Abundance of Selected Lipopolysaccharide-Rich Bacteria and Levels of Toll-like Receptor 4 and Interleukin 8 Expression Are Significantly Associated with Live Attenuated Rotavirus Vaccine Shedding among South African Infants
Abstract
1. Background
2. Methodology
2.1. Study Design and Participants
2.2. Ethics
2.3. Specimen Collection and Storage
2.4. Stool Viral RNA and Genomic DNA Extraction
2.5. Human and Bacterial mRNA Extraction
2.6. Detection of Rotavirus Vaccine Virus Shedding in Stool RNA Samples
2.7. Primer and Probe Design for Selected Bacterial LPSs and N-acetylglucosamine Genes
2.8. Detection of the Selected LPS-Rich Bacteria in Stool DNA Samples
2.9. Bacterial Quantification
2.10. Bacterial and Host Gene Expression via Real-Time PCR
2.11. Statistical Analysis
3. Results
3.1. Demographics and Baseline Characteristics
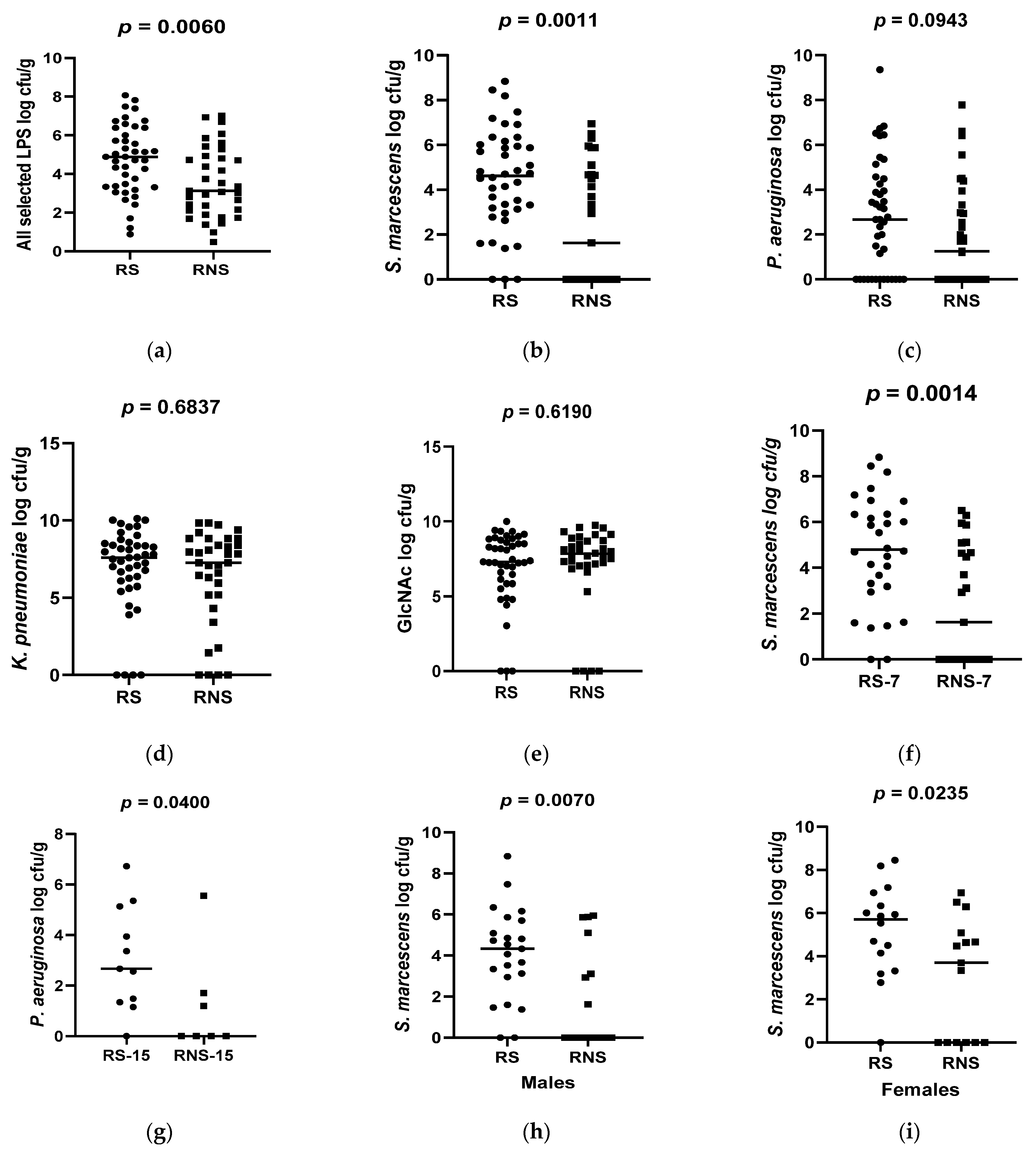
3.2. Abundances of Selected LPS-Rich Bacteria and Bacterial N-acetylglucosamine in Stool Samples
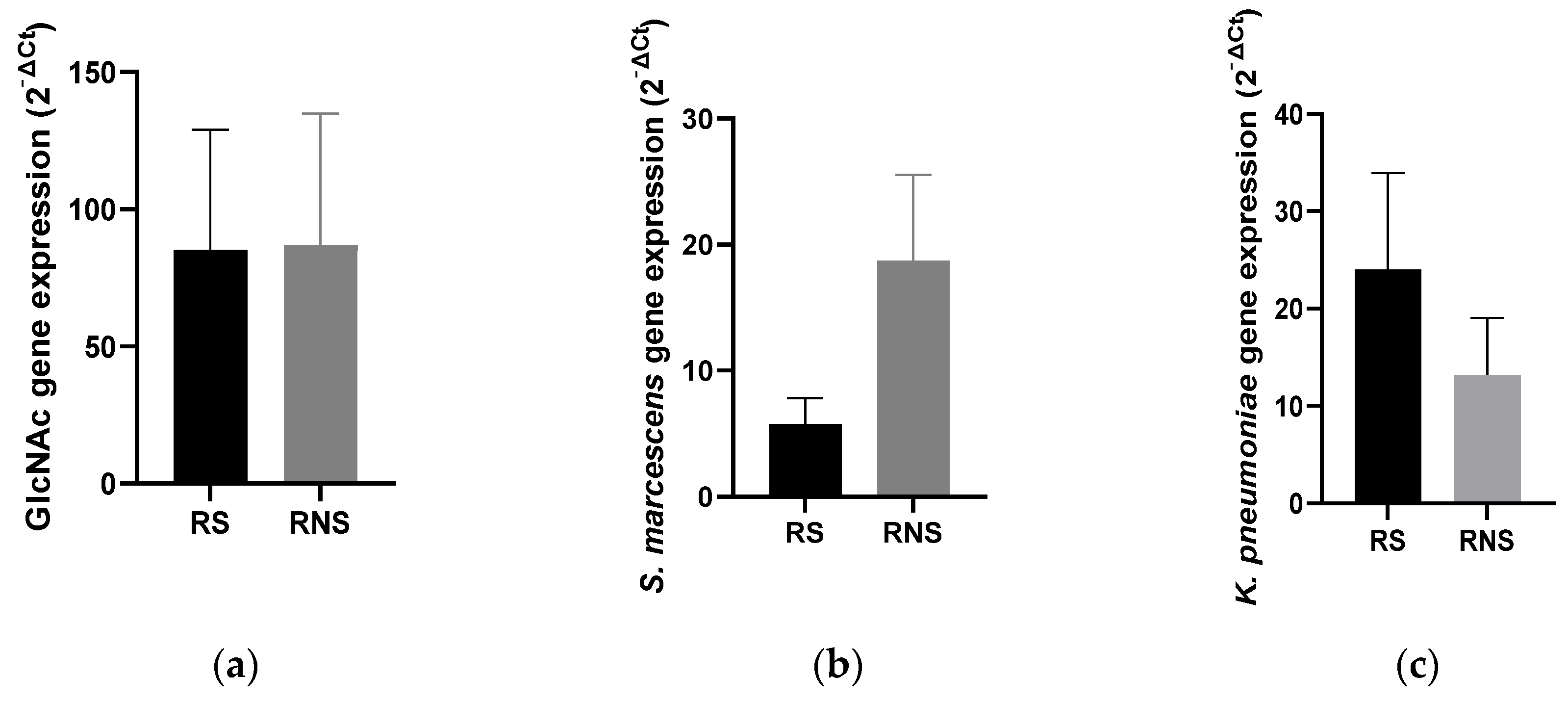
3.3. Expression of Selected Bacterial LPS and N-acetylglucosamine Genes in Vaccine Shedders versus Non-Shedders
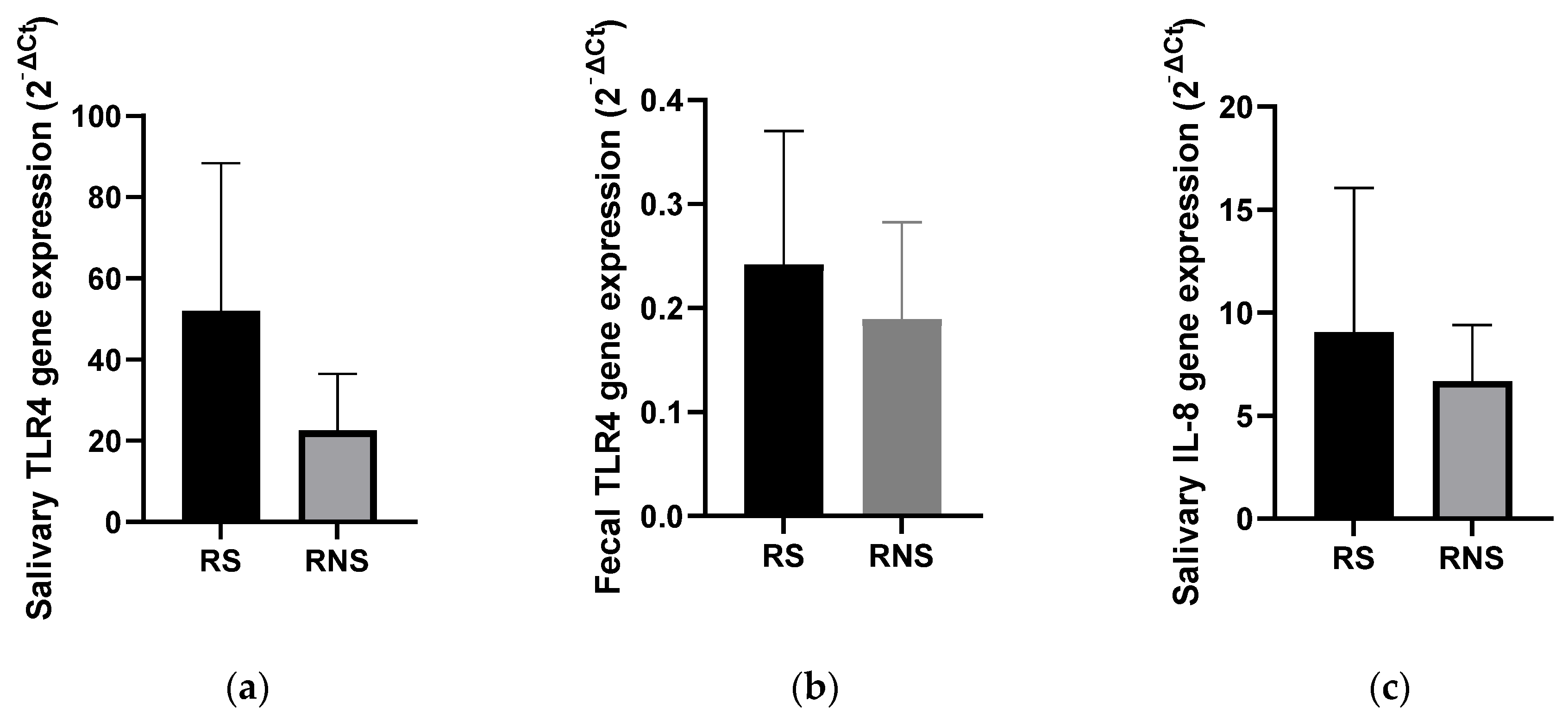
3.4. Levels of TLR4 and IL-8 Gene Expressions
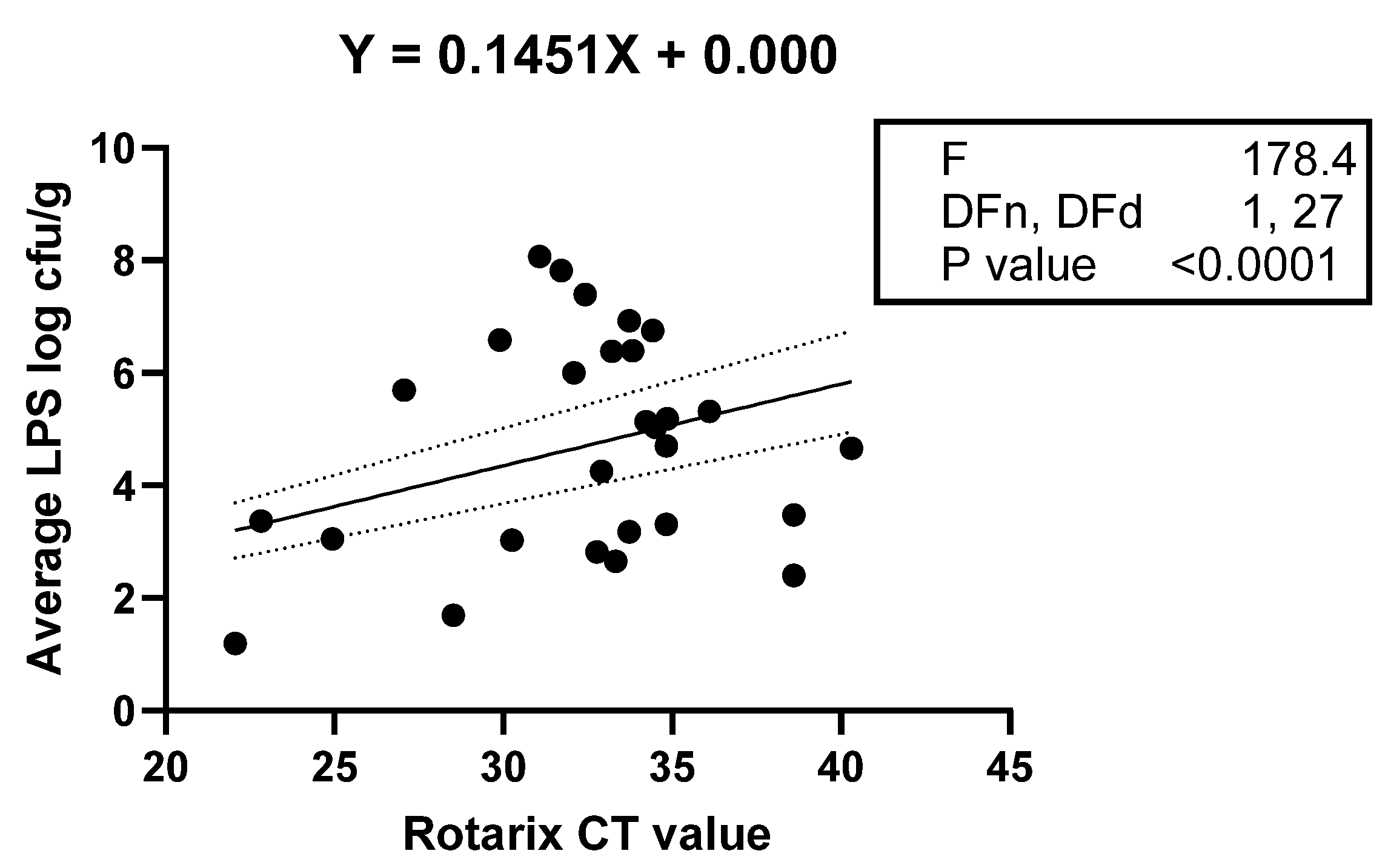
3.5. Association between Abundances of Selected LPS-Rich Bacteria and Vaccine Shedding
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef]
- Vesikari, T.; Karvonen, A.; Prymula, R.; Schuster, V.; Tejedor, J.C.; Cohen, R.; Meurice, F.; Han, H.H.; Damaso, S.; Bouckenooghe, A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: Randomised, double-blind controlled study. Lancet 2007, 370, 1757–1763. [Google Scholar] [CrossRef]
- Armah, G.E.; Sow, S.O.; Breiman, R.F.; Dallas, M.J.; Tapia, M.D.; Feikin, D.R.; Binka, F.N.; Steele, A.D.; Laserson, K.F.; Ansah, N.A.; et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 376, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Anh, D.D.; Victor, J.C.; Shin, S.; MBBS, Y.; Dallas, M.J.; Podder, G.; Thiem, V.D.; Mai, L.T.P.; Luby, S.P.; et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 376, 615–623. [Google Scholar] [CrossRef]
- Madhi, S.; Kirsten, M.; Louw, C.; Bos, P.; Aspinall, S.; Bouckenooghe, A.; Neuzil, K.; Steele, A. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: A randomized, double-blind, placebo-controlled trial. Vaccine 2012, 30 (Suppl. S1), 44–51. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Johnson, H.; Steele, A.D.; Tate, J.E. Health Impact of Rotavirus Vaccination in Developing Countries: Progress and Way Forward. Clin. Infect. Dis. 2016, 62, S91–S95. [Google Scholar] [CrossRef]
- Becker-Dreps, S.; Vilchez, S.; Velasquez, D.M.; Moon, S.-S.; Hudgens, M.G.; Zambrana, L.E.; Jiang, B.D. Rotavirus-Specific IgG Antibodies from Mothers’ Serum may Inhibit Infant Immune Responses to the Pentavalent Rotavirus Vaccine. Pediatr. Infect. Dis. J. 2016, 34, 115–116. [Google Scholar] [CrossRef]
- Kazi, A.M.; Cortese, M.M.; Yu, Y.; Lopman, B.; Morrow, A.L.; Fleming, J.A.; McNeal, M.M.; Steele, A.D.; Parashar, U.D.; Zaidi, A.K.M.; et al. Secretor and salivary ABO blood group antigen status predict rotavirus vaccine take in infants. J. Infect. Dis. 2017, 215, 786–789. [Google Scholar] [CrossRef]
- Magwira, C.A.; Kgosana, L.P.; Esona, M.D.; Seheri, M.L. Low fecal rotavirus vaccine virus shedding is significantly associated with non-secretor histo-blood group antigen phenotype among infants in northern Pretoria, South Africa. Vaccine 2020, 38, 8260–8263. [Google Scholar] [CrossRef]
- Moon, S.-S.; Tate, J.E.; Ray, P.; Dennehy, P.H.; Archary, D.; Coutsoudis, A.; Bland, R.M.; Newell, M.-L.; Glass, R.I.; Parashar, U.M.B.; et al. Differential Profiles and Inhibitory Effect on Rotavirus Vaccines of Nonantibody Components in Breast Milk from Mothers in Developing and Developed Countries. Pediatr. Infect. Dis. J. 2013, 32, 863–870. [Google Scholar] [CrossRef]
- Harris, V.C.; Haak, B.W.; Handley, S.A.; Jiang, B.; Velasquez, D.E.; Hykes, B.L.; Droit, L.; Berbers, G.A.; Kemper, E.M.; van Leeuwen, E.M.; et al. Effect of Antibiotic-Mediated Microbiome Modulation on Rotavirus Vaccine Immunogenicity: A Human, Randomized-Control Proof-of-Concept Trial. Cell Host Microbe 2018, 24, 197–207.e4. [Google Scholar] [CrossRef]
- Harris, V.; Ali, A.; Fuentes, S.; Korpela, K.; Kazi, M.; Tate, J.; Parashar, U.; Wiersinga, W.J.; Giaquinto, C.; de Weerth, C.; et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes 2018, 9, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.C.; Armah, G.; Fuentes, S.; Korpela, K.E.; Parashar, U.; Victor, J.C.; Tate, J.; de Weerth, C.; Giaquinto, C.; Wiersinga, W.J.; et al. Significant Correlation between the Infant Gut Microbiome and Rotavirus Vaccine Response in Rural Ghana. J. Infect. Dis. 2017, 215, 34–41. [Google Scholar] [CrossRef]
- Uchiyama, R.; Chassaing, B.; Zhang, B.; Gewirtz, A.T. Antibiotic Treatment Suppresses Rotavirus Infection and Enhances Specific Humoral Immunity. J. Infect. Dis. 2014, 210, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.K.; Yi, H.; Kearns, D.B.; Mainou, B.A. Bacteria and bacterial envelope components enhance mammalian reovirus thermostability. PLoS Pathog. 2017, 13, e1006768. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Jesudhasan, P.R.; Pfeiffer, J.K. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 2014, 15, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kuss, S.K.; Best, G.T.; Etheredge, C.A.; Pruijssers, A.J.; Frierson, J.M.; Hooper, L.V.; Dermody, T.S.; Pfeiffer, J.K. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 2011, 334, 249–252. [Google Scholar] [CrossRef]
- Pulendran, B.; Kumar, P.; Cutler, C.W.; Mohamadzadeh, M.; Van Dyke, T.; Banchereau, J. Lipopolysaccharides from Distinct Pathogens Induce Different Classes of Immune Responses In Vivo. J. Immunol. 2001, 167, 5067–5076. [Google Scholar] [CrossRef]
- He, W.; Qu, T.; Yu, Q.; Wang, Z.; Lv, H.; Zhang, J.; Zhao, X.; Wang, P. LPS induces IL-8 expression through TLR4, MyD88, NF-kappaB and MAPK pathways in human dental pulp stem cells. Int. Endod. J. 2013, 46, 128–136. [Google Scholar] [CrossRef]
- Chen, S.M.; Lin, C.P.; Tsai, J.D.; Chao, Y.H.; Sheu, J.N. The Significance of Serum and Fecal Levels of Interleukin-6 and Interleukin-8 in Hospitalized Children with Acute Rotavirus and Norovirus Gastroenteritis. Pediatr. Neonatol. 2014, 55, 120–126. [Google Scholar] [CrossRef][Green Version]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kaczor-Urbanowicz, K.E.; Sun, J.; Majem, B.; Lo, H.-C.; Kim, Y.; Koyano, K.; Rao, S.L.; Kang, S.Y.; Kim, S.M.; et al. Characterization of human salivary extracellular RNA by next-generation sequencing. Clin. Chem. 2018, 64, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Mijatovic-Rustempasic, S.; Esona, M.D.; Tam, K.I.; Quaye, O.; Bowen, M.D. One-step multiplex real-time RT-PCR assay for detecting and genotyping wild-type group A rotavirus strains and vaccine strains (Rotarix® and RotaTeq®) in stool samples. PeerJ 2016, 2016, e1560. [Google Scholar] [CrossRef] [PubMed]
- Magwira, C.A.; Steele, D.; Seheri, M.L. Norovirus diarrhea is significantly associated with higher counts of fecal histo-blood group antigen expressing Enterobacter cloacae among black South African infants. Gut Microbes 2021, 13, 1979876. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Deng, W.; Zhao, B.; Xu, Y.; Wang, X.; Fang, Y.; Xiao, H. FOXO3-induced lncRNA LOC554202 contributes to hepatocellular carcinoma progression via the miR-485-5p/BSG axis. Cancer Gene Ther. 2022, 29, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Parker, E.P.; Praharaj, I.; Zekavati, A.; Lazarus, R.P.; Giri, S.; Operario, D.J.; Liu, J.; Houpt, E.; Iturriza-Gómara, M.; Kampmann, B.; et al. Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in South India. Vaccine 2018, 36, 264–272. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; Van den Brandt, P.A.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Huhta, H.; Helminen, O.; Kauppila, J.H.; Salo, T.; Porvari, K.; Saarnio, J.; Lehenkari, P.P.; Karttunen, T.J. The Expression of Toll-like Receptors in Normal Human and Murine Gastrointestinal Organs and the Effect of Microbiome and Cancer. J. Histochem. Cytochem. 2016, 64, 470–482. [Google Scholar] [CrossRef]
- Gómez-Rial, J.; Curras-Tuala, M.J.; Talavero-González, C.; Rodríguez-Tenreiro, C.; Vilanova-Trillo, L.; Gómez-Carballa, A.; Rivero-Calle, I.; Justicia-Grande, A.; Pardo-Seco, J.; Redondo-Collazo, L.; et al. Salivary epidermal growth factor correlates with hospitalization length in rotavirus infection. BMC Infect. Dis. 2017, 17, 370. [Google Scholar] [CrossRef]
- Rodrigues, A.; Queiróz, D.B.C.; Honda, L.; Silva, E.J.R.; Hall, S.H.; Avellar, M.C.W. Activation of Toll-Like Receptor 4 (TLR4) by In Vivo and In Vitro Exposure of Rat Epididymis to Lipopolysaccharide from Escherichia coli. Biol. Reprod. 2008, 79, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.; Case, L.K.; Kopaskie, K.; Kozlova, A.; MacDearmid, C.; Chervonsky, A.V.; Golovkina, T.V. Successful transmission of a retrovirus depends on the commensal microbiota. Science 2011, 334, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Wilks, J.; Lien, E.; Jacobson, A.N.; Fischbach, M.A.; Qureshi, N.; Chervonsky, A.V.; Golovkina, T.V. Mammalian Lipopolysaccharide Receptors Incorporated into the Retroviral Envelope Augment Virus Transmission. Cell Host Microbe 2015, 18, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Moon, S.; Wang, Y.; Jiang, B. Multiple virus infection alters rotavirus replication and expression of cytokines and Toll-like receptors in intestinal epithelial cells. Virus Res. 2012, 167, 48–55. [Google Scholar] [CrossRef]
| Bacteria/Compound | Primer Name and Sequences | Target Gene | Reference |
|---|---|---|---|
| P. aeruguinosa | WaaL F-CCAGATCAGCGAGCATCCAT | WaaL | This study |
| WaaL R-CGAAAAGCACACCCAGTTCG | |||
| Waal P-Texas red-CGGCTACGATCATCCGAT-BHQ-2 | |||
| S. marcescens | Waa F-TCGACGGTAAACAGGGGTTG | kdtX | This study |
| Waa R-CGAACGTCCCGGGATAGATG | |||
| Waa P-Hex-TAGCGGTGGTCAACGCGCAATATA | |||
| K. pneumoniae | WaaEF-TCGTTATAGCGGTAACGGGC | WaaE | This study |
| WaaER-TCGCCCGCCGTAACTATTTT | |||
| WaaEP-Hex-ATACCAACCGCTGTGGCGCATAAA-BHQ-1 | |||
| N-acetylglucosamine | GlmU-F-GTGATGTAGTATTCGCCCTGAG | GlmU | This study |
| GlmU-R-AAGATGCCACCGACGAGCAG | |||
| GlmU-P FAM-TTGTTGGTCAGCTTCGCCAG-BHQ-1 | |||
| Interleukin 8 | IL-8 F–CACCGGAAGGAACCATCTCACT | IL-8 | This study |
| IL-8 R–ACCTTCACACAGAGCTGCAGA | |||
| Toll-like receptor 4 | TLR4 F–GATTGCTCAGACCTGGCAGTT | TLR4 | This study |
| TLR4 R-GTCCTCCCACTCCAGGTAAGT | |||
| Glyceraldehyde 3-phosphate dehydrogenase | Gapdh-F–CAAGGTCATCCATGACAACTTTG | Gapdh | [25] |
| Gapdh-R–GTCCACCACCCTGTTGCTGTAG |
| Characteristic | Vaccine Shedders (n = 43), n (%) | Vaccine Non-Shedders (n = 35), n (%) | p Value |
|---|---|---|---|
| Sex Male | 25 (58.14) | 19 (54.29) | 0.8198 |
| Female | 18 (41.86) | 16 (45.71) | |
| Age | |||
| 7 weeks | 32 (74.42) | 27 (77.14) | >0.9999 |
| 15 weeks | 11 (25.58) | 8 (22.86) | |
| Birth method | |||
| Natural birth | 27 (62.79) | 27 (77.14) | 0.2204 |
| Caesarean | 16 (37.21) | 8 (22.86) | |
| Feeding type | |||
| Breast milk | 37 (86.05) | 30 (85.71) | >0.9999 |
| Formula | 6 (13.95) | 5 (14.29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kgosana, L.P.; Seheri, M.L.; Magwira, C.A. Abundance of Selected Lipopolysaccharide-Rich Bacteria and Levels of Toll-like Receptor 4 and Interleukin 8 Expression Are Significantly Associated with Live Attenuated Rotavirus Vaccine Shedding among South African Infants. Vaccines 2024, 12, 273. https://doi.org/10.3390/vaccines12030273
Kgosana LP, Seheri ML, Magwira CA. Abundance of Selected Lipopolysaccharide-Rich Bacteria and Levels of Toll-like Receptor 4 and Interleukin 8 Expression Are Significantly Associated with Live Attenuated Rotavirus Vaccine Shedding among South African Infants. Vaccines. 2024; 12(3):273. https://doi.org/10.3390/vaccines12030273
Chicago/Turabian StyleKgosana, Lerato P., Mapaseka L. Seheri, and Cliff A. Magwira. 2024. "Abundance of Selected Lipopolysaccharide-Rich Bacteria and Levels of Toll-like Receptor 4 and Interleukin 8 Expression Are Significantly Associated with Live Attenuated Rotavirus Vaccine Shedding among South African Infants" Vaccines 12, no. 3: 273. https://doi.org/10.3390/vaccines12030273
APA StyleKgosana, L. P., Seheri, M. L., & Magwira, C. A. (2024). Abundance of Selected Lipopolysaccharide-Rich Bacteria and Levels of Toll-like Receptor 4 and Interleukin 8 Expression Are Significantly Associated with Live Attenuated Rotavirus Vaccine Shedding among South African Infants. Vaccines, 12(3), 273. https://doi.org/10.3390/vaccines12030273






