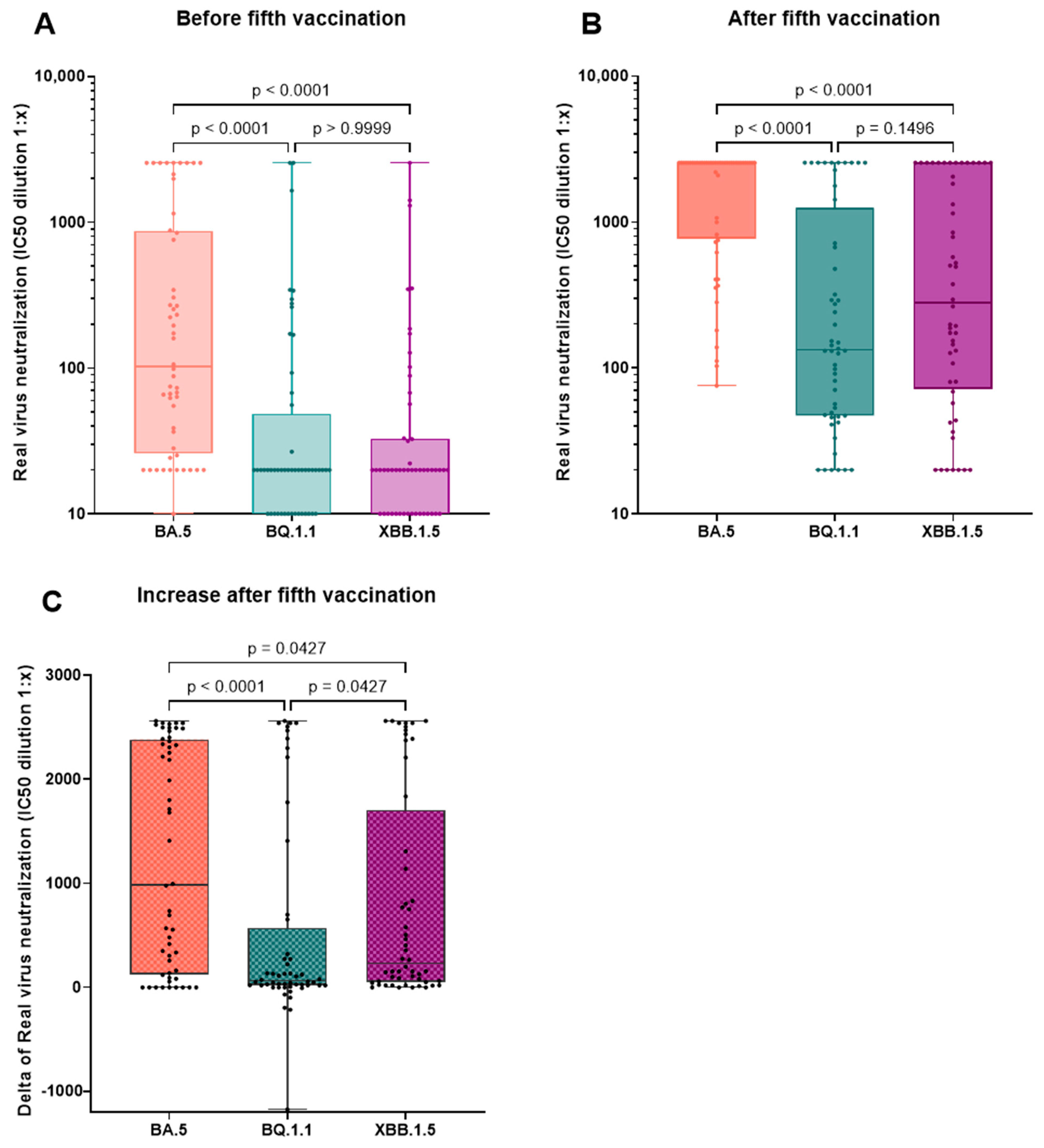
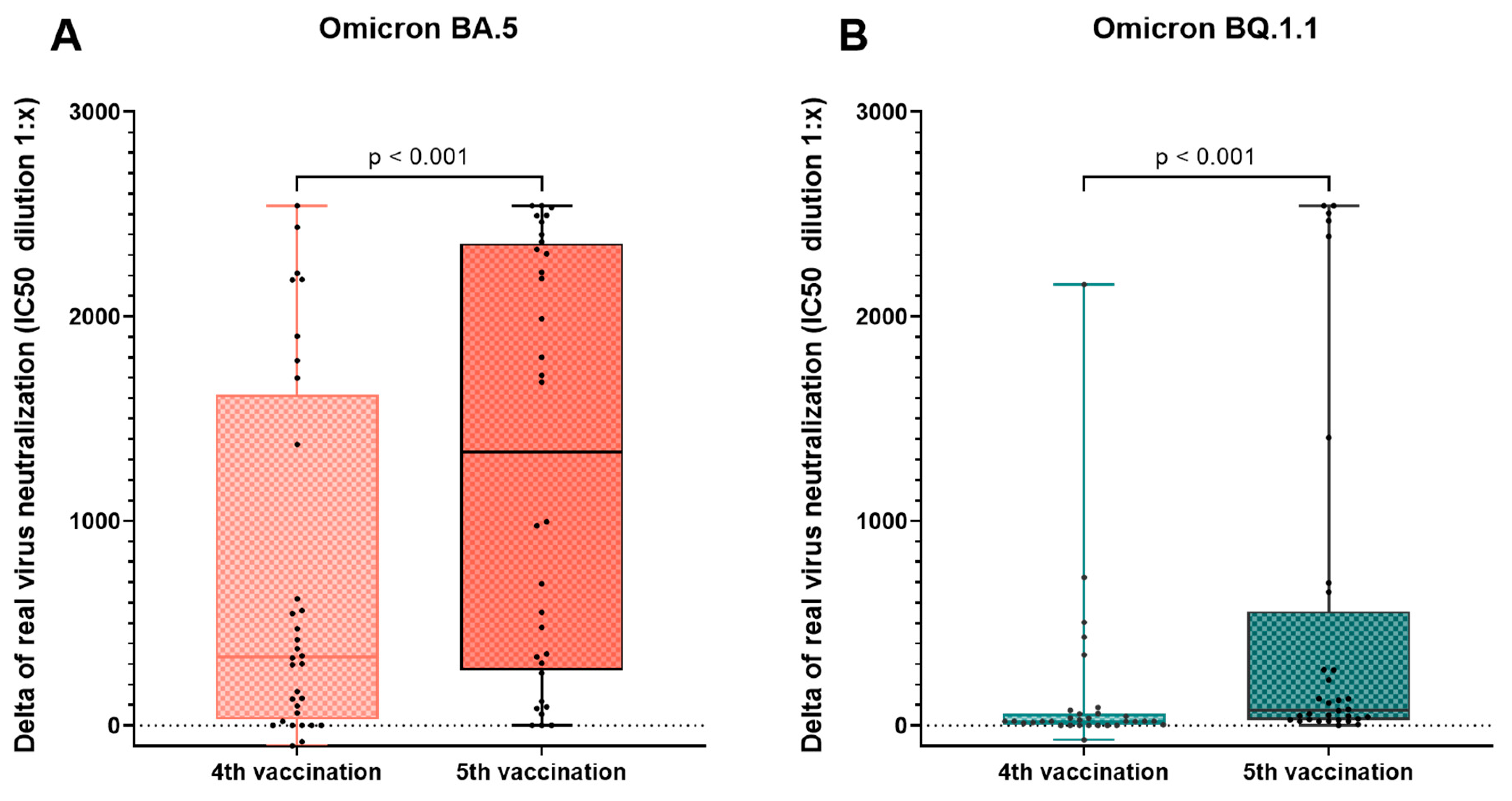
3.4. Effect of the Fourth and Fifth Vaccination
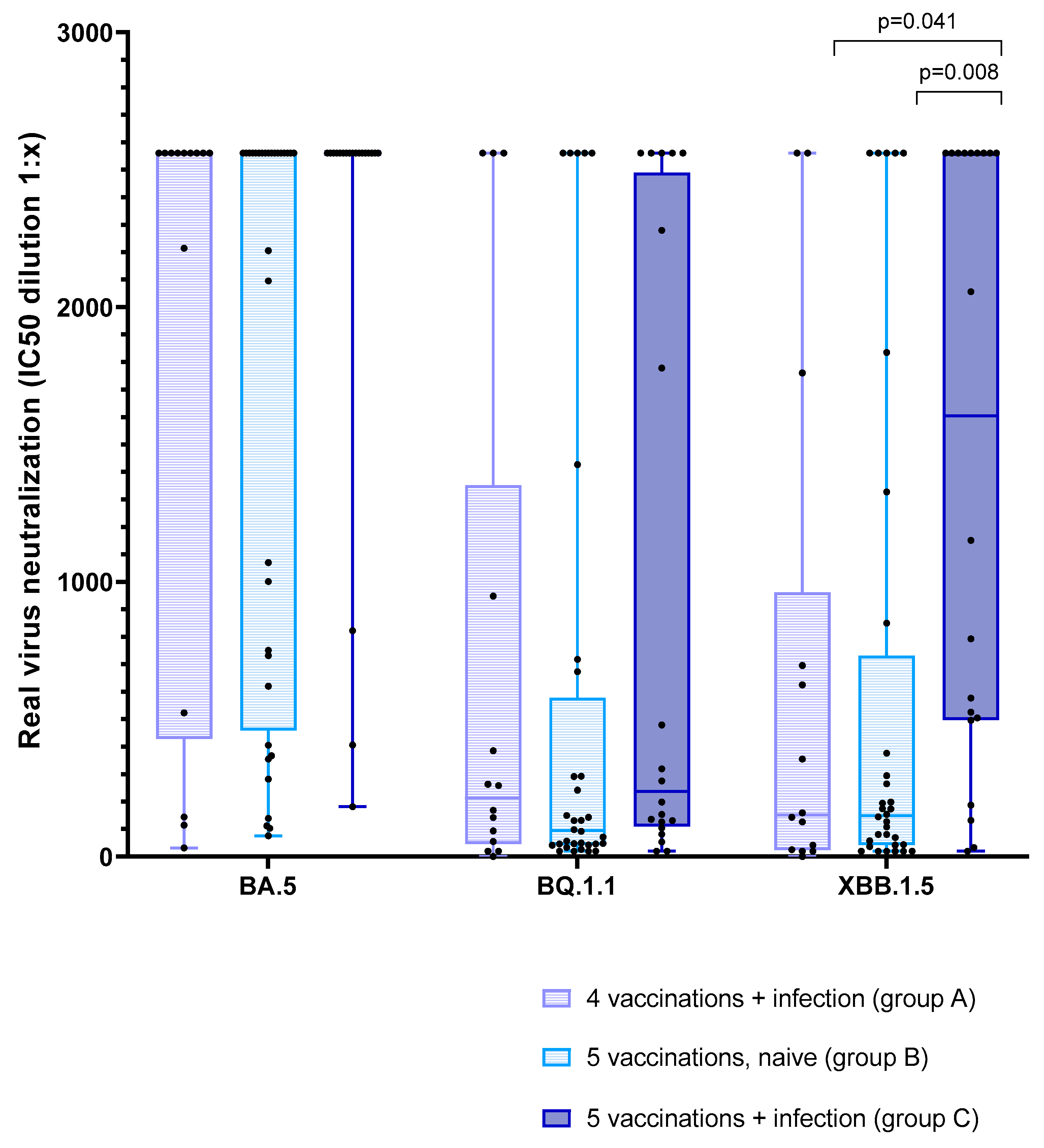
3.5. Effect of Vaccination and Breakthrough Infections
4. Discussion
5. Conclusions
6. Key Learning Points
6.1. What Is Already Known about This Subject?
- Hemodialysis patients are impaired in their immune response against SARS-CoV-2 and have an elevated morbidity and mortality risk compared with the general population.
- COVID-19 vaccinations activated immunity against SARS-CoV-2 in hemodialysis patients, but this is weaning over time, necessitating monitoring of immune status and regular booster vaccinations.
- Emerging new variants of SARS-CoV-2 usually possess immune-evasive properties and increased transmissibility, requiring the regular analysis of patients’ immunity against these new variants.
6.2. What Does This Study Add?
- In hemodialysis patients, a fifth vaccination with a BA.4-5-adapted COVID-19 vaccine leads to an improved humoral immunity against the original and evolved SARS-CoV-2 strains eight months after the last vaccination.
- The increase in neutralizing capacity after the vaccination with the BA.4-5-adapted vaccine is significantly lower for more recent VoCs like BQ.1.1 and XBB.1.5 compared to BA.5.
- Humoral immunity towards the original and recent SARS-CoV-2 variants did not differ between fourfold-vaccinated HD patients with a recent history of infection and SARS-CoV-2-naïve patients after a fifth vaccination with an adapted COVID-19 vaccine.
6.3. What Impact May This Have on Practice?
- A fifth vaccination seems sensible in hemodialysis patients who did not experience a spike exposition within eight months following the fourth vaccination.
- The exposition to a SARS-CoV-2 spike protein of more recent VoCs, e.g., in an XBB-adapted vaccine, might provide patients with better immunity against current variants.
- Comparable to COVID-19 vaccines, breakthrough infections provide patients with a broadened immunity against current VoCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharif, M.R.; Chitsazian, Z.; Moosavian, M.; Raygan, F.; Nikoueinejad, H.; Sharif, A.R.; Einollahi, B. Immune disorders in hemodialysis patients. Iran. J. Kidney Dis. 2015, 9, 84–96. [Google Scholar]
- Takazono, T.; Ngwe Tun, M.M.; Funakoshi, S.; Morimoto, S.; Ota, K.; Torigoe, K.; Abe, S.; Muta, K.; Ito, Y.; Ashizawa, N.; et al. Long-Term Neutralizing Antibody Titers After BNT162b2 Vaccination in Hemodialysis Patients. Kidney Int. Rep. 2023, 8, 1883–1886. [Google Scholar] [CrossRef]
- Li, P.; Guan, Y.; Zhou, S.; Wang, E.; Sun, P.; Fei, G.; Zeng, D.; Wang, R. Mortality and risk factors for COVID-19 in hemodialysis patients: A systematic review and meta-analysis. Sci. Prog. 2022, 105, 368504221110858. [Google Scholar] [CrossRef]
- Weinhandl, E.D.; Liu, J.; Gilbertson, D.T.; Wetmore, J.B.; Johansen, K.L. Associations of COVID-19 Outcomes with Dialysis Modalities and Settings. Clin. J. Am. Soc. Nephrol. 2022, 17, 1526–1534. [Google Scholar] [CrossRef]
- Robert-Koch-Institut. Mitteilung der Ständigen Impfkommission beim Robert Koch-Institut Beschluss der STIKO zur 4. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung—Aktualisierung vom 1. April 2021. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/Ausgaben/16_21.pdf?__blob=publicationFile (accessed on 20 September 2023).
- Wijkstrom, J.; Caldinelli, A.; Bruchfeld, A.; Nowak, A.; Artborg, A.; Stendahl, M.; Segelmark, M.; Lindholm, B.; Bellocco, R.; Rydell, H.; et al. Results of the first nationwide cohort study of outcomes in dialysis and kidney transplant patients before and after vaccination for COVID-19. Nephrol. Dial. Transplant. 2023, 38, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Platen, L.; Liao, B.-H.; Tellenbach, M.; Cheng, C.-C.; Holzmann-Littig, C.; Christa, C.; Dächert, C.; Kappler, V.; Bester, R.; Werz, M.L.; et al. Longitudinal SARS-CoV-2 neutralization of Omicron BA.1, BA.5 and BQ.1.1 after four vaccinations and the impact of breakthrough infections in haemodialysis patients. Clin. Kidney J. 2023, 16, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- European-Medicines-Agency. Comirnaty: EMA Recommends Approval of Adapted COVID-19 Vaccine Targeting Omicron XBB.1.5. Available online: https://www.ema.europa.eu/en/news/comirnaty-ema-recommends-approval-adapted-covid-19-vaccine-targeting-omicron-xbb15 (accessed on 20 September 2023).
- Robert-Koch-Institut. SARS-CoV-2 Varianten in Deutschland. Available online: https://public.data.rki.de/t/public/views/IGS_Dashboard/DashboardVariants?%3Aembed=y&%3AisGuestRedirectFromVizportal=y (accessed on 20 September 2023).
- European-Medicines-Agency. Spikevax: EMA Recommends Approval of Adapted COVID-19 Vaccine Targeting Omicron XBB.1.5|European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/spikevax-ema-recommends-approval-adapted-covid-19-vaccine-targeting-omicron-xbb15 (accessed on 20 September 2023).
- European-Medicines-Agency. Adapted Vaccine Targeting BA.4 and BA.5 Omicron Variants and Original SARS-CoV-2 Recommended for Approval. Available online: https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omicron-variants-original-sars-cov-2-recommended-approval (accessed on 20 September 2023).
- Robert-Koch-Institut. Stellungnahme der STIKO anlässlich der Zulassung von XBB.1.5-Varianten-Adaptierten COVID-19-Impfstoffen für die Auffrischimpfung von Personen mit erhöhtem Risiko für einen schweren Krankheits verlauf—Stand: 18 September 2023. Available online: https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/Stellungnahme-COVID-19-Varianten-adaptierte-Impfstoffe.html (accessed on 20 September 2023).
- Cheng, C.-C.; Platen, L.; Christa, C.; Tellenbach, M.; Kappler, V.; Bester, R.; Liao, B.-H.; Holzmann-Littig, C.; Werz, M.; Schönhals, E.; et al. Improved SARS-CoV-2 Neutralization of Delta and Omicron BA.1 Variants of Concern after Fourth Vaccination in Hemodialysis Patients. Vaccines 2022, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Robert-Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19) 10 March 2022—AKTUALISIERTER STAND FÜR DEUTSCHLAND. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2022-03-10.pdf?__blob=publicationFile (accessed on 5 September 2023).
- Robert-Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19) 18 August 2022—AKTUALISIERTER STAND FÜR DEUTSCHLAND. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2022-08-18.pdf?__blob=publicationFile (accessed on 5 September 2023).
- Robert-Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19) 15 December 2022—AKTUALISIERTER STAND FÜR DEUTSCHLAND. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2022-12-15.pdf?__blob=publicationFile (accessed on 5 September 2023).
- Perkmann, T.; Perkmann-Nagele, N.; Koller, T.; Mucher, P.; Radakovics, A.; Marculescu, R.; Wolzt, M.; Wagner, O.F.; Binder, C.J.; Haslacher, H. Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: A Head-to-Head Comparison of Five Quantitative Assays. Microbiol. Spectr. 2021, 9, e0024721. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Tani, Y.; Takita, M.; Wakui, M.; Saito, H.; Nishiuchi, T.; Zhao, T.; Yamamoto, C.; Kawamura, T.; Sugiyama, A.; Nakayama, A.; et al. Five doses of the mRNA vaccination potentially suppress ancestral-strain stimulated SARS-CoV2-specific cellular immunity: A cohort study from the Fukushima vaccination community survey, Japan. Front. Immunol. 2023, 14, 1240425. [Google Scholar] [CrossRef]
- Anft, M.; Skrzypczyk, S.; Frahnert, M.; Fricke, L.; Zapka, J.; Kühn, D.; Koos, B.; Adamzik, M.; Pfaender, S.; Stervbo, U.; et al. Immunogenicity of Bivalent Omicron BA.4/5-Adapted Vaccine in Hemodialysis Patients. Kidney Int. Rep. 2023, 8, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Huth, L.; Schäfer, L.; Almanzar, G.; Lupoli, G.; Bischof, M.; Wratil, P.R.; Stövesand, T.; Drechsler, C.; Keppler, O.T.; Prelog, M. Immunologic Effect of Bivalent mRNA Booster in Patients Undergoing Hemodialysis. N. Eng. J. Med. 2023, 388, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Bartenschlager, M.; Kim, H.; Kalble, F.; Nusshag, C.; Buylaert, M.; Reichel, P.; Schaier, M.; Morath, C.; Zeier, M.; et al. Live-virus serum neutralization after bivalent SARS-CoV-2 mRNA vaccination in hemodialysis patients. J. Med. Virol. 2023, 95, e29303. [Google Scholar] [CrossRef]
- Affeldt, P.; Brensing, K.A.; Heger, E.; Wirtz, M.; Steger, G.; Koehler, F.C.; Benzing, T.; Stippel, D.; Klein, F.; Kurschat, C.; et al. Neutralizing response against SARS-CoV-2 Omicron BA.5 and XBB.1.5 in hemodialysis patients. Clin. Kidney J. 2023, 16, 2757–2759. [Google Scholar] [CrossRef] [PubMed]
- Kaku, Y.; Kosugi, Y.; Uriu, K.; Ito, J.; Hinay, A.A., Jr.; Kuramochi, J.; Sadamasu, K.; Yoshimura, K.; Asakura, H.; Nagashima, M.; et al. Antiviral efficacy of the SARS-CoV-2 XBB breakthrough infection sera against omicron subvariants including EG.5. Lancet Infect. Dis. 2023, 23, e395–e396. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Yasuhara, A.; Ito, M.; Akasaka, O.; Nakamura, M.; Nakachi, I.; Koga, M.; Mitamura, K.; Yagi, K.; Maeda, K.; et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine 2021, 32, 100734. [Google Scholar] [CrossRef]
- World-Health-Organisation. EG.5 Initial Risk Evaluation, 9 August 2023. Available online: https://www.who.int/docs/default-source/coronaviruse/09082023eg.5_ire_final.pdf (accessed on 20 September 2023).
- Abbasi, J. What to Know about EG.5, the Latest SARS-CoV-2 “Variant of Interest”. JAMA 2023, 330, 900–901. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Jacob-Dolan, C.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature 2022, 603, 493–496. [Google Scholar] [CrossRef]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Khan, S.R.; Sasson, S.C.; Kent, S.J.; Khoury, D.S.; Davenport, M.P. Predicting vaccine effectiveness against severe COVID-19 over time and against variants: A meta-analysis. Nat. Commun. 2023, 14, 1633. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; Caggiano, G.; Squiccimarro, E.; Losappio, V.; Fiorentino, M.; Alfieri, C.; Stallone, G.; Gesualdo, L.; Castellano, G. Enhancing Immune Protection in Hemodialysis Patients: Role of the Polymethyl Methacrylate Membrane. Blood Purif. 2023, 52, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Total n = 66 | 4 Vaccinations + Infection (Group A) n = 14 | 5 Vaccinations, Infection-Naïve (Group B) n = 32 | 5 Vaccinations + Infection (Group C) n = 20 | p | |
|---|---|---|---|---|---|
| Age (years) | 72.7 (60.3–81.5) | 69.7 (58.5–79.5) | 74.2 (64.8–82.1) | 75.5 (59.8–80.6) | ns |
| Female | 21 (31.8%) | 2 (14.3%) | 11 (34.4%) | 8 (40%) | ns |
| Dialysis vintage (months) | 42.0 (15.5–65.5) | 17.2 (8.5–32.6) | 55.4 (34.2–79.0) | 33.6 (14.8–65.6) | p = 0.0023 (A vs. B) * |
| Immunosuppressive medication | 10 (15.2%) | 2 (14.3%) | 5 (15.6%) | 3 (15%) | na |
| Charlson Comorbidity Index [18] | 7.0 (5.0–9.0) | 7.5 (5.2–8.8) | 8.0 (6.0–9.0) | 6.0 (5.0–9.0) | ns |
| Renal diagnosis | na | ||||
| Glomerulopathy | 12 (18.2%) | 3 (21.4%) | 7 (21.9%) | 2 (10.0%) | |
| Diabetic nephropathy | 12 (18.2%) | 4 (28.6%) | 5 (15.6%) | 3 (15.0%) | |
| Hypertensive nephropathy | 8 (12.1%) | 1 (7.1%) | 5 (15.6%) | 2 (10.0%) | |
| Congenital or cystic renal disease | 7 (10.6%) | 1 (7.1%) | 4 (12.5%) | 1 (5.0%) | |
| Tubulointerstitial disease | 1 (1.5%) | 0 (0.0%) | 1 (3.1%) | 0 (0.0%) | |
| Reflux nephropathy | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Other | 6 (9.1%) | 1 (7.1%) | 2 (6.3%) | 3 (15.0%) | |
| Nephropathy of unkown origin | 20 (30.3%) | 4 (28.6%) | 8 (25.0%) | 8 (40.0%) | |
| History of kidney transplantation | 5 (7.6%) | 2 (14.3%) | 3 (9.4%) | 0 (0.0%) | na |
| 4 Vaccinations + Infection (Group A) | 5 Vaccinations, Infection-Naïve (Group B) | 5 Vaccinations + Infection (Group C) | Group-Wise Comparison (Kruskal–Wallis Test and Dunn’s test) | |||
|---|---|---|---|---|---|---|
| p (Adjusted p) A vs. B | p (Adjusted p) A vs. C | p (Adjusted p) B vs. C | ||||
| Neutralizing antibodies (BAU/mL) | ||||||
| Before 5th vac./analysis 3 | ≥800 (147.5–≥800) | 773.5 (506.2–≥800) | ≥800 (675.2–≥800) | 0.784 | 0.508 | 0.321 |
| After 5th vac./analysis 4 | ≥800 (≥800–≥800) | ≥800 (≥800–≥800) | ≥800 (≥800–≥800) | 0.088 | 0.925 | 0.052 |
| anti-spike (S) IgG (BAU/mL) | ||||||
| Before 5th vac./analysis 3 | 9205.0 (2387.5–34,979.5) | 7948.5 (4310.2–23,892.5) | 34,197.5 (10,497.2–≥40,000) | 0.644 | 0.162 | 0.058 |
| After 5th vac./analysis 4 | 36,955.5 (20,258.5–≥40,000) | ≥40,000 (35,322.2–≥40,000) | ≥40,000 (≥40,000–≥40,000) | 0.121 | 0.046 | 0.388 |
| Virus neutralization of Omicron BA.5 (IC50) | ||||||
| Before 5th vac./analysis 3 | 130.3 (68.4–461.5) | 67.3 (23.2–258.2) | 286.9 (70.8–≥2560) | 0.276 | 0.369 | 0.037 |
| After 5th vac./analysis 4 | ≥2560 (945.6–≥2560) | ≥2560 (566.3–≥2560) | ≥2560 (≥2560–≥2560) | 0.655 | 0.138 | 0.023 |
| Virus neutralization of Omicron BQ.1.1 (IC50) | ||||||
| Before 5th vac./analysis 3 | 20.0 (5.0–111.4) | 20.0 (0.0–20.0) | 47.2 (0.0–308.1) | 0.385 | 0.380 | 0.026 |
| After 5th vac./analysis 4 | 213.9 (64.6–807.7) | 95.0 (45.0–387.6) | 236.6 (120.7–2349.2) | 0.458 | 0.571 | 0.072 |
| Virus neutralization of Omicron XBB.1.5 (IC50) | ||||||
| Before 5th vac./analysis 3 | 20.0 (0.0–44.5) | 20.0 (0.0–20.0) | 32.1 (15.0–175.7) | 0.562 | 0.301 | 0.010 |
| After 5th vac./analysis 4 | 151.2 (29.3–678.1) | 149.5 (43.4–494.6) | 1603.5 (502.3–≥2560) | 0.948 | 0.017 (0.041) | 0.002 (0.008) |
| Group | Infection Subgroup | n | IgG (AU/mL) | NAb (BAU/mL) | Anti-Spike (S) IgG (BAU/mL) | BA.5 (IC50) | BQ.1.1 (IC50) | XBB.1.5 (IC50) |
|---|---|---|---|---|---|---|---|---|
| 4 vaccinations + infection (group A) | early | 3 | 4 | ≥800 | 29,413 | 523 | 55 | 41 |
| late | 3 | 7 | ≥800 | ≥40,000 | 2214 | 142 | 143 | |
| very late | 8 | 20 | ≥800 | 36,956 | 2560 | 606 | 428 | |
| 5 vaccinations, infection-naïve (group B) | all | 32 | 1 | ≥800 | ≥40,000 | 2560 | 95 | 150 |
| 5 vaccinations + infection (group C) | early | 5 | 4 | ≥800 | ≥40,000 | 2560 | 153 | 792 |
| late | 8 | 11 | ≥800 | ≥40,000 | 2560 | 237 | 1604 | |
| very late | 7 | 65 | ≥800 | ≥40,000 | 2560 | 320 | 2560 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, B.-H.; Platen, L.; Grommes, M.; Cheng, C.-C.; Holzmann-Littig, C.; Christa, C.; Haller, B.; Kappler, V.; Bester, R.; Werz, M.L.; et al. SARS-CoV-2 Neutralization Capacity in Hemodialysis Patients with and without a Fifth Vaccination with the Updated Comirnaty Original/Omicron BA.4-5 Vaccine. Vaccines 2024, 12, 308. https://doi.org/10.3390/vaccines12030308
Liao B-H, Platen L, Grommes M, Cheng C-C, Holzmann-Littig C, Christa C, Haller B, Kappler V, Bester R, Werz ML, et al. SARS-CoV-2 Neutralization Capacity in Hemodialysis Patients with and without a Fifth Vaccination with the Updated Comirnaty Original/Omicron BA.4-5 Vaccine. Vaccines. 2024; 12(3):308. https://doi.org/10.3390/vaccines12030308
Chicago/Turabian StyleLiao, Bo-Hung, Louise Platen, Myriam Grommes, Cho-Chin Cheng, Christopher Holzmann-Littig, Catharina Christa, Bernhard Haller, Verena Kappler, Romina Bester, Maia Lucia Werz, and et al. 2024. "SARS-CoV-2 Neutralization Capacity in Hemodialysis Patients with and without a Fifth Vaccination with the Updated Comirnaty Original/Omicron BA.4-5 Vaccine" Vaccines 12, no. 3: 308. https://doi.org/10.3390/vaccines12030308
APA StyleLiao, B.-H., Platen, L., Grommes, M., Cheng, C.-C., Holzmann-Littig, C., Christa, C., Haller, B., Kappler, V., Bester, R., Werz, M. L., Platen, E., Eggerer, P., Tréguer, L., Küchle, C., Schmaderer, C., Heemann, U., Renders, L., Protzer, U., & Braunisch, M. C. (2024). SARS-CoV-2 Neutralization Capacity in Hemodialysis Patients with and without a Fifth Vaccination with the Updated Comirnaty Original/Omicron BA.4-5 Vaccine. Vaccines, 12(3), 308. https://doi.org/10.3390/vaccines12030308






