Long-Term Immunological Alertness and Response to COVID-19 Vaccination—Conditions for Prevention in Early Palliative Oncological Care Patients
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Setting
2.3. Participants
2.4. Serum Antibody Determination
2.5. Virus Neutralization Assay
2.6. Data Collection
2.7. Data Analysis
2.8. Ethics
3. Results
3.1. Patients, Antitumor Therapy, and COVID-19 Vaccination
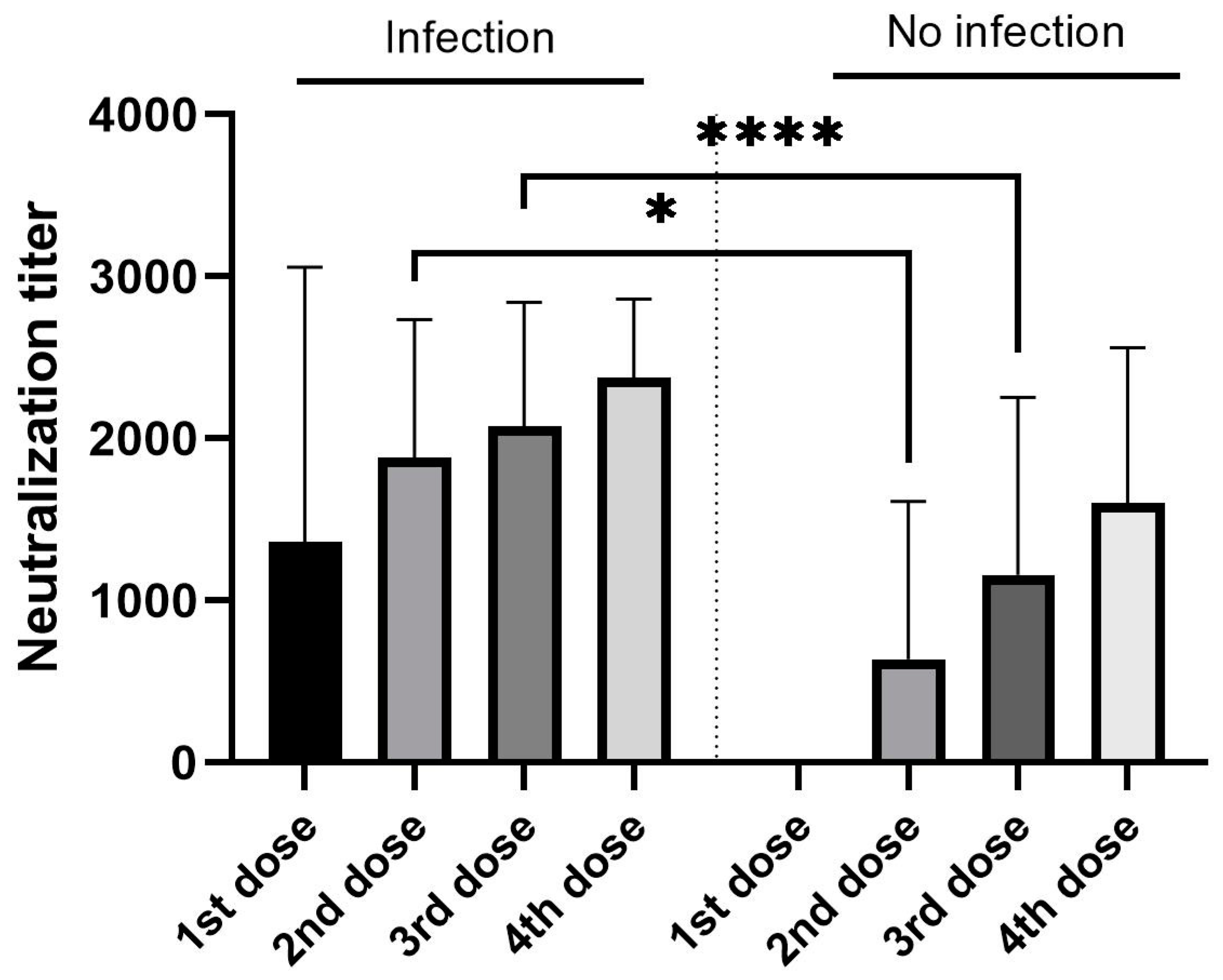
3.2. Antibody Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Mohseni Afshar, Z.; Hosseinzadeh, R.; Barary, M.; Ebrahimpour, S.; Alijanpour, A.; Sayad, B.; Hosseinzadeh, D.; Rouhollah Miri, S.; Sio, T.T.; Sullman, J.M.S.; et al. Chalanges posed by COVID-19 in cancer patients: A narrative review. Cancer Med. 2022, 11, 1119–1135. [Google Scholar] [CrossRef]
- Bakitas, M.A.; Tosteson, T.D.; Li, Z.; Lyons, K.D.; Hull, J.G.; Li, Z.; Dione-Odom, N.; Frost, J.; Dragnev, H.K.; Hegel, T.M.; et al. Early versus delayed initiation of concurrent palliative oncology care: Patient outcomes in the ENABLE III Randomized controlled trial. Clin. Oncol. 2015, 33, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-M.; Huang, Y.-T.; Lai, M.-Y.; Liu, H.-E.; Shiao, C.-C. Optimal timing for hospice-shared care initiation in terminal cancer patients. Support. Care Cancer 2021, 29, 6871–6880. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, L.; Knaul, F.M.; De Lima, L.; De Joncheere, C.; Bhadelia, A. The key role of palliative care in response to the COVID-19 tsunami of suffering. Lancet 2020, 395, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The immune response to SARS-CoV-2 and COVID-19 immunopathology—Current perspectives. Pulmonology 2021, 27, 423–437. [Google Scholar] [CrossRef]
- Vabret, N.; Britton, G.J.; Gruber, C.; Hegde, S.; Kim, J.; Kuksin, M.; Levantovsky, R.; Malle, L.; Moreira, A.; Park, M.D.; et al. Immunology of COVID-19: Current State of the Science. Immunity 2020, 52, 910–941. [Google Scholar] [CrossRef]
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef]
- Caturegli, G.; Materi, J.; Howard, B.M.; Caturegli, P. Clinical validity of serum antibodies to SARS-CoV-2: A case-control study. Ann. Intern. Med. 2020, 173, 614–622. [Google Scholar] [CrossRef]
- Sakurai, A.; Sasaki, T.; Kato, S.; Hayasgi, M.; Sei-Ichiro, T.; Ishihara, T.; Iwata, M.; Morise, Z.; Doi, Y. Natural history of asymptomatic SARS-CoV-2 infection. N. Engl. J. Med. 2020, 383, 885–886. [Google Scholar] [CrossRef]
- Perkmann, T.; Perkman-Nagele, N.; Koller, T.; Mucher, P.; Radakovics, A.; Marculescu, R.; Woltz, M.; Wagner, F.O.; Binder, C.J.; Haslacher, H. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: A head-to-head comparison of five quantitative assays. Microbiol. Spectr. 2021, 9, e0024721. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Cosma, C.; Bonfante, F.; Della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall´Olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing antibody titres six months after Comirnaty vaccination kinetics and comparison with SARS-CoV-2 immunoassays. Clin. Chem. Lab. Med. 2021, 60, 456–463. [Google Scholar] [CrossRef]
- Favresse, J.; Gillot, C.; Di Chiaro, L.; Eucher, C.; Elsen, M.; Van Eeckhoudt, S.; David, C.; Morimont, L.; Dogné, J.-M.; Douxfils, J. Neutralizing antibodies in COVID-19 patients and vaccine recipients after two doses of BNT162b2. Viruses 2021, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Post, N.; Eddy, D.; Huntley, C.; Van Schalkwyk, I.C.M.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, V.S.; Bermingham, W.H.; Kellam, P.; et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2021, 15, e0244126. [Google Scholar] [CrossRef]
- Wang, Z.; Muecksch, F.; Schaefer-Badajev, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Baldo, P.; Fornasier, G.; Ciolfi, L.; Sartor, I.; Francescon, S. Pharmacovigilance in oncology. Int. J. Clin. Pharm. 2018, 40, 832–841. [Google Scholar] [CrossRef]
- Yu, W.-D.; Sun, G.; Li, J.; Xu, J.; Wang, X. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett. 2019, 452, 66–70. [Google Scholar] [CrossRef]
- Desai, A.; Gainor, J.F.; Hegde, A.; Schram, M.A.; Curigliano, G.; Pal, S.; Liu, V.S.; Halmos, B.; Groisberg, R.; Grade, E.; et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat. Rev. Clin. Oncol. 2021, 18, 313–319. [Google Scholar] [CrossRef]
- Schmidt, A.L.; Labaki, C.; Hsu, C.Y.; Bakouny, Z.; Berg, S.A.; Blau, S.; Daher, A.; El Zarif, T.; Friese, C.R.; Griffiths, A.E.; et al. COVID-19 vaccination and breakthrough infection in patients with cancer. Ann. Oncol. 2022, 33, 340–346. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Labaki, C.; Bakouny, Z.; Hsu, C.-Y.; Schmidt, A.L.; De Lima Lopes, G., Jr.; Hwang, C.; Singh, K.R.S.; Jani, C.; Weissmann, L.B.; et al. Breakthrough SRS-CoV-2 infections among patients with cancer following two and three doses of covid-19 mRNA vaccines: A retrospective observational study from the COVID-19 and cancer consortium. Lancet Reg. Health Am. 2023, 19, 100445. [Google Scholar]
- Subbiah, V.A. Global effort to understand the riddles of COVID-19 and cancer. Nat. Cancer 2020, 1, 943–945. [Google Scholar] [CrossRef]
- Desai, A.; Gupta, R.; Advani, S.; Ouellette, L.; Kuderer, N.M.; Lyman, G.H.; Li, A. Mortality in hospitalized patients with cancer and coronavirus disease 2019: A systematic review and meta- analysis of cohort studies. Cancer 2021, 127, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sachdeva, S.; Parekh, T.; Desai, R. COVID-19 and cancer: Lessons from a pooled meta-analysis. JCO Glob. Oncol. 2020, 6, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, R.D.; Shete, S.; Hsu, C.-Y.; Desai, A.; De Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Strang, P. Palliative oncology and palliative care. Mol. Oncol. 2022, 16, 3399–3409. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, L.; Johnsaton, B.; Kotronoulas, G.; Finlay, F.; Keeley, P.; McKeown, A. COVID-19 and hospital palliative care—A service evaluation exploring the symptoms and outcomes of 186 patients and the impact of the pandemic on specialist hospital palliative care. Pall. Med. 2020, 34, 1256–1262. [Google Scholar] [CrossRef]
- Bausewein, C.; Hodiamont, F.; Berges, N.; Ullrich, A.; Gerlach, C.; Oechsle, K.; Pauli, B.; Weber, J.; Stiel, S.; Schneider, N.; et al. National strategy for palliative care of severely ill and dying people and their relatives in pandemics (PallPan) in Germany—Study protocol of mixed-methods project. BMC Pall. Care 2022, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Grana, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD0154477. [Google Scholar]
- Fendler, A.; De Vries, E.G.; Geurtsvan-Kessel, C.H.; Haanen, J.B.; Wörmann, B.; Turajlic, S.; van Lilienfeld-Toal, M. COVID-19 vaccines in patients with cancer: Immunogenicity, efficacy and safety. Nat. Rev. Clin. Oncol. 2022, 19, 385–401. [Google Scholar] [CrossRef]
- Scherrens, A.L.; Cohen, J.; Mahieu, A.; Deliens, L.; Deforche, B.; Beernaert, K. The perception of people with cancer of starting a conversation about palliative care: A qualitative interview study. Eur. J. Cancer Care 2020, 29, e13282. [Google Scholar] [CrossRef]
- Bandieri, E.; Borelli, E.; Gilioli, F.; Bigi, S.; Mucciarini, C.; Ferrari, U.; Eliardo, S.; Pinto, L.; Porro, C.A.; Efficace, F.; et al. Stigma of palliative care among patients with advanced cancer and their caregivers on early palliative care. Cancers 2023, 15, 3656. [Google Scholar] [CrossRef]
- Mukai, K.; Tsunoda, H.; Imai, R.; Numata, A.; Kida, K.; Oba, K.; Yagishita, K.; Yamauchi, H.; Kanomata, N.; Kurihara, Y. The location of unilateral axillary lymphadenopathy after COVID-19 vaccination compared with that of metastasis from breast cancer without vaccination. Jpn. J. Radiol. 2023, 41, 617–624. [Google Scholar] [CrossRef]
- Martinez-Cannon, B.A.; Garcia-Ronquillo, K.; Leon-Rodriguez, E. Vaccination status and attitudes towards COVID-19 vaccination in patients undergoing active cancer treatment in a referral center in Mexico: A survey study. Support. Care Cancer 2023, 31, 209. [Google Scholar] [CrossRef]
- Piler, P.; Thon, V.; Andrýsková, L.; Dolezel, K.; Kostka, D.; Pavlík, T.; Dusek, L.; Pikhart, H.; Bobak, M.; Matic, S.; et al. Nationwide increases in anti-SARS-CoV-2 IgG antibodies between October 2020 and March 2021 in the unvaccinated Czech population. Commun. Med. 2022, 2, 19. [Google Scholar] [CrossRef]
- Thon, V.; Piler, P.; Pavlík, T.; Andrysova, L.; Dolezel, K.; Kostka, D.; Pikhart, H.; Bobak, M.; Klamova, J. Investigation of SARS-CoV-2 seroprevalence in relation to natural infection and vaccination between October 2020 and September 2021 in the Czech Republic: A prospective national cohort study. BMJ Open 2023, 13, e068258. [Google Scholar] [CrossRef]
- Grivas, A.; Khaki, A.R.; Wise-Draper, T.M.; French, B.; Hennessy, C.; Hsu, C.Y.; Shyr, Y.; Li, X.; Choueiri, T.K.; Painter, C.A.; et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: A report from the COVID-19 and cancer consortium. Ann. Oncol. 2021, 32, 787–800. [Google Scholar] [CrossRef]
- Ali, H.; Alahmad, B.; Al-Shammari, A.A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.A.; Mohammad, A.; et al. Previous COVID-19 infection and antibody levels after vaccination. Front. Public Health 2021, 9, 778234. [Google Scholar] [CrossRef] [PubMed]
- Glück, V.; Grobecker, S.; Köstler, J.; Tydykov, L.; Bertok, M.; Weidlich, T.; Gottwald, C.; Salzberger, B.; Wagner, R.; Zeman, F.; et al. Immunity after COVID-19 and vaccination: Follow-up study over 1 year among medical personnel. Infection 2022, 50, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Rank, A.; Tzortzini, A.; Kling, E.; Schmid, C.; Claus, R.; Löll, E.; Burger, R.; Römmele, C.; Dhillon, C.; Müler, K.; et al. One year after mild COVID-19: The majority of patients maintain specific immunity, but one in four still suffer from long-term symptoms. J. Clin. Med. 2021, 10, 3305. [Google Scholar] [CrossRef] [PubMed]
- Sarrigeorgiou, I.; Moschandreou, D.; Dimitriadis, A.; Tsinti, G.; Sotiropoulou, E.; Ntoukaki, E.; Eliadis, P.; Backovic, M.; Labropoulou, S.; Escriou, N.; et al. Combined monitoring if IgG and IgA anti-spike and anti-receptor binding domain long term responses following BNT162b2 mRNA vaccination in Greek healthcare workers. PLoS ONE 2022, 17, e0277827. [Google Scholar] [CrossRef]
- Choj, H.-W.; Jung, Y.; Kim, U.J.; Lee, S.-C.; Kwon, J.H.; Kim, H.; Kim, S.; Lee, Y.; Shim, H.-J.; Cho, S.-H.; et al. Comparative study on the immunogenicity of COVI-19 mRNA vaccines in patients receiving adjuvant and palliative chemotherapy. Cho. Med. J. 2024, 60, 69–77. [Google Scholar]
- Tran, S.; Truong, H.T.; Narendran, A. Evaluation of COVIDE-19 vaccine response in patients with cancer: An interim analysis. Eur. J. Cancer 2021, 159, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Caramujo, C.; Gomes, I.; Fraga, T.; Paulo, J.; Broco, S.; Cunha, N.; Madeira, P.; Carvalho, T.; Teixeira, M.; Sousa, G. Immune response to SARS-CoV-2 Vaccination in cancer patients: A prospective study. Cureus 2023, 15, e37014. [Google Scholar] [CrossRef] [PubMed]
- Fajfr, M.; Pajer, P.; Ruzek, D.; Sleha, R.; Janovska, S.; Bohonek, M.; Kabickova, H.; Kubicková, P.; Stefanik, M.; Strakova, P.; et al. Multicentric evaluation of sensitivity of eight commercial anti-SARS-CoV-2 antibody assays and their correlation to virus neutralisation titers in seropositive subjects. Sci. Rep. 2024, 14, 1421. [Google Scholar] [CrossRef]
- Schwedler, C.; Grzeski, M.; Kappert, K.; Rust, J.; Heymann, G.; Hoppe, B.; Blanchard, V. Coronavirus disease 2019—Related alteration of total and anti-spike IgG glycosylation in relation to age and anti-spike IgG titer. Front. Microbiol. 2022, 13, 775186. [Google Scholar] [CrossRef]
- Limbu, Y.B.; Gautam, K.R. The determinants of COVID-19 vaccination intention: A meta-review. Front. Public Health 2023, 11, e1162861. [Google Scholar] [CrossRef]
- Pradani, K.I.P.; Weerasekara, I.; Damayanthi, H.D.W.T. COVID-19 vaccine acceptance and hesitancy among patients with cancer: A systematic review and meta-analysis. Front. Public Health 2022, 212, 66–75. [Google Scholar] [CrossRef]
- Xie, Z.; Tak-Fai Lau, J.; Liang, Y.; Ouyang, Q.; Chen, J.; Lin, S.; Yao, K.; Hu, X.; Lin, H.; Yu, Y.; et al. Prevalence and factors of COVID-19 vaccine refusal among solid cancer patients in China: An application of the health belief model. Front. Public Health 2023, 11, e1236376. [Google Scholar] [CrossRef]
| Patients (n = 147) | |
|---|---|
| Female 54.4% (n = 80) | Average age 68.2 years (ranged 39–98 years) |
| Male 45.6% (n = 67) | Average age 69.4 years (ranged 22–94 years) |
| ECOG Performance Status Scale (PS) | PS 0–3; median 2 |
| Diseases | |
| Colorectal cancer | 24 (16.3%) |
| Breast cancer | 23 (15.6%) |
| Urinary cancer | 17 (11.6%) |
| Pancreatic cancer | 14 (9.5%) |
| Head–neck cancer | 10 (6.8%) |
| Ovarian cancer | 10 (6.8%) |
| Liver cancer | 9 (6.2%) |
| Stomach cancer | 8 (5.4%) |
| Tumors of unknown origin | 7 (4.8%) |
| Lung cancer | 6 (4.1%) |
| Other tumors | 19 (12.9%) |
| COVID-19 Infection * | Fisher’s Test | Statistical Significance | |||
|---|---|---|---|---|---|
| Number of Vaccinations | Yes | Yes (%) | No | ||
| One dose | 2 | 100 | 0 | 0.5005 | ns |
| Two doses | 16 | 57.1 | 12 | 0.8343 | ns |
| Three doses | 55 | 53.4 | 48 | 0.7216 | ns |
| Four doses | 7 | 50.0 | 7 | 0.7828 | ns |
| TOTAL | 80 | 54.4 | 67 | ||
| Number of Vaccination Doses * | One | Two | Three | Four |
|---|---|---|---|---|
| N (0–50 AU/mL) | 0 | 3 (10.7%) | 2 (1.9%) | 0 |
| L (51–1000 AU/mL) | 1 (50%) | 4 (14.3%) | 14 (13.6%) | 0 |
| H (1000–10,000 AU/mL) | 1 (50%) | 10 (35.7%) | 32 (31.1%) | 5 (35.7%) |
| VH (10,001–40,000 AU/mL) | 0 | 11 (39.3%) | 55 (53.4%) | 9 (64.3%) |
| TOTAL | 2 | 28 | 103 | 14 |
| Anti-S IgG Level | VNT Titer * | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 40 | 80 | 160 | 320 | 640 | 1280 | 2560 | >2560 | |
| N (0–50 AU/mL) | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| L (51–1000 AU/mL) | 3 | 7 | 4 | 5 | 0 | 0 | 0 | 0 | 0 |
| H (1000–10,000 AU/mL) | 0 | 2 | 4 | 1 | 3 | 7 | 16 | 3 | 12 |
| VH (10,001–40,000 AU/mL) | 0 | 0 | 0 | 0 | 1 | 2 | 12 | 2 | 58 |
| TOTAL | 7 | 9 | 8 | 6 | 4 | 9 | 29 | 5 | 70 |
| Days after Vaccination * | N (0–50 AU/mL) | L (51–1000 AU/mL) | H (1000–10,000 AU/mL) | VH (10,001–40,000 AU/mL) |
|---|---|---|---|---|
| 1–100 | 0 | 0 | 0 | 4 |
| 101–200 | 0 | 2 | 3 | 17 |
| 201–300 | 1 | 5 | 12 | 12 |
| 301–400 | 1 | 7 | 11 | 12 |
| 401–500 | 1 | 2 | 14 | 11 |
| 501–600 | 1 | 3 | 5 | 15 |
| 601–700 | 1 | 0 | 3 | 2 |
| 701–800 | 0 | 0 | 0 | 2 |
| TOTAL | 5 (3.4%) | 19 (12.9%) | 48 (32.7%) | 75 (51.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priester, P.; Fajfr, M.; Molnarova, V.; Sleha, R.; Janovska, S.; Bostik, P.; Filip, S. Long-Term Immunological Alertness and Response to COVID-19 Vaccination—Conditions for Prevention in Early Palliative Oncological Care Patients. Vaccines 2024, 12, 299. https://doi.org/10.3390/vaccines12030299
Priester P, Fajfr M, Molnarova V, Sleha R, Janovska S, Bostik P, Filip S. Long-Term Immunological Alertness and Response to COVID-19 Vaccination—Conditions for Prevention in Early Palliative Oncological Care Patients. Vaccines. 2024; 12(3):299. https://doi.org/10.3390/vaccines12030299
Chicago/Turabian StylePriester, Peter, Miroslav Fajfr, Veronika Molnarova, Radek Sleha, Sylva Janovska, Pavel Bostik, and Stanislav Filip. 2024. "Long-Term Immunological Alertness and Response to COVID-19 Vaccination—Conditions for Prevention in Early Palliative Oncological Care Patients" Vaccines 12, no. 3: 299. https://doi.org/10.3390/vaccines12030299
APA StylePriester, P., Fajfr, M., Molnarova, V., Sleha, R., Janovska, S., Bostik, P., & Filip, S. (2024). Long-Term Immunological Alertness and Response to COVID-19 Vaccination—Conditions for Prevention in Early Palliative Oncological Care Patients. Vaccines, 12(3), 299. https://doi.org/10.3390/vaccines12030299






