Reduction in Interferon-Stimulated Genes Contributes to High-Yield Production of Influenza Virus in Suspension MDCK Cells
Abstract
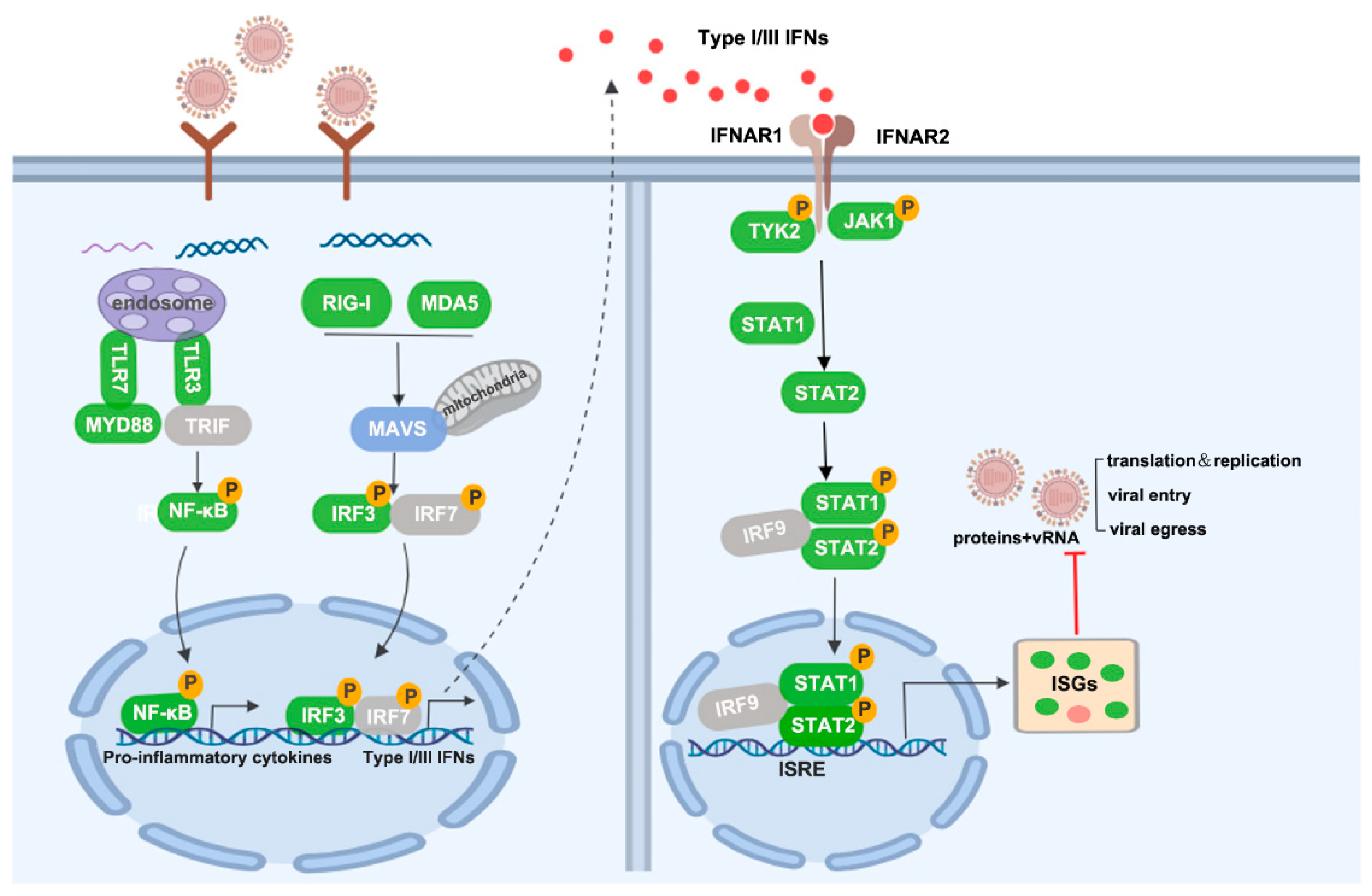
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Sialic Acids (SAs) Detection Assay
2.3. Plasmids and Molecular Cloning
2.4. Cell Culture Infection and Transfection
2.5. Determination of Virus Titer
2.6. Western Blotting Assay
2.7. RT-qPCR
2.8. RNA Interference Assay
2.9. JAK Inhibition
2.10. RNA-seq and Data Analysis
2.11. Statistical Analysis
3. Results
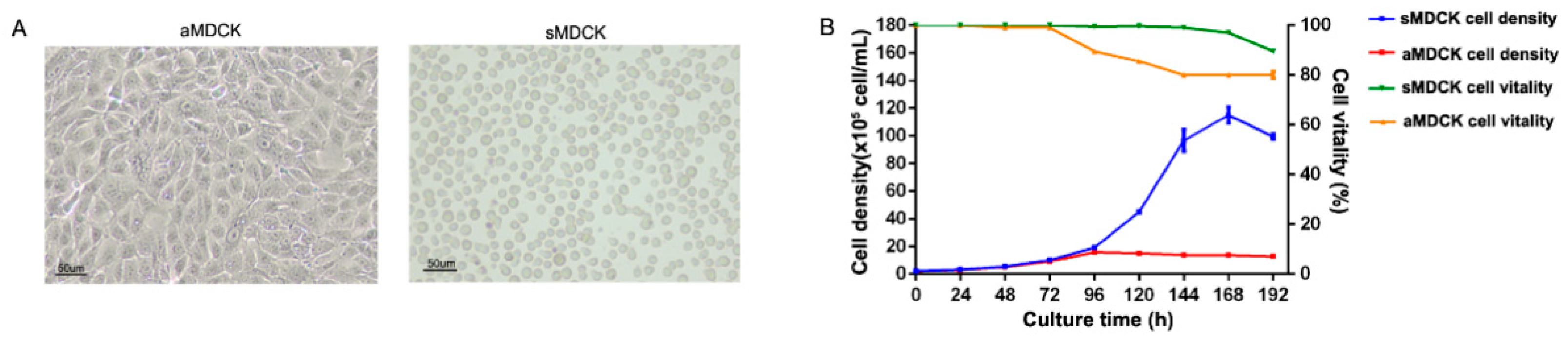
3.1. Biological Characteristics Analysis of sMDCK Cells
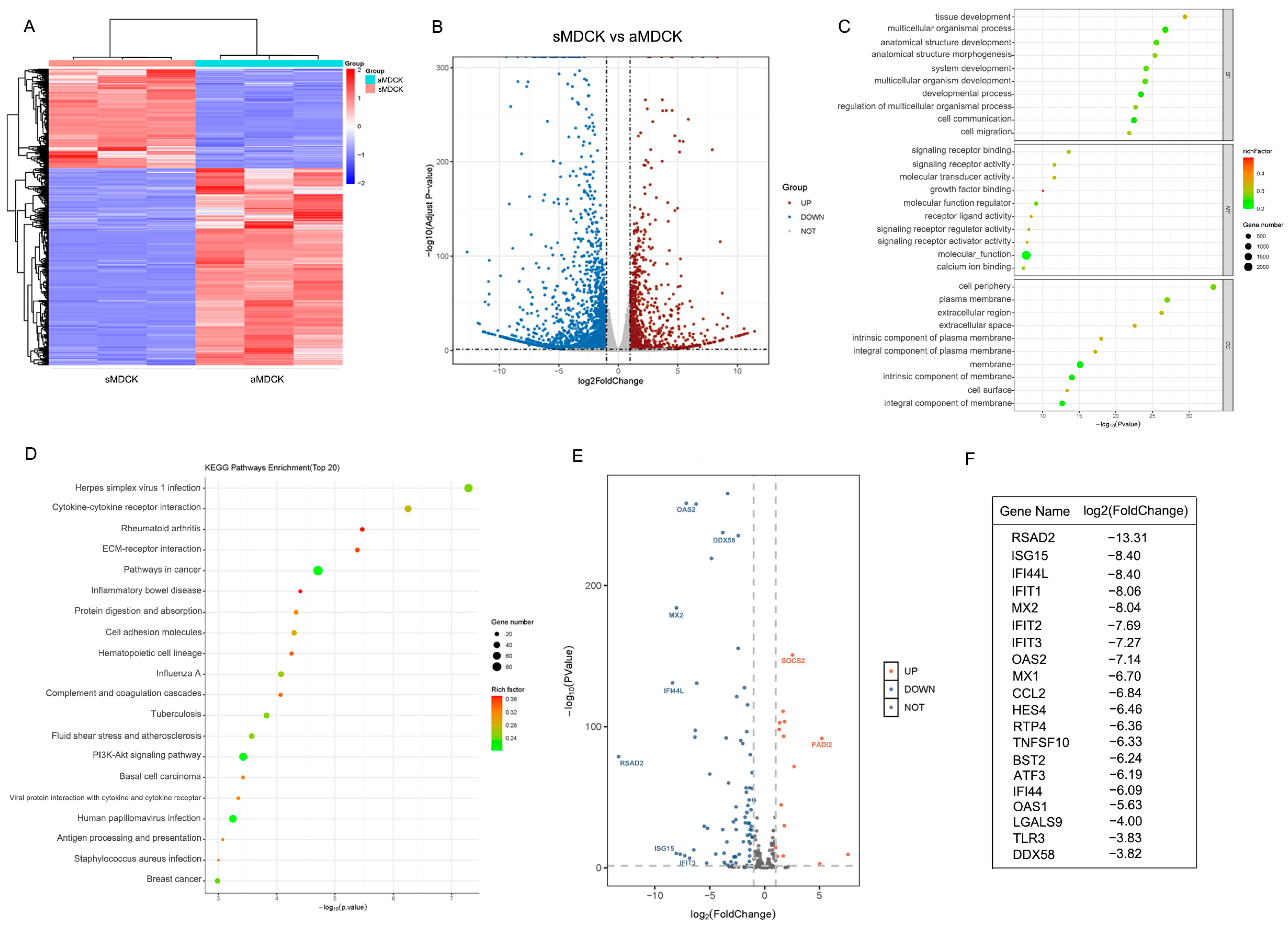
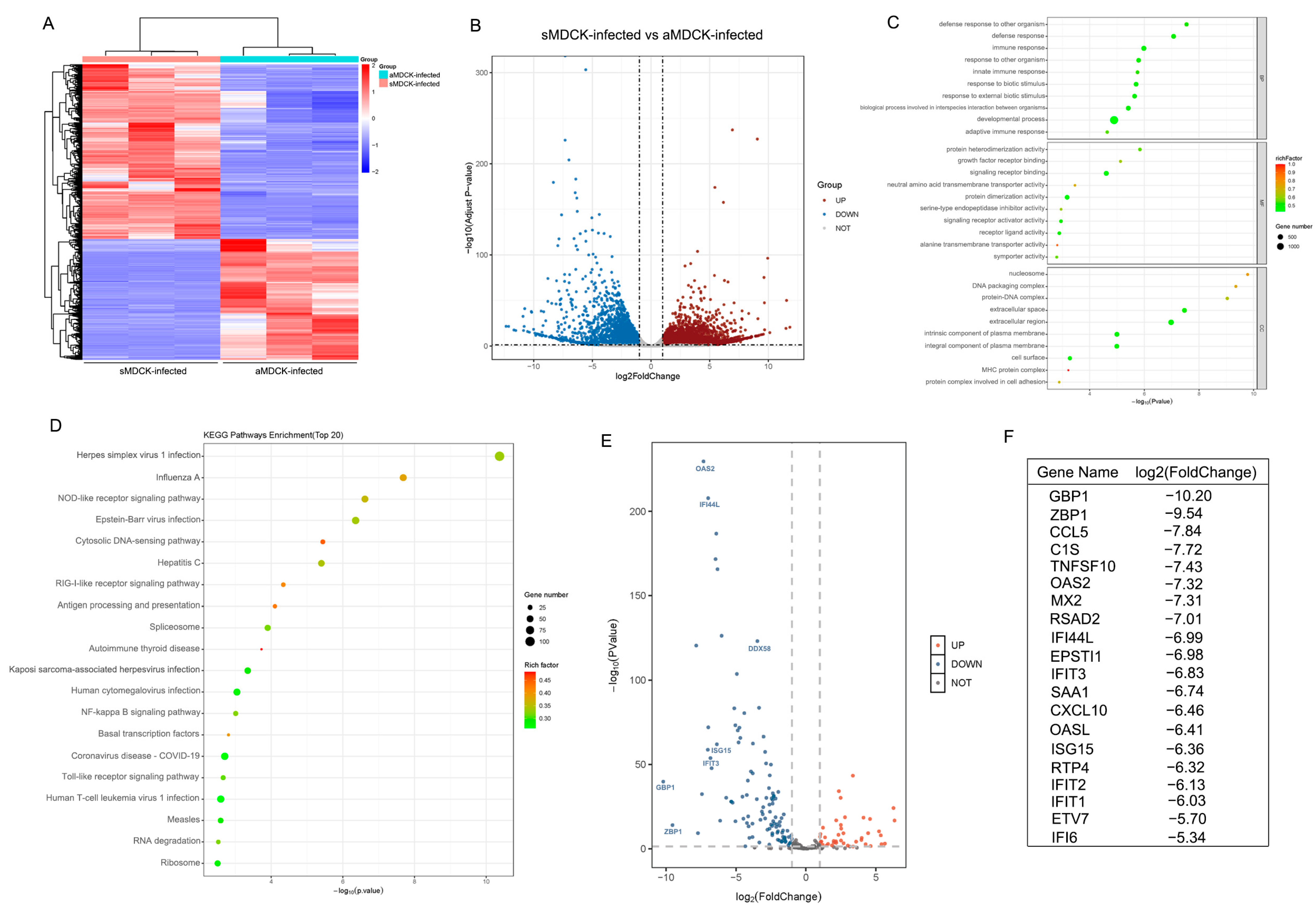
3.2. Transcriptomic Analysis of sMDCK vs. aMDCK Cells
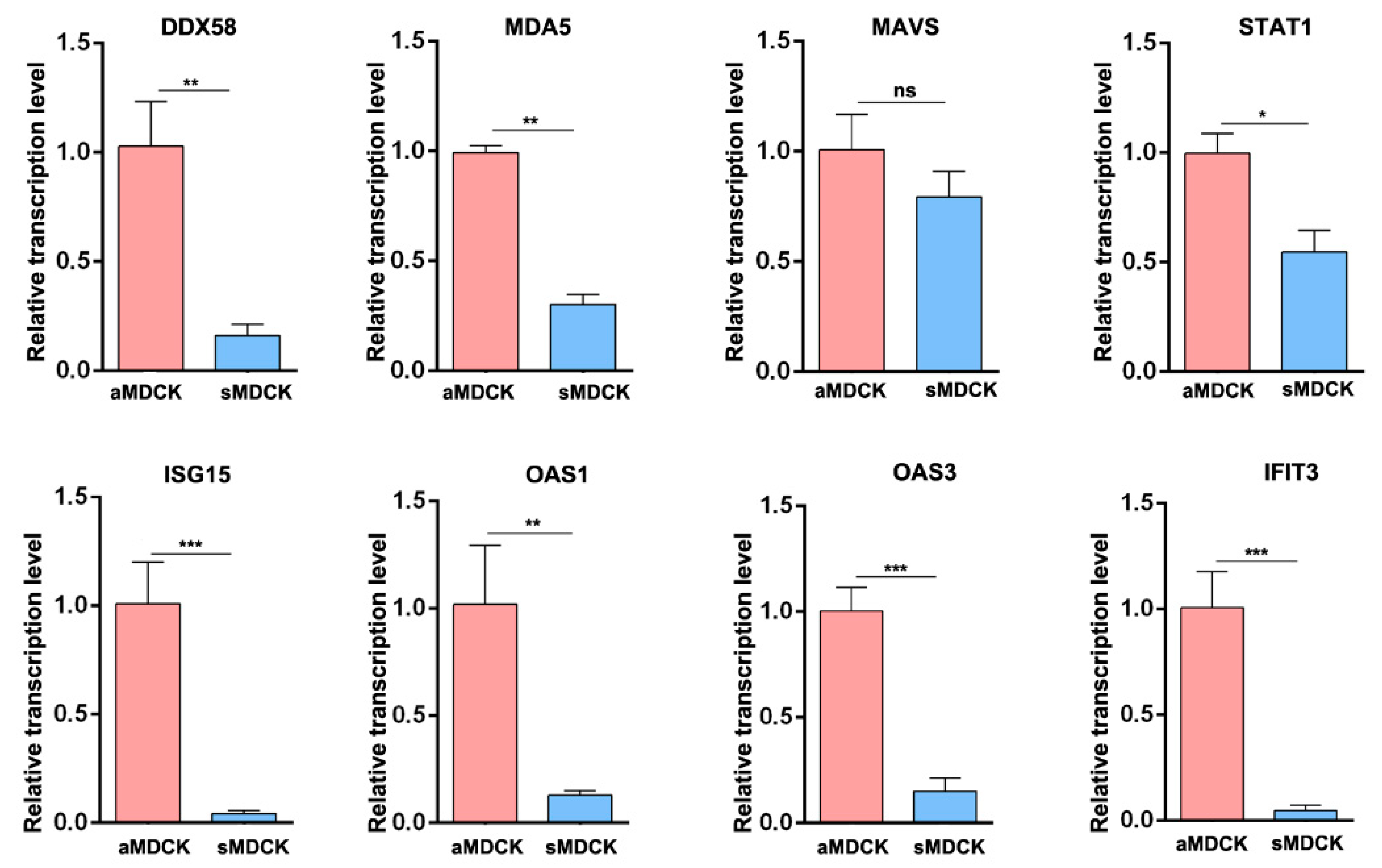
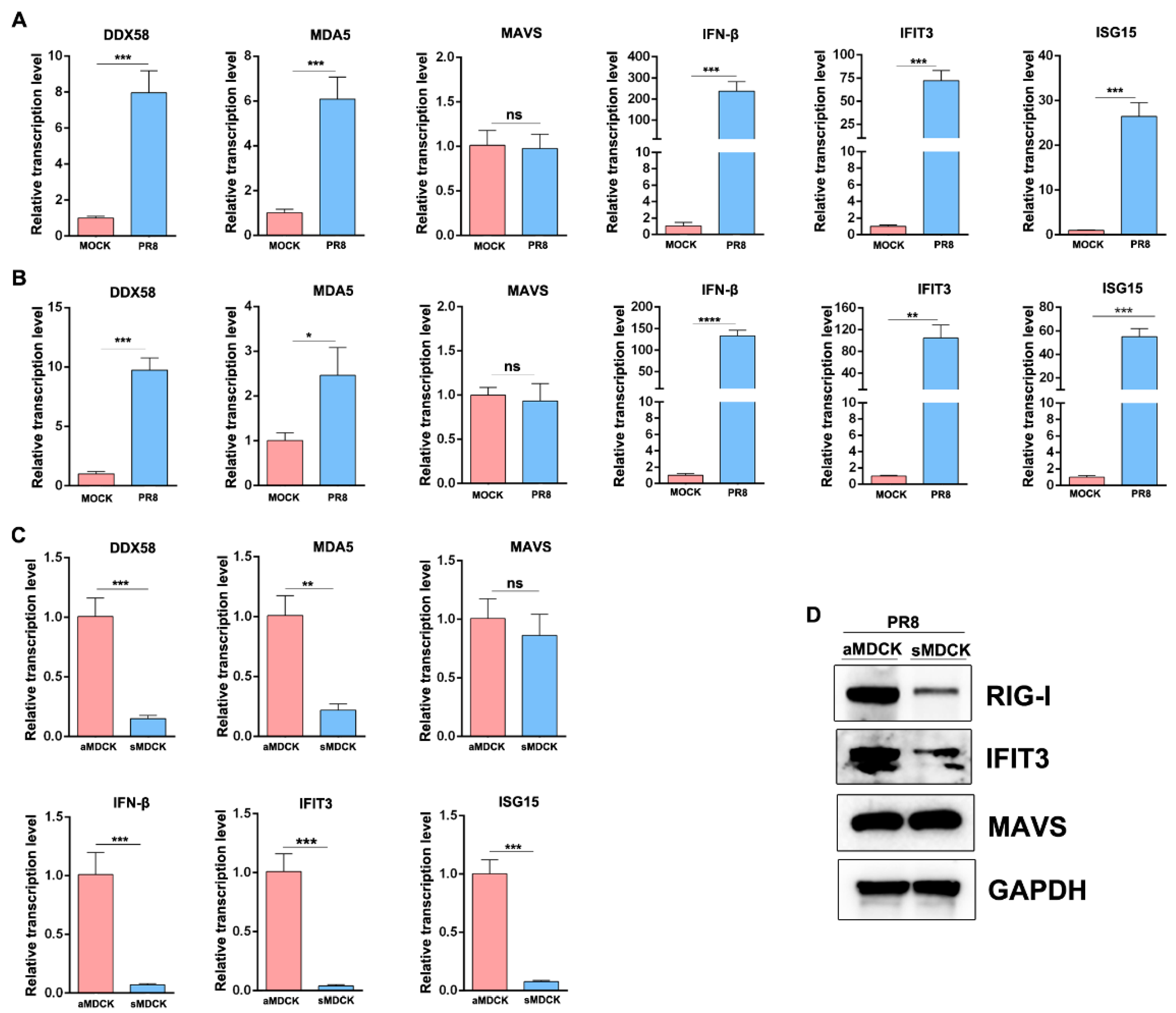
3.3. Validation of Gene Expression by RT-qPCR
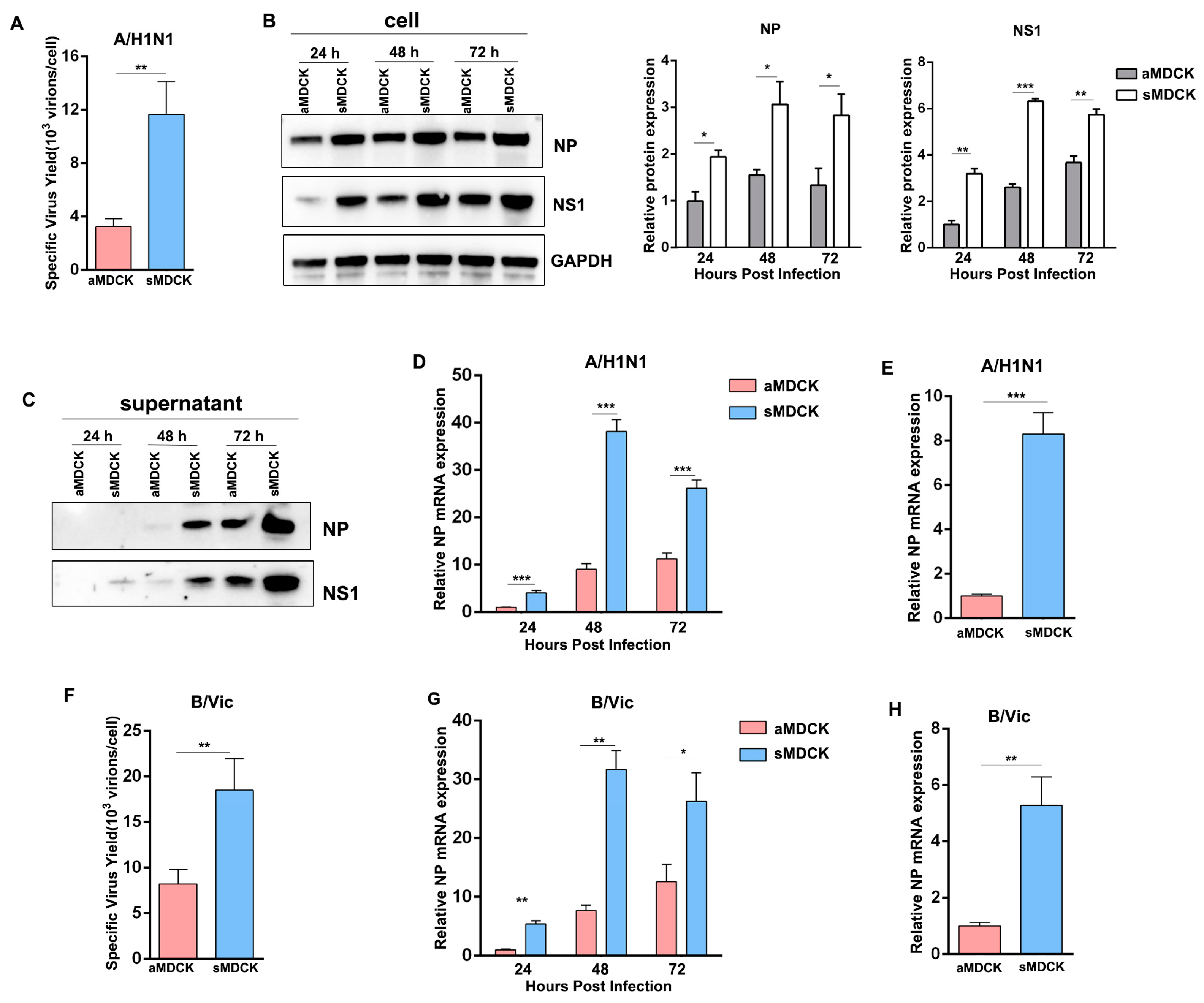
3.4. Viral Yields of Influenza A/B Virus Were Higher in sMDCK Cells Than in aMDCK Cells
3.5. Transcriptomic Analysis of sMDCK vs. aMDCK after Viral Infection
3.6. The Induced ISG in sMDCK Cells Significantly Decreases Compared with aMDCK Cells
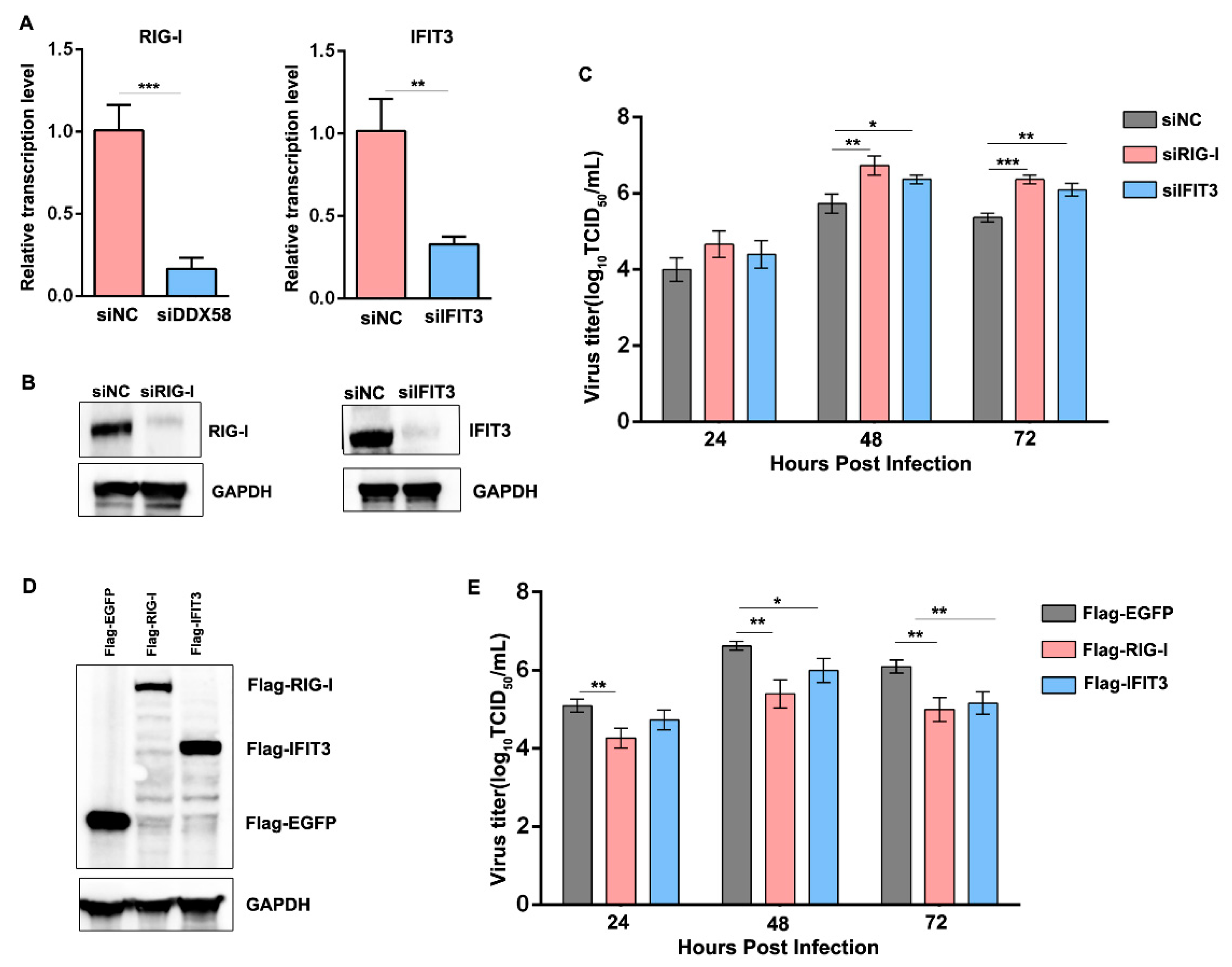
3.7. Reduction in the Induced RIG-I and IFIT3 in sMDCK Cells Promotes Viral Production
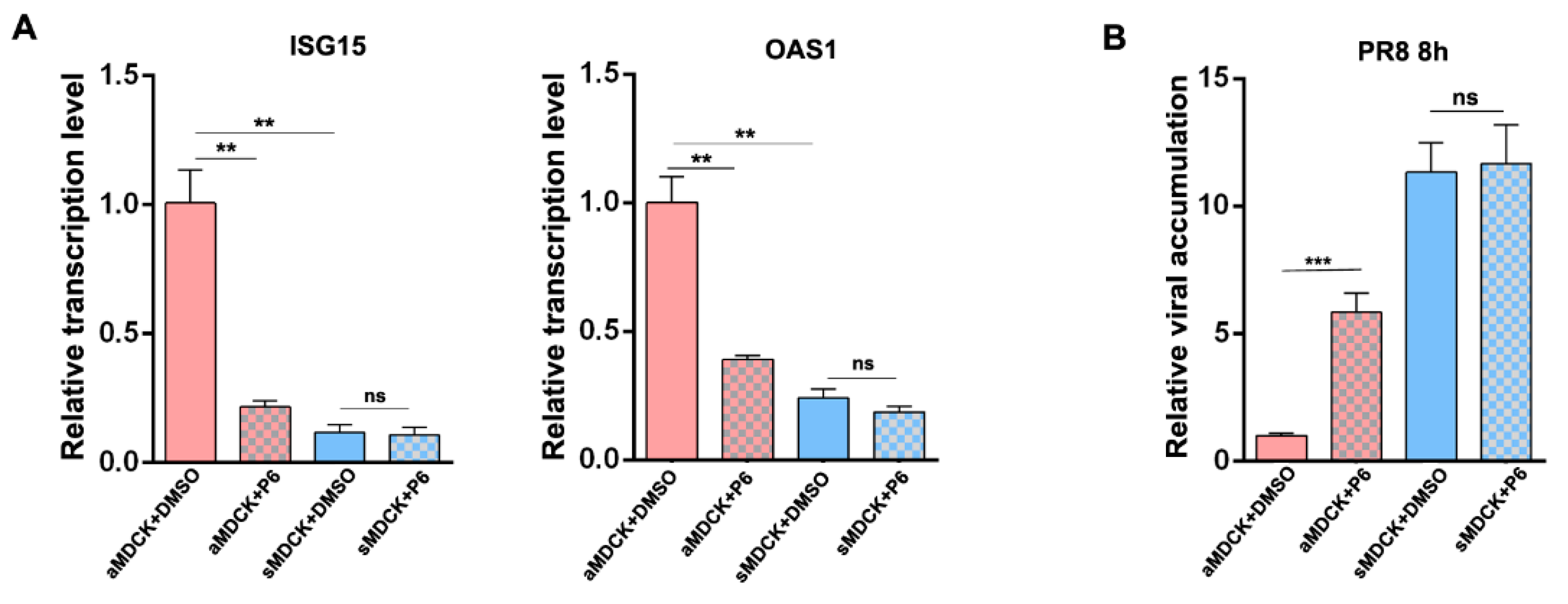
3.8. Inhibition of JAK/STAT Pathway Increased the Production of IAV in aMDCK Cells Instead of sMDCK Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, S.-S.; Webby, R.J. Traditional and New Influenza Vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef]
- Parker, L.; Wharton, S.A.; Martin, S.R.; Cross, K.; Lin, Y.; Liu, Y.; Feizi, T.; Daniels, R.S.; McCauley, J.W. Effects of egg-adaptation on receptor-binding and antigenic properties of recent influenza A (H3N2) vaccine viruses. J. Gen. Virol. 2016, 97, 1333–1344. [Google Scholar] [CrossRef]
- Wu, N.C.; Lv, H.; Thompson, A.J.; Wu, D.C.; Ng, W.W.S.; Kadam, R.U.; Lin, C.-W.; Nycholat, C.M.; McBride, R.; Liang, W.; et al. Preventing an Antigenically Disruptive Mutation in Egg-Based H3N2 Seasonal Influenza Vaccines by Mutational Incompatibility. Cell Host Microbe 2019, 25, 836–844.e835. [Google Scholar] [CrossRef]
- Milián, E.; Kamen, A.A. Current and Emerging Cell Culture Manufacturing Technologies for Influenza Vaccines. BioMed Res. Int. 2015, 2015, 504831. [Google Scholar] [CrossRef] [PubMed]
- Hütter, J.; Rödig, J.V.; Höper, D.; Seeberger, P.H.; Reichl, U.; Rapp, E.; Lepenies, B. Toward Animal Cell Culture–Based Influenza Vaccine Design: Viral Hemagglutinin N-Glycosylation Markedly Impacts Immunogenicity. J. Immunol. 2013, 190, 220–230. [Google Scholar] [CrossRef]
- Palache, A.M.; Brands, R.; Scharrenburg, G.J.M.v. Immunogenicity and Reactogenicity of Influenza Subunit Vaccines Produced in MDCK Cells or Fertilized Chicken Eggs. J. Infect. Dis. 1997, 176, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.G.; Ophorst, C.; Koldijk, M.H.; Schouten, G.; Mehtali, M.; Uytdehaag, F. The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine 2001, 19, 2716–2721. [Google Scholar] [CrossRef]
- Kistner, O.; Barrett, P.N.; Mundt, W.; Reiter, M.; Schober-Bendixen, S.; Dorner, F. Development of a mammalian cell (Vero) derived candidate influenza virus vaccine. Vaccine 1998, 16, 960–968. [Google Scholar] [CrossRef]
- Rindler, M.J.; Chuman, L.M.; Shaffer, L.; Saier, M.H., Jr. Retention of differentiated properties in an established dog kidney epithelial cell line (MDCK). J. Cell Biol. 1979, 81, 635–648. [Google Scholar] [CrossRef]
- Saier, M.H. Growth and differentiated properties of a kidney epithelial cell line (MDCK). Am. J. Physiol. Cell Physiol. 1981, 240, C106–C109. [Google Scholar] [CrossRef] [PubMed]
- Taub, M.; Chuman, L.; Saier, M.H.; Sato, G. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc. Natl. Acad. Sci. USA 1979, 76, 3338–3342. [Google Scholar] [CrossRef]
- Kluge, S.; Benndorf, D.; Genzel, Y.; Scharfenberg, K.; Rapp, E.; Reichl, U. Monitoring changes in proteome during stepwise adaptation of a MDCK cell line from adherence to growth in suspension. Vaccine 2015, 33, 4269–4280. [Google Scholar] [CrossRef]
- van Wielink, R.; Kant-Eenbergen, H.C.M.; Harmsen, M.M.; Martens, D.E.; Wijffels, R.H.; Coco-Martin, J.M. Adaptation of a Madin–Darby canine kidney cell line to suspension growth in serum-free media and comparison of its ability to produce avian influenza virus to Vero and BHK21 cell lines. J. Virol. Methods 2011, 171, 53–60. [Google Scholar] [CrossRef]
- Chu, C.; Lugovtsev, V.; Golding, H.; Betenbaugh, M.; Shiloach, J. Conversion of MDCK cell line to suspension culture by transfecting with human siat7e gene and its application for influenza virus production. Proc. Natl. Acad. Sci. USA 2009, 106, 14802–14807. [Google Scholar] [CrossRef]
- Tapia, F.; Vogel, T.; Genzel, Y.; Behrendt, I.; Hirschel, M.; Gangemi, J.D.; Reichl, U. Production of high-titer human influenza A virus with adherent and suspension MDCK cells cultured in a single-use hollow fiber bioreactor. Vaccine 2014, 32, 1003–1011. [Google Scholar] [CrossRef]
- Peschel, B.; Frentzel, S.; Laske, T.; Genzel, Y.; Reichl, U. Comparison of influenza virus yields and apoptosis-induction in an adherent and a suspension MDCK cell line. Vaccine 2013, 31, 5693–5699. [Google Scholar] [CrossRef]
- Genzel, Y.; Vogel, T.; Buck, J.; Behrendt, I.; Ramirez, D.V.; Schiedner, G.; Jordan, I.; Reichl, U. High cell density cultivations by alternating tangential flow (ATF) perfusion for influenza A virus production using suspension cells. Vaccine 2014, 32, 2770–2781. [Google Scholar] [CrossRef] [PubMed]
- Kluge, S.; Genzel, Y.; Laus, K.; Serve, A.; Pflugmacher, A.; Peschel, B.; Rapp, E.; Reichl, U. Ezrin and HNRNP expression correlate with increased virus release rate and early onset of virus-induced apoptosis of MDCK suspension cells. Biotechnol. J. 2016, 11, 1332–1342. [Google Scholar] [CrossRef]
- Weber-Gerlach, M.; Weber, F. Standing on three legs: Antiviral activities of RIG-I against influenza viruses. Curr. Opin. Immunol. 2016, 42, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ye, S.; Yao, C.; Wang, J.; Mao, J.; Xu, L.; Liu, Y.; Fu, C.; Lu, G.; Li, S. Antiviral Activity of Canine RIG-I against Canine Influenza Virus and Interactions between Canine RIG-I and CIV. Viruses 2021, 13, 2048. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef]
- Pekosz, A.; Khaperskyy, D.A.; Emara, M.M.; Johnston, B.P.; Anderson, P.; Hatchette, T.F.; McCormick, C. Influenza A Virus Host Shutoff Disables Antiviral Stress-Induced Translation Arrest. PLoS Pathog. 2014, 10, e1004217. [Google Scholar] [CrossRef]
- Gil, J.; García, M.A.; Gomez-Puertas, P.; Guerra, S.; Rullas, J.; Nakano, H.; Alcamí, J.; Esteban, M. TRAF Family Proteins Link PKR with NF-κB Activation. Mol. Cell. Biol. 2023, 24, 4502–4512. [Google Scholar] [CrossRef]
- Everitt, A.R.; Clare, S.; Pertel, T.; John, S.P.; Wash, R.S.; Smith, S.E.; Chin, C.R.; Feeley, E.M.; Sims, J.S.; Adams, D.J.; et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 2012, 484, 519–523. [Google Scholar] [CrossRef]
- Zhou, A.; Dong, X.; Liu, M.; Tang, B. Comprehensive Transcriptomic Analysis Identifies Novel Antiviral Factors Against Influenza A Virus Infection. Front. Immunol. 2021, 12, 632798. [Google Scholar] [CrossRef]
- Mayuramart, O.; Poomipak, W.; Rattanaburi, S.; Khongnomnan, K.; Anuntakarun, S.; Saengchoowong, S.; Chavalit, T.; Chantaravisoot, N.; Payungporn, S. IRF7-deficient MDCK cell based on CRISPR/Cas9 technology for enhancing influenza virus replication and improving vaccine production. PeerJ 2022, 10, e13989. [Google Scholar] [CrossRef]
- Qiao, Z.; Liao, Y.; Pei, M.; Qiu, Z.; Liu, Z.; Jin, D.; Zhang, J.; Ma, Z.; Yang, X. RSAD2 Is an Effective Target for High-Yield Vaccine Production in MDCK Cells. Viruses 2022, 14, 2587. [Google Scholar] [CrossRef]
- Ye, Q.; Phan, T.; Hu, W.-S.; Liu, X.; Fan, L.; Tan, W.-S.; Zhao, L. Transcriptomic Characterization Reveals Attributes of High Influenza Virus Productivity in MDCK Cells. Viruses 2021, 13, 2200. [Google Scholar] [CrossRef] [PubMed]
- Kalbfuss, B.; Knöchlein, A.; Kröber, T.; Reichl, U. Monitoring influenza virus content in vaccine production: Precise assays for the quantitation of hemagglutination and neuraminidase activity. Biologicals 2008, 36, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Bock, A.; Schulze-Horsel, J.; Schwarzer, J.; Rapp, E.; Genzel, Y.; Reichl, U. High-density microcarrier cell cultures for influenza virus production. Biotechnol. Prog. 2011, 27, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty PER Cent Endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Pedranzini, L.; Dechow, T.; Berishaj, M.; Comenzo, R.; Zhou, P.; Azare, J.; Bornmann, W.; Bromberg, J. Pyridone 6, A Pan-Janus–Activated Kinase Inhibitor, Induces Growth Inhibition of Multiple Myeloma Cells. Cancer Res. 2006, 66, 9714–9721. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus–sialic acid interactions. Nat. Rev. Microbiol. 2014, 12, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, Z. An overview of influenza A virus receptors. Crit. Rev. Microbiol. 2011, 37, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Lenschow, D.J.; Lai, C.; Frias-Staheli, N.; Giannakopoulos, N.V.; Lutz, A.; Wolff, T.; Osiak, A.; Levine, B.; Schmidt, R.E.; García-Sastre, A.; et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl. Acad. Sci. USA 2007, 104, 1371–1376. [Google Scholar] [CrossRef]
- Haller, O.; Kochs, G. Mx genes: Host determinants controlling influenza virus infection and trans-species transmission. Hum. Genet. 2019, 139, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.; Greenhalgh, C.J.; Viney, E.; Willson, T.A.; Starr, R.; Nicola, N.A.; Hilton, D.J.; Alexander, W.S. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature 2000, 405, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wu, S.; Wu, Y.; Liu, T.; Zhao, X.; Li, Y. MicroRNA-196a/-196b regulate the progression of hepatocellular carcinoma through modulating the JAK/STAT pathway via targeting SOCS2. Cell Death Dis. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Inoue, H.; Fukuyama, S.; Yoshida, H.; Moriwaki, A.; Matsumoto, T.; Matsumoto, K.; Asai, Y.; Kubo, M.; Yoshimura, A.; et al. Effects of a Janus kinase inhibitor, pyridone 6, on airway responses in a murine model of asthma. Biochem. Biophys. Res. Commun. 2011, 404, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Hinay, A.A.; Kakee, S.; Kageyama, S.; Tsuneki-Tokunaga, A.; Perdana, W.Y.; Akena, Y.; Nishiyama, S.; Kanai, K. Pro-Inflammatory Cytokines and Interferon-Stimulated Gene Responses Induced by Seasonal Influenza A Virus with Varying Growth Capabilities in Human Lung Epithelial Cell Lines. Vaccines 2022, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.E.; Cubbon, R.M.; Cummings, R.T.; Wicker, L.S.; Frankshun, R.; Cunningham, B.R.; Cameron, P.M.; Meinke, P.T.; Liverton, N.; Weng, Y.; et al. Photochemical preparation of a pyridone containing tetracycle: A jak protein kinase inhibitor. Bioorganic Med. Chem. Lett. 2002, 12, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Skehel, J.J.; Wiley, D.C. Receptor Binding and Membrane Fusion in Virus Entry: The Influenza Hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Zhang, M.; Wang, P.; Yuan, C.; Qi, J.; Meng, H.; Gao, C. Tripartite Motif-Containing Protein 38 Negatively Regulates TLR3/4- and RIG-I–Mediated IFN-β Production and Antiviral Response by Targeting NAP1. J. Immunol. 2012, 188, 5311–5318. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Kim, I.; Seong, S.; Kim, N. TRIM38 regulates NF-κB activation through TAB2 degradation in osteoclast and osteoblast differentiation. Bone 2018, 113, 17–28. [Google Scholar] [CrossRef]
- Hu, M.-M.; Liao, C.-Y.; Yang, Q.; Xie, X.-Q.; Shu, H.-B. Innate immunity to RNA virus is regulated by temporal and reversible sumoylation of RIG-I and MDA5. J. Exp. Med. 2017, 214, 973–989. [Google Scholar] [CrossRef]
- Chikhalya, A.; Dittmann, M.; Zheng, Y.; Sohn, S.-Y.; Rice, C.M.; Hearing, P.; Shenk, T. Human IFIT3 Protein Induces Interferon Signaling and Inhibits Adenovirus Immediate Early Gene Expression. mBio 2021, 12, e0282921. [Google Scholar] [CrossRef]
- Adamson, A.L.; Hsu, Y.-L.; Shi, S.-F.; Wu, W.-L.; Ho, L.-J.; Lai, J.-H. Protective Roles of Interferon-Induced Protein with Tetratricopeptide Repeats 3 (IFIT3) in Dengue Virus Infection of Human Lung Epithelial Cells. PLoS ONE 2013, 8, e79518. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Chen, W.; Wei, B.; Shan, Y.-F.; Wang, C. IFN-Induced TPR Protein IFIT3 Potentiates Antiviral Signaling by Bridging MAVS and TBK1. J. Immunol. 2011, 187, 2559–2568. [Google Scholar] [CrossRef]
- Mears, H.V.; Sweeney, T.R. Better together: The role of IFIT protein–protein interactions in the antiviral response. J. Gen. Virol. 2018, 99, 1463–1477. [Google Scholar] [CrossRef]
- Tran, V.; Ledwith, M.P.; Thamamongood, T.; Higgins, C.A.; Tripathi, S.; Chang, M.W.; Benner, C.; García-Sastre, A.; Schwemmle, M.; Boon, A.C.M.; et al. Influenza virus repurposes the antiviral protein IFIT2 to promote translation of viral mRNAs. Nat. Microbiol. 2020, 5, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Stark, G.R.; Darnell, J.E. The JAK-STAT Pathway at Twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2013, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Xin, Z.-t.; Liang, Y.; Ly, H.; Liang, Y. NF-κB Signaling Differentially Regulates Influenza Virus RNA Synthesis. J. Virol. 2008, 82, 9880–9889. [Google Scholar] [CrossRef]
| Primer Name | Sequence (5′ to 3′) |
|---|---|
| cRIG-I-F | ATGACGGCCGAGGAGCGG |
| cRIG-I-R | TCATTCGGGTATTTCTGGAGAAC |
| cIFIT3-F | ATGAGTGAGGTCAACAAGAATTC |
| cIFIT3-R | TCAGGTCTCAGGAGCTCTGAG |
| EGFP-F | ATGGTGAGCAAGGGCGAGGAG |
| EGFP-R | TTACTTGTACAGCTCGTCCATG |
| Primer Name | Sequence (5′ to 3′) |
|---|---|
| cRIG-I mRNA-F | AGAGCAAGTTCAGTCAACTGTG |
| cRIG-I mRNA-R | ACTGAAGGTGGACATGGGTC |
| cSTAT1 mRNA-F | GGAACGTTCAGAGCACTGTG |
| cSTAT1-mRNA-R | ATGTTCTCGGTTCTGCAAGG |
| cIFIT3 mRNA-F | CTGGCCATAGCAATGTACTG |
| cIFIT3 mRNA-R | CTCTGTAATTTCAGGGCCAAG |
| cMAVS mRNA-F | TACCAGAGTCCCTGGAAGAG |
| cMAVS mRNA-R | AGTCAGAGGGTTGACAGCTG |
| cOAS1 mRNA-F | TCTTCAGGCAAAGGCACGAC |
| cOAS1 mRNA-R | ACTCTGGACCTCAAAGTACAC |
| cOAS3 mRNA-F | CCATCCCCGGTCTGAACTTTG |
| cOAS3 mRNA-R | CTGCCCCAGAGCGTCAAAG |
| cIFNB mRNA-F | GAGCAACGACTTGCTTCGATC |
| cIFNB mRNA-R | CTGGAACTGGCGTGATTTCTC |
| cISG15 mRNA-F | CATGGCTGGGAACCTGACTG |
| cISG15 mRNA-R | GAGATCCCATCCTGCAGCAC |
| cGAPDH mRNA-F | CCAAGAGGGTCATCATCTCTGC |
| cGAPDH mRNA-R | TGCCGAAGTGGTCATGGATG |
| PR8 NP-F | CTCTTGTTCGCACCGGAATG |
| PR8 NP-R | GTTCCGATCATTGATCCCACG |
| BVR-26 NP-F | CAGAGATAAAGAAGAGCGTCTAC |
| BVR-26 NP-R | TTCTTGTCATCAGTGGCAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Luo, J.; Li, B.; Ye, Q.; Xu, W.; Gao, F.; Zhou, L.; Lu, W.; Tan, W.-S.; Li, X. Reduction in Interferon-Stimulated Genes Contributes to High-Yield Production of Influenza Virus in Suspension MDCK Cells. Vaccines 2024, 12, 287. https://doi.org/10.3390/vaccines12030287
Wang Q, Luo J, Li B, Ye Q, Xu W, Gao F, Zhou L, Lu W, Tan W-S, Li X. Reduction in Interferon-Stimulated Genes Contributes to High-Yield Production of Influenza Virus in Suspension MDCK Cells. Vaccines. 2024; 12(3):287. https://doi.org/10.3390/vaccines12030287
Chicago/Turabian StyleWang, Qi, Jian Luo, Beibei Li, Qian Ye, Wenting Xu, Feixia Gao, Linting Zhou, Wenyue Lu, Wen-Song Tan, and Xiuling Li. 2024. "Reduction in Interferon-Stimulated Genes Contributes to High-Yield Production of Influenza Virus in Suspension MDCK Cells" Vaccines 12, no. 3: 287. https://doi.org/10.3390/vaccines12030287
APA StyleWang, Q., Luo, J., Li, B., Ye, Q., Xu, W., Gao, F., Zhou, L., Lu, W., Tan, W.-S., & Li, X. (2024). Reduction in Interferon-Stimulated Genes Contributes to High-Yield Production of Influenza Virus in Suspension MDCK Cells. Vaccines, 12(3), 287. https://doi.org/10.3390/vaccines12030287






