Application of Recombinant Lactic Acid Bacteria (LAB) Live Vector Oral Vaccine in the Prevention of F4+ Enterotoxigenic Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Plasmid Construction and the Induction of Recombinant LAB
2.3. Western Blot
2.4. Oral Vaccine Preparation
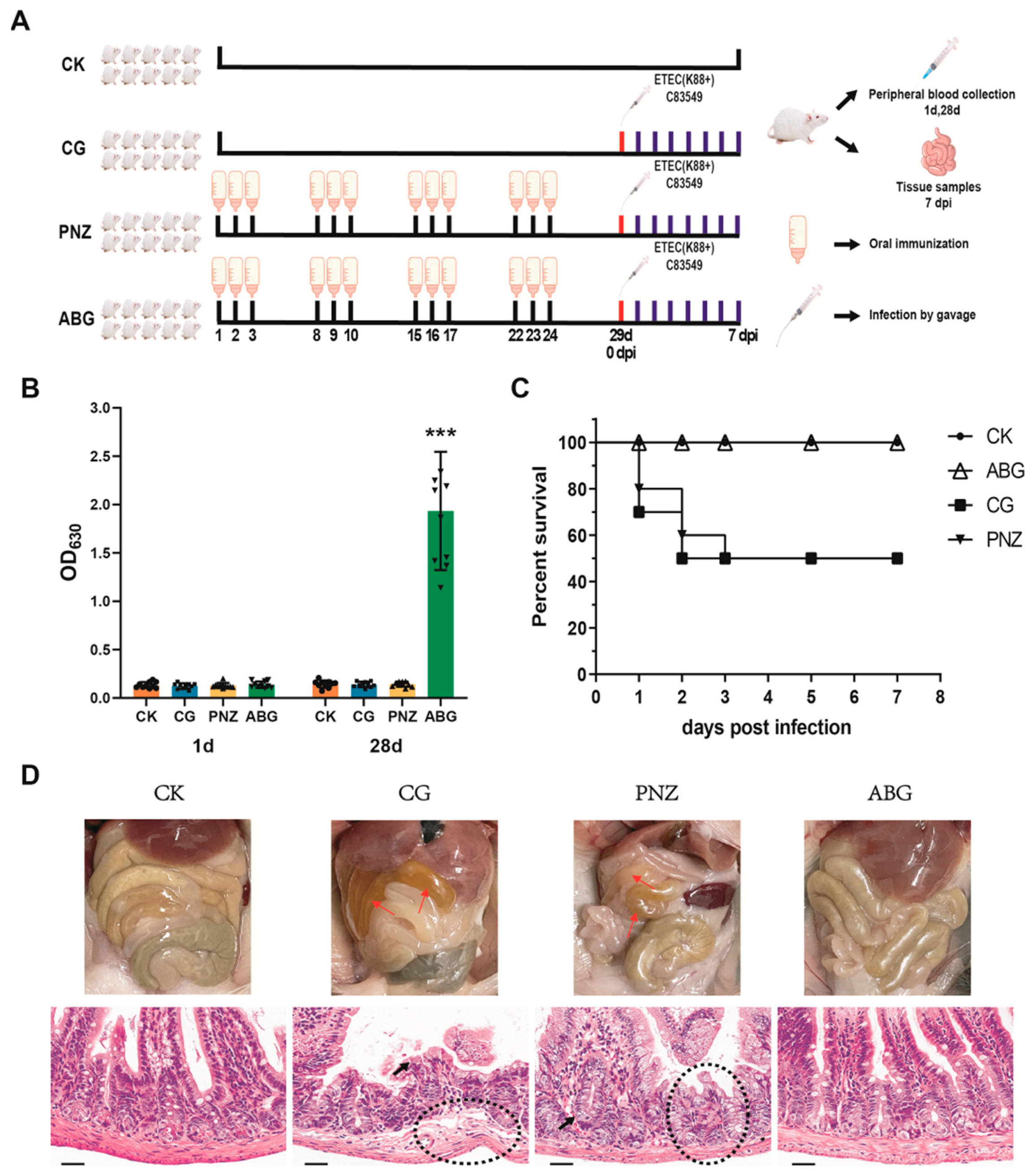
2.5. Mouse Immunoprotection Assay
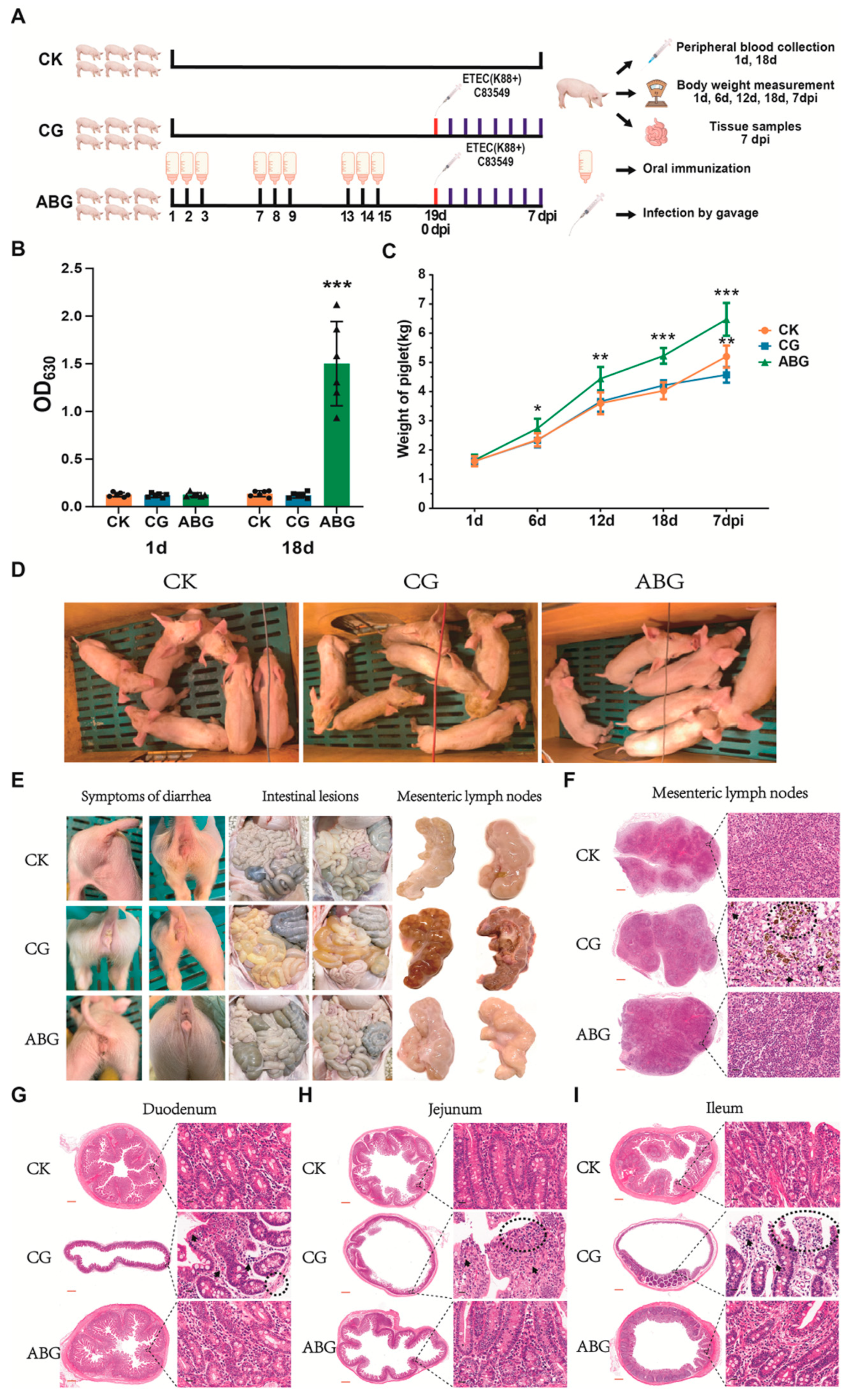
2.6. Immunoprotection Assay for Piglets without Maternal Antibodies
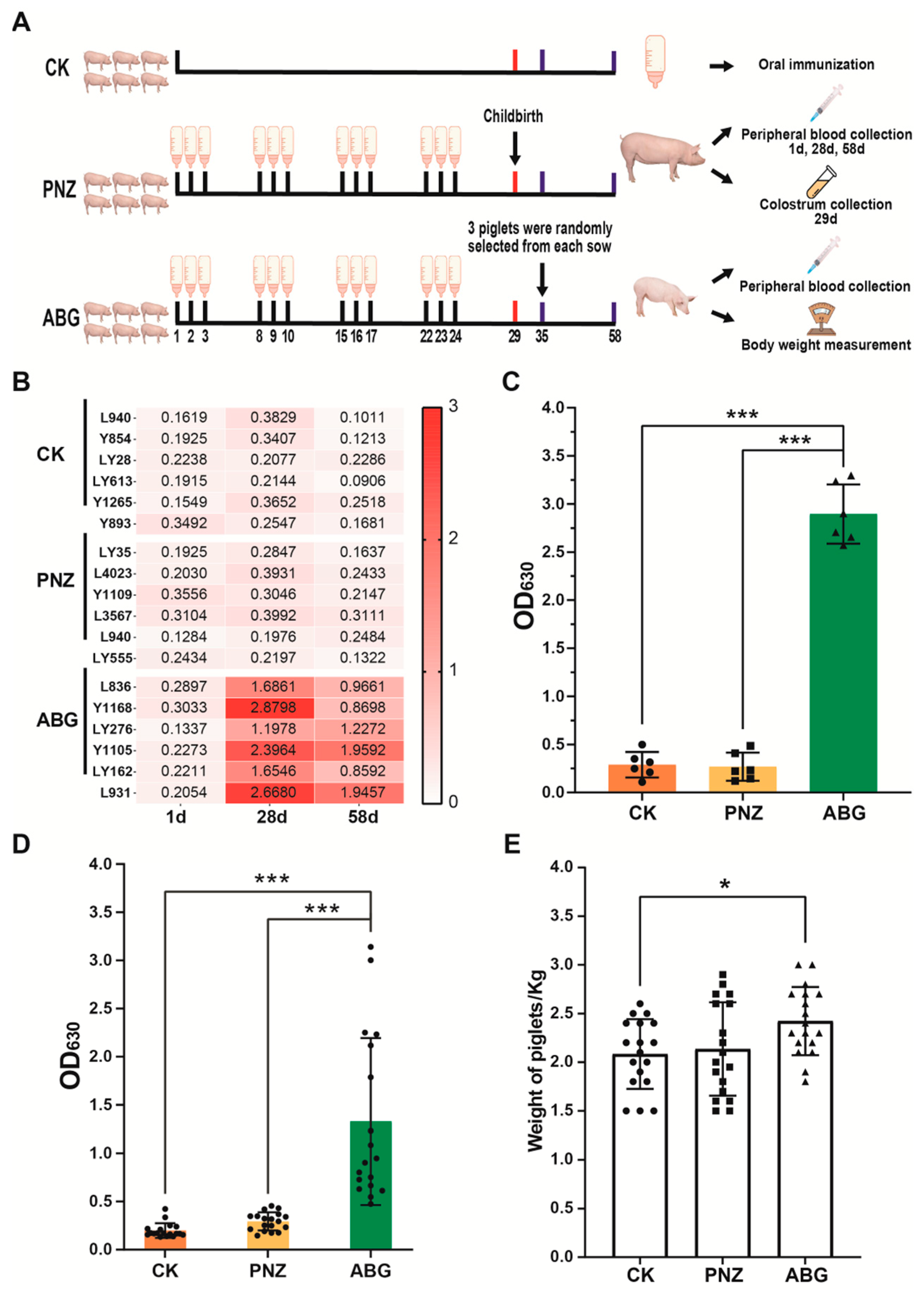
2.7. Immunoprotection Assessment for Sows in Late Stage of Pregnancy
2.8. Immunoprotection Assay for Lactating Piglets with Maternal Antibodies
2.9. Booster Immunization of Piglets with Maternal Antibodies
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Histopathological Examination
2.12. DNA Extraction and Microbiota Analysis
2.13. Data Analysis
2.14. Ethics Statement
3. Results
3.1. Construction of Three Recombinant Lactic Acid Bacteria (LAB) Strains
3.2. Oral Immunization with a Recombinant LAB Live Vector Vaccine Protected Mice from F4+ ETEC Infection
3.3. Recombinant LAB Live Vector Oral Vaccine Provides Protection against F4+ ETEC Infection in Weaned Piglets Lacking Maternal Antibodies
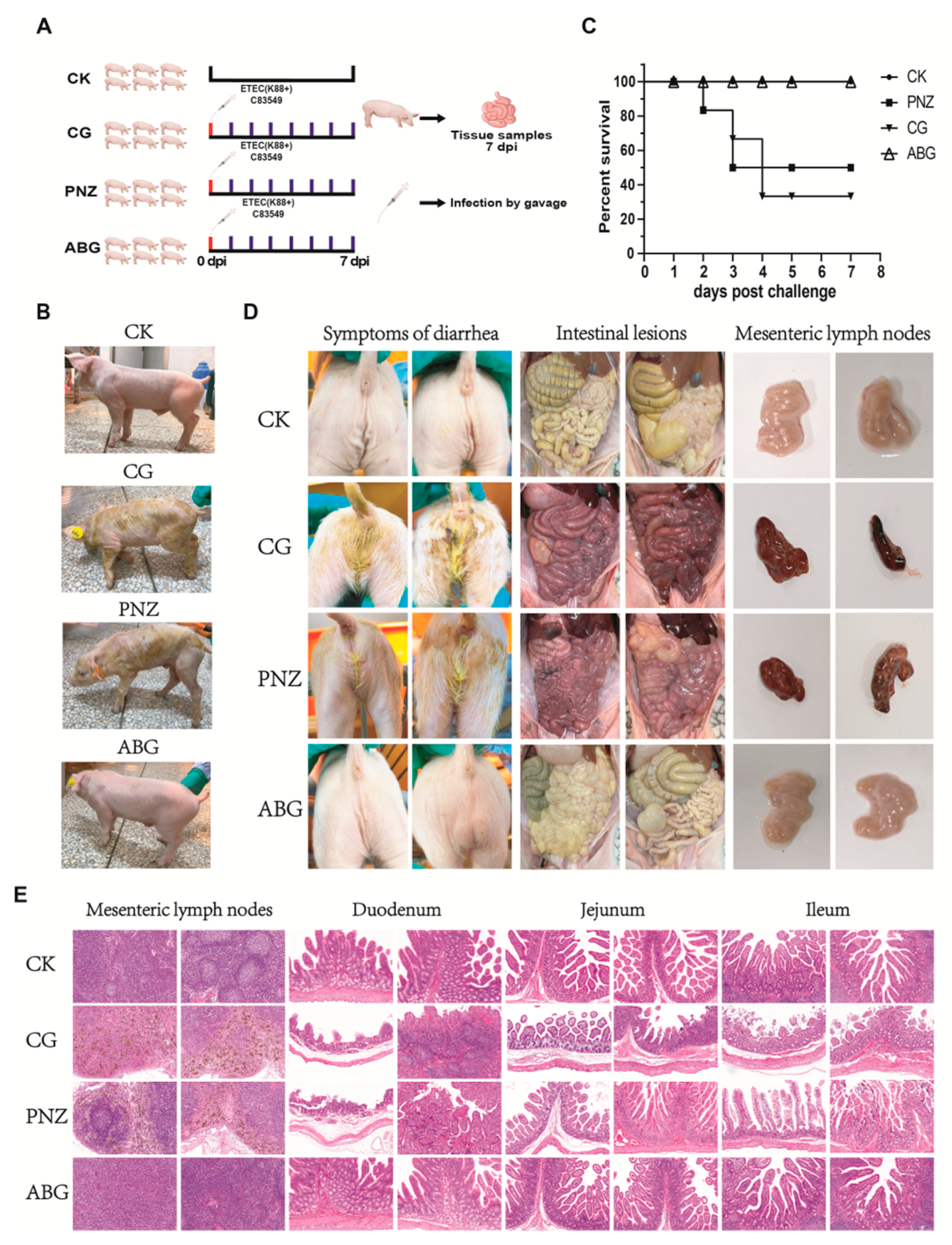
3.4. Immunization of Sows with the Recombinant LAB Live Vector Oral Vaccine during Late Pregnancy Effectively Enhances the Transfer of Maternal Antibodies to Piglets
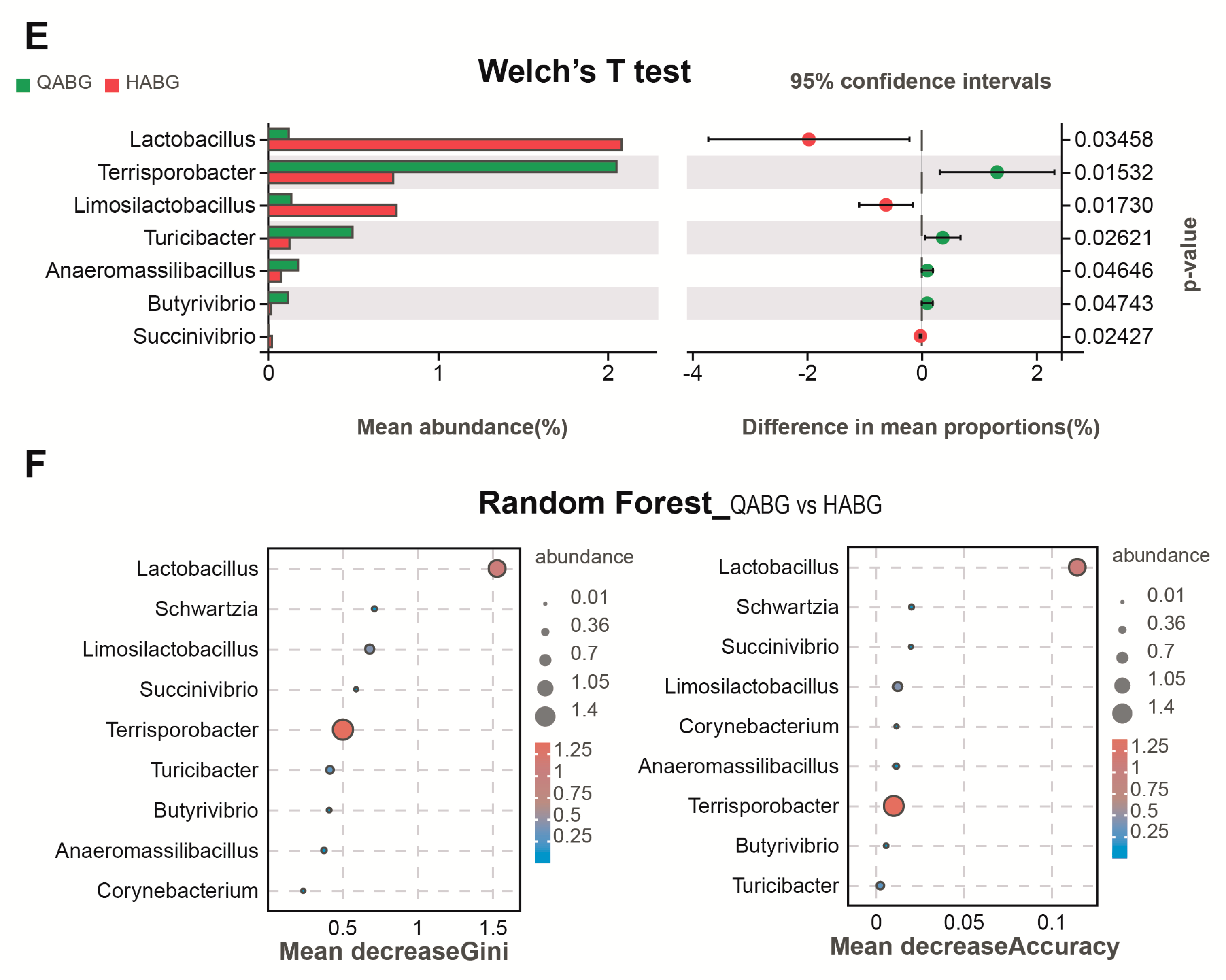
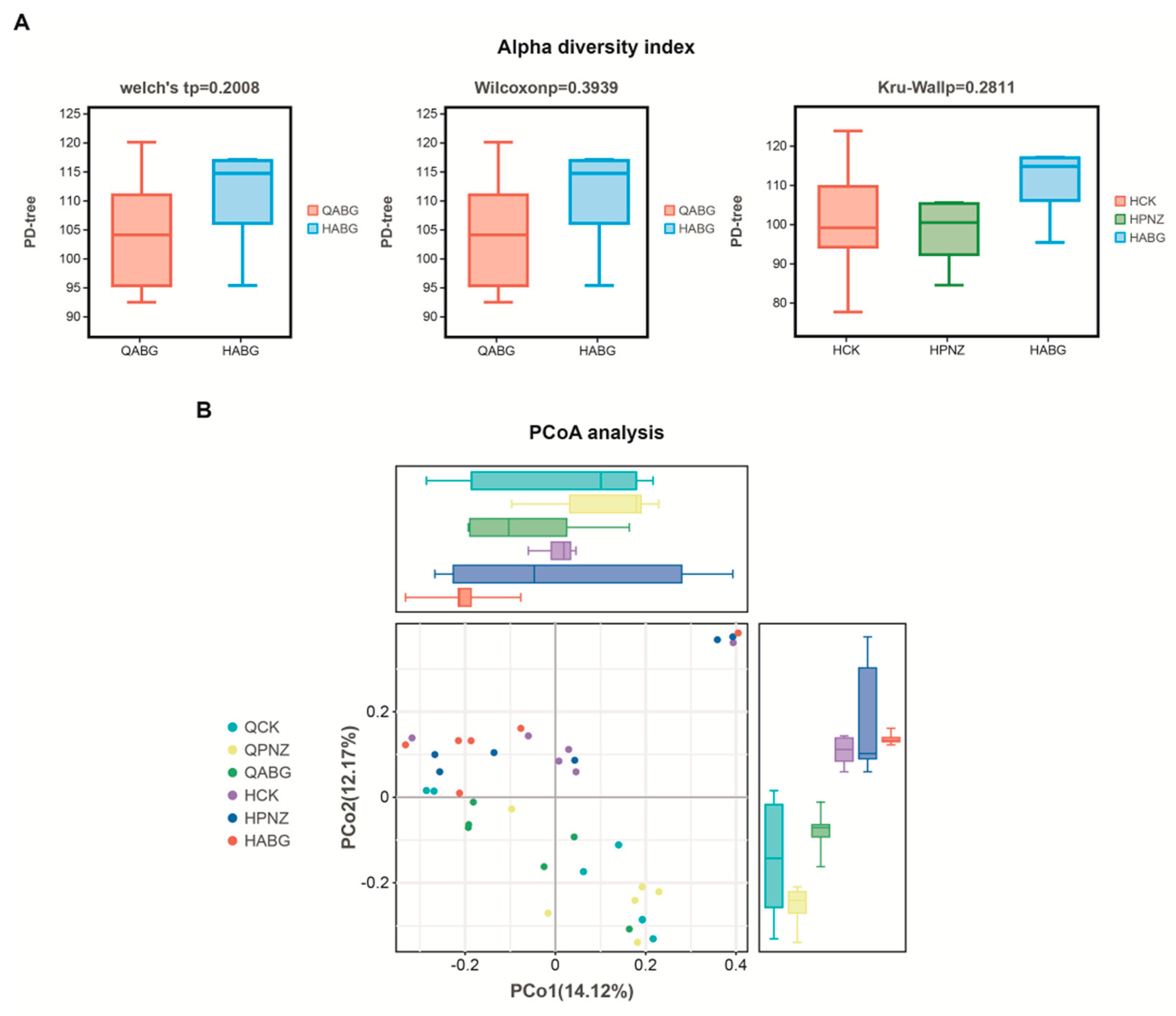
3.5. Immunization of the Recombinant LAB Live Vector Oral Vaccine Significantly Enhanced the Abundance of Lactobacillus in the Intestinal Tract of Sows during Late Pregnancy
3.6. The Richness and Uniformity of the Intestinal Microbiota in Late-Pregnant Sows Were Improved after Four Rounds of Immunization with a Recombinant Live LAB Vector Oral Vaccine
3.7. Maternal Antibodies from Orally Immunized Sows Protected Suckling Piglets (8 d of Age) from F4+ ETEC Infection
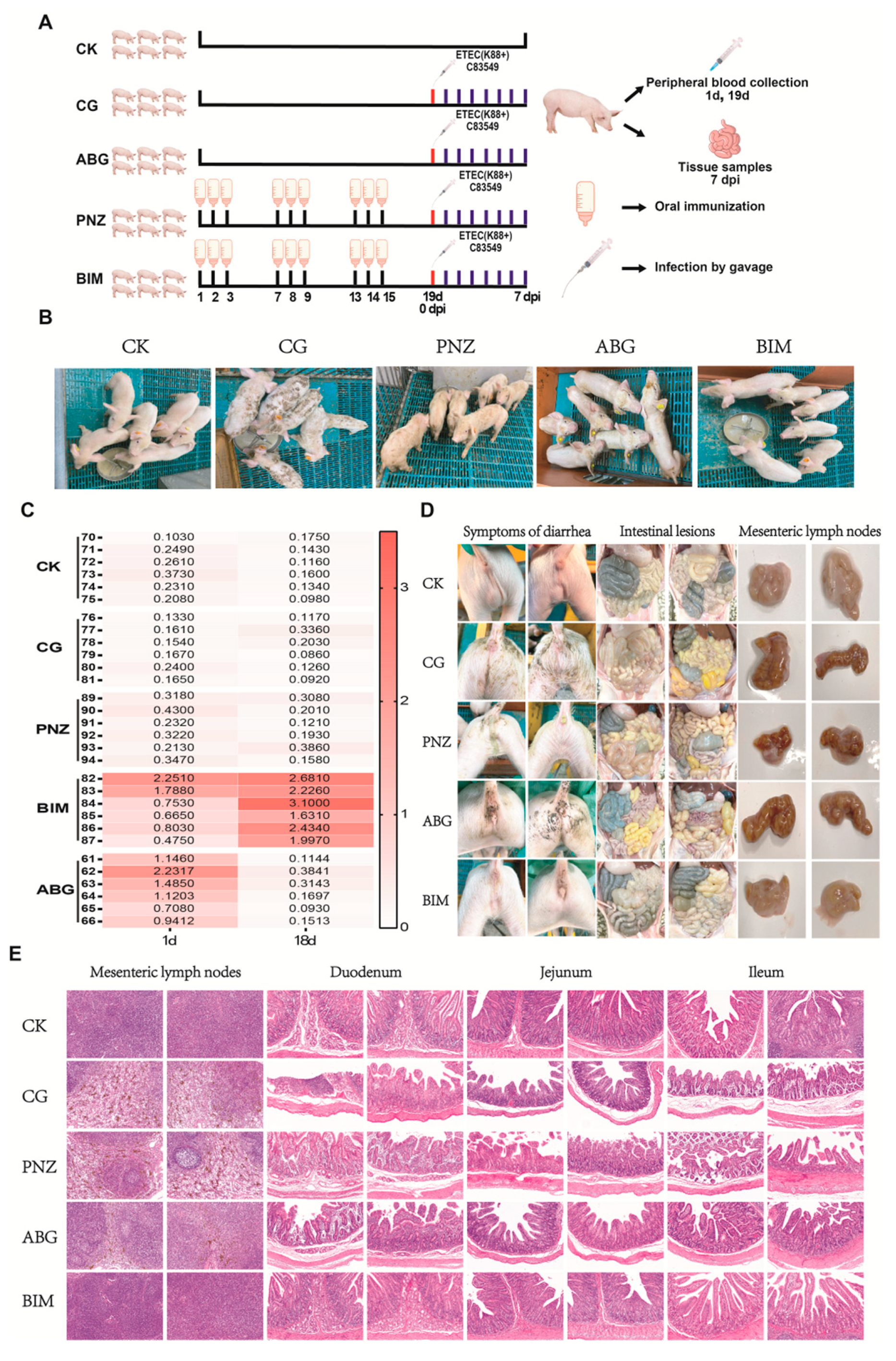
3.8. Booster Immunization of the Live Oral Recombinant LAB Vector Vaccine in Piglets with Maternal Antibodies during Lactation Enhances Their Resistance against F4+ ETEC Infection at Weaning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli in veterinary medicine. Int. J. Med. Microbiol. 2005, 295, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Kopic, S.; Geibel, J.P. Toxin mediated diarrhea in the 21 century: The pathophysiology of intestinal ion transport in the course of ETEC, V. cholerae and rotavirus infection. Toxins 2010, 2, 2132–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Zhang, Z.; You, J.; Yang, Y.; Li, X. Protective immunity of a Multivalent Vaccine Candidate against piglet diarrhea caused by enterotoxigenic Escherichia coli (ETEC) in a pig model. Vaccine 2018, 36, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, P.; Zhao, Y.; Ma, X. Enterotoxigenic Escherichia coli: Intestinal pathogenesis mechanisms and colonization resistance by gut microbiota. Gut Microbes 2022, 14, 2055943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W. Escherichia coli K88ac fimbriae expressing heat-labile and heat-stable (STa) toxin epitopes elicit antibodies that neutralize cholera toxin and STa toxin and inhibit adherence of K88ac fimbrial E. coli. Clin. Vaccine Immunol. 2010, 17, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.W.; Kohler, E.M.; Schneider, R.A.; Whipp, S.C. Prevalence of pilus antigens, enterotoxin types, and enteropathogenicity among K88-negative enterotoxigenic Escherichia coli from neonatal pigs. Infect. Immun. 1980, 27, 222–230. [Google Scholar] [CrossRef]
- Wittig, W.; Klie, H.; Gallien, P.; Lehmann, S.; Timm, M.; Tschäpe, H. Prevalence of the fimbrial antigens F18 and K88 and of enterotoxins and verotoxins among Escherichia coli isolated from weaned pigs. Zentralbl. Bakteriol. 1995, 283, 95–104. [Google Scholar] [CrossRef]
- Lu, T.; Moxley, R.A.; Zhang, W. Mapping the Neutralizing Epitopes of Enterotoxigenic Escherichia coli K88 (F4) Fimbrial Adhesin and Major Subunit FaeG. Appl. Environ. Microbiol. 2019, 85, e00329-19. [Google Scholar] [CrossRef]
- Isaacson, R.E.; Nagy, B.; Moon, H.W. Colonization of porcine small intestine by Escherichia coli: Colonization and adhesion factors of pig enteropathogens that lack K88. J. Infect. Dis. 1977, 135, 531–539. [Google Scholar] [CrossRef]
- Xia, P.; Wu, Y.; Lian, S.; Quan, G.; Wang, Y.; Zhu, G. Deletion of FaeG alleviated Enterotoxigenic Escherichia coli F4ac-induced apoptosis in the intestine. AMB Express 2021, 11, 44. [Google Scholar] [CrossRef]
- Xia, P.; Song, Y.; Zou, Y.; Yang, Y.; Zhu, G. F4+ enterotoxigenic Escherichia coli (ETEC) adhesion mediated by the major fimbrial subunit FaeG. J. Basic Microbiol. 2015, 55, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Tumala, B.; Vickers, T.J.; Martin, J.C.; Rosa, B.A.; Sabui, S.; Basu, S.; Simoes, R.D.; Mitreva, M.; Storer, C.; et al. Enterotoxigenic Escherichia coli heat-labile toxin drives enteropathic changes in small intestinal epithelia. Nat. Commun. 2022, 13, 6886. [Google Scholar] [CrossRef]
- Duan, Q.; Xia, P.; Nandre, R.; Zhang, W.; Zhu, G. Review of Newly Identified Functions Associated with the Heat-Labile Toxin of Enterotoxigenic Escherichia coli. Front. Cell Infect. Microbiol. 2019, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Pizza, M.; Giuliani, M.M.; Fontana, M.R.; Monaci, E.; Douce, G.; Dougan, G.; Mills, K.H.; Rappuoli, R.; Del Giudice, G. Mucosal vaccines: Non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine 2001, 19, 2534–2541. [Google Scholar] [CrossRef]
- Molina Estupiñan, J.L.; Aradottir Pind, A.A.; Foroutan Pajoohian, P.; Jonsdottir, I.; Bjarnarson, S.P. The adjuvants dmLT and mmCT enhance humoral immune responses to a pneumococcal conjugate vaccine after both parenteral or mucosal immunization of neonatal mice. Front. Immunol. 2022, 13, 1078904. [Google Scholar] [CrossRef]
- Wyszyńska, A.; Kobierecka, P.; Bardowski, J.; Jagusztyn-Krynicka, E.K. Lactic acid bacteria--20 years exploring their potential as live vectors for mucosal vaccination. Appl. Microbiol. Biotechnol. 2015, 99, 2967–2977. [Google Scholar] [CrossRef]
- Qiao, N.; Du, G.; Zhong, X.; Sun, X. Recombinant lactic acid bacteria as promising vectors for mucosal vaccination. Exploration 2021, 1, 20210026. [Google Scholar] [CrossRef] [PubMed]
- Szatraj, K.; Szczepankowska, A.K.; Chmielewska-Jeznach, M. Lactic acid bacteria—Promising vaccine vectors: Possibilities, limitations, doubts. J. Appl. Microbiol. 2017, 123, 325–339. [Google Scholar] [CrossRef]
- Lu, W.W.; Wang, T.; Wang, Y.; Xin, M.; Kong, J. A food-grade fimbrial adhesin FaeG expression system in Lactococcus lactis and Lactobacillus casei. Can. J. Microbiol. 2016, 62, 241–248. [Google Scholar] [CrossRef]
- García-Fruitós, E. Lactic Acid Bacteria: A promising alternative for recombinant protein production. Microb. Cell. Fact. 2012, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.S.; Savarino, S.J. Moving beyond a heat-labile enterotoxin-based vaccine against enterotoxigenic Escherichia coli. Lancet Infect. Dis. 2014, 14, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.L.; Wierzba, T.F.; Walker, R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 2016, 34, 2880–2886. [Google Scholar] [CrossRef]
- Seo, H.; Zhang, W. Development of effective vaccines for enterotoxigenic Escherichia coli. Lancet Infect. Dis. 2020, 20, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, J.D. Pig vaccination strategies based on enterotoxigenic Escherichia coli toxins. Braz. J. Microbiol. 2021, 52, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Peng, K.; She, Y.; Fu, F.; Shi, Q.; Lin, Y.; Xu, C. Preparation of novel trivalent vaccine against enterotoxigenic Escherichia coli for preventing newborn piglet diarrhea. Am. J. Vet. Res. 2023, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qi, R.; Chen, C.; Yin, J.; Ma, S.; Shi, W.; Wu, Y.; Ge, J.; Jiang, Y.; Tang, L.; et al. Immunogenicity of recombinant Lactobacillus casei-expressing F4 (K88) fimbrial adhesin FaeG in conjunction with a heat-labile enterotoxin A (LTAK63) and heat-labile enterotoxin B (LTB) of enterotoxigenic Escherichia coli as an oral adjuvant in mice. J. Appl. Microbiol. 2017, 122, 506–515. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; Cheng, D.; Feng, Y.; Yang, Y.; Sun, H.; Ding, J.; Wang, F. An Attenuated Escherichia coli K88ac LT(S63K)ΔSTb Efficiently Provides Protection Against Enterotoxigenic Escherichia coli in the Mouse Model. Front. Vet. Sci. 2020, 7, 620255. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, J.; Luo, P.; Zhang, W.J.; Zou, Q.M. LT(K63/R72), a new mutant of Escherichia coli heat-labile enterotoxin, exhibits characteristics more similar to LT(K63) than LT(R72). Acta Biochim. Biophys. Sin. 2005, 37, 126–132. [Google Scholar] [CrossRef]
- Roslan, A.M.; Mustafa Kamil, A.; Chandran, C.; Song, A.A.; Yusoff, K.; Abdul Rahim, R. Secretion of recombinant xylanase in Lactococcus lactis using signal peptides Usp45 and Spk1. Biotechnol. Lett. 2020, 42, 1727–1733. [Google Scholar] [CrossRef]
- Pham, M.L.; Tran, A.M.; Kittibunchakul, S.; Nguyen, T.T.; Mathiesen, G.; Nguyen, T.H. Immobilization of β-Galactosidases on the Lactobacillus Cell Surface Using the Peptidoglycan-Binding Motif LysM. Catalysts 2019, 9, 443. [Google Scholar] [CrossRef]
- Hur, J.; Lee, K.M.; Lee, J.H. Age-dependent competition of porcine enterotoxigenic E. coli (ETEC) with different fimbria genes—Short communication. Acta Vet. Hung. 2011, 59, 411–417. [Google Scholar] [CrossRef]
- Wen, L.J.; Hou, X.L.; Wang, G.H.; Yu, L.Y.; Wei, X.M.; Liu, J.K.; Liu, Q.; Wei, C.H. Immunization with recombinant Lactobacillus casei strains producing K99, K88 fimbrial protein protects mice against enterotoxigenic Escherichia coli. Vaccine 2012, 30, 3339–3349. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Xu, Z. Induction of specific immune responses in piglets by intramuscular immunization with fimbrial adhesin FaeG expressed in Lactococcus lactis. Res. Vet. Sci. 2013, 95, 130–136. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Y.; Tong, P.; Li, C.; Yang, W.; Hu, J.; Ye, L.; Gu, W.; Shi, C.; Shan, B.; et al. Alleviation of enterotoxigenic Escherichia coli challenge by recombinant Lactobacillus plantarum expressing a FaeG- and DC-targeting peptide fusion protein. Benef. Microbes 2017, 8, 379–391. [Google Scholar] [CrossRef]
- Verdonck, F.; Cox, E.; Van der Stede, Y.; Goddeeris, B.M. Oral immunization of piglets with recombinant F4 fimbrial adhesin FaeG monomers induces a mucosal and systemic F4-specific immune response. Vaccine 2004, 22, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Norton, E.B.; Branco, L.M.; Clements, J.D. Evaluating the A-Subunit of the Heat-Labile Toxin (LT) As an Immunogen and a Protective Antigen Against Enterotoxigenic Escherichia coli (ETEC). PLoS ONE 2015, 10, e0136302. [Google Scholar] [CrossRef][Green Version]
- Norton, E.B.; Lawson, L.B.; Mahdi, Z.; Freytag, L.C.; Clements, J.D. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect. Immun. 2012, 80, 2426–2435. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Angulo, C.; Meza, B. Food-Grade Organisms as Vaccine Biofactories and Oral Delivery Vehicles. Trends Biotechnol. 2016, 34, 124–136. [Google Scholar] [CrossRef]
- Berlec, A.; Ravnikar, M.; Strukelj, B. Lactic acid bacteria as oral delivery systems for biomolecules. Pharmazie 2012, 67, 891–898. [Google Scholar] [PubMed]
- LeCureux, J.A.-O.; Dean, G.A.-O. Lactobacillus Mucosal Vaccine Vectors: Immune Responses against Bacterial and Viral Antigens. mSphere 2018, 3, e00061-18. [Google Scholar] [CrossRef]
- Ribelles, P.; Benbouziane, B.; Langella, P.; Suárez, J.E.; Bermúdez-Humarán, L.G. Protection against human papillomavirus type 16-induced tumors in mice using non-genetically modified lactic acid bacteria displaying E7 antigen at its surface. Appl. Microbiol. Biotechnol. 2013, 97, 1231–1239. [Google Scholar] [CrossRef]
- Wu, C.M.; Chung, T.C. Mice protected by oral immunization with Lactobacillus reuteri secreting fusion protein of Escherichia coli enterotoxin subunit protein. FEMS Immunol. Med. Microbiol. 2007, 50, 354–365. [Google Scholar] [CrossRef]
- Hernandez-Valdes, J.A.; Huang, C.; Kok, J.; Kuipers, O.P. Another Breaker of the Wall: The Biological Function of the Usp45 Protein of Lactococcus lactis. Appl. Environ. Microbiol. 2020, 86, e00903-20. [Google Scholar] [CrossRef] [PubMed]
- Michon, C.; Langella, P.; Eijsink, V.G.; Mathiesen, G.; Chatel, J.M. Display of recombinant proteins at the surface of lactic acid bacteria: Strategies and applications. Microb. Cell Fact. 2016, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Buist, G.; Steen, A.; Kok, J.; Kuipers, O.P. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol. Microbiol. 2008, 68, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Hou, X.L.; Wei, C.H.; Yu, L.Y.; He, X.J.; Wang, G.H.; Lee, J.S.; Kim, C.J. Induction of immune responses in mice after oral immunization with recombinant Lactobacillus casei strains expressing enterotoxigenic Escherichia coli F41 fimbrial protein. Appl. Environ. Microbiol. 2009, 75, 4491–4497. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.H.; Liu, J.K.; Hou, X.L.; Yu, L.Y.; Lee, J.S.; Kim, C.J. Immunogenicity and protective efficacy of orally or intranasally administered recombinant Lactobacillus casei expressing ETEC K99. Vaccine 2010, 28, 4113–4118. [Google Scholar] [CrossRef]
- Craig, K.; Dai, X.; Li, A.; Lu, M.; Xue, M.; Rosas, L.; Gao, T.Z.; Niehaus, A.; Jennings, R.; Li, J. A Lactic Acid Bacteria (LAB)-Based Vaccine Candidate for Human Norovirus. Viruses 2019, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Lee, J.H. Comparative evaluation of a vaccine candidate expressing enterotoxigenic Escherichia coli (ETEC) adhesins for colibacillosis with a commercial vaccine using a pig model. Vaccine 2012, 30, 3829–3833. [Google Scholar] [CrossRef]
- Melkebeek, V.; Goddeeris, B.M.; Cox, E. ETEC vaccination in pigs. Vet. Immunol. Immunopathol. 2013, 152, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Rutter, J.M.; Jones, G.W. Protection against enteric disease caused by Escherichia coli—A model for vaccination with a virulence determinant? Nature 1973, 242, 531–532. [Google Scholar] [CrossRef]
- Alp, D.; Kuleaşan, H. Adhesion mechanisms of lactic acid bacteria: Conventional and novel approaches for testing. World J. Microbiol. Biotechnol. 2019, 35, 156. [Google Scholar] [CrossRef]
- Vesa, T.; Pochart, P.; Marteau, P. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment. Pharmacol. Ther. 2000, 14, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; Pomorska-Mól, M. Vaccination Failures in Pigs-The Impact of Chosen Factors on the Immunisation Efficacy. Vaccines 2023, 11, 230. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Fu, J.; Liu, H.; Kang, C.; Wang, Z.; Jin, Y.; Wu, S.; Li, T.; Yang, R.; Jin, M.; et al. Application of Recombinant Lactic Acid Bacteria (LAB) Live Vector Oral Vaccine in the Prevention of F4+ Enterotoxigenic Escherichia coli. Vaccines 2024, 12, 304. https://doi.org/10.3390/vaccines12030304
Yu J, Fu J, Liu H, Kang C, Wang Z, Jin Y, Wu S, Li T, Yang R, Jin M, et al. Application of Recombinant Lactic Acid Bacteria (LAB) Live Vector Oral Vaccine in the Prevention of F4+ Enterotoxigenic Escherichia coli. Vaccines. 2024; 12(3):304. https://doi.org/10.3390/vaccines12030304
Chicago/Turabian StyleYu, Jiangxu, Jiyang Fu, Hongshuo Liu, Chao Kang, Zesong Wang, Yancheng Jin, Shuxuan Wu, Tianzhi Li, Ruicheng Yang, Meilin Jin, and et al. 2024. "Application of Recombinant Lactic Acid Bacteria (LAB) Live Vector Oral Vaccine in the Prevention of F4+ Enterotoxigenic Escherichia coli" Vaccines 12, no. 3: 304. https://doi.org/10.3390/vaccines12030304
APA StyleYu, J., Fu, J., Liu, H., Kang, C., Wang, Z., Jin, Y., Wu, S., Li, T., Yang, R., Jin, M., Chen, H., & Wang, X. (2024). Application of Recombinant Lactic Acid Bacteria (LAB) Live Vector Oral Vaccine in the Prevention of F4+ Enterotoxigenic Escherichia coli. Vaccines, 12(3), 304. https://doi.org/10.3390/vaccines12030304






