Potential Protectivity of a Conjugated COVID-19 Vaccine against Tetanus
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
- (1)
- PCovac-1D: COVID-19-vaccinated individuals who received one dose of the PastoCovac vaccine.
- (2)
- PCovac-2D: COVID-19-vaccinated individuals who received two doses of the PastoCovac vaccine.
- (3)
- PCovac-3D: COVID-19-vaccinated individuals who received three doses of the PastoCovac vaccine.
- (4)
- PCovac + Td (CT): COVID-19-vaccinated individuals who received one dose of the PastoCovac vaccine, followed by a dose of the Td vaccine.
- (5)
- Td + PCovac (TC): COVID-19-vaccinated individuals who received a dose of the Td vaccine, followed by one dose of the PastoCovac vaccine.
- (6)
- Tetanus: COVID-19-vaccinated individuals from different platforms, except the PastoCovac vaccine, who received a dose of the Td vaccine.
2.2. Vaccines
2.3. Immunogenicity Assessment
2.4. Vaccine Safety
2.5. Statistical Analysis
3. Results
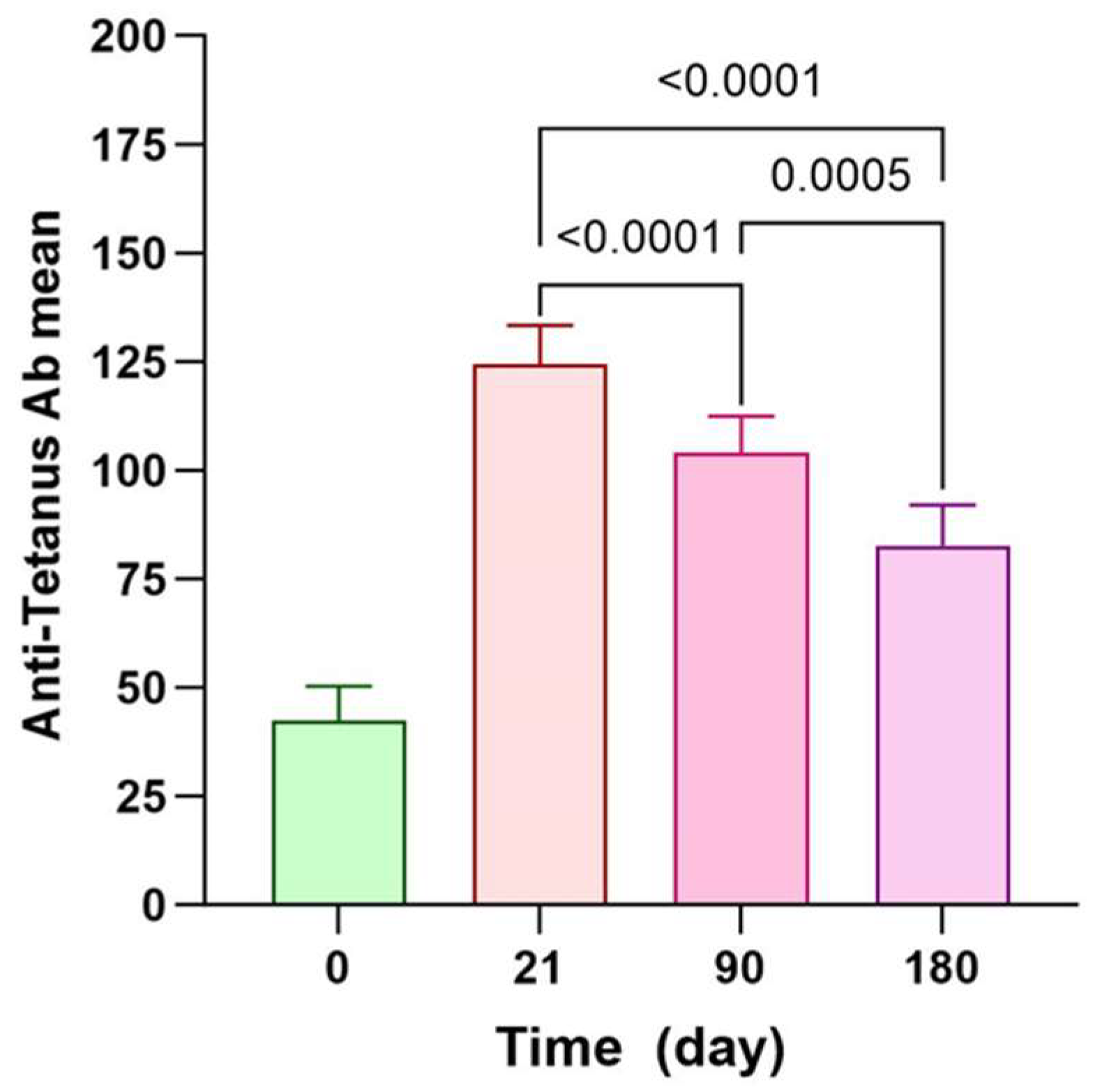
3.1. Antitetanus Persistency among PastoCovac Recipients
3.2. Assessment of Adverse Events (AEs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ab | Antibody |
| ACIP | Advisory Committee on Immunization Practices |
| AEs | Adverse Events |
| CDC | Centers for Disease Control and Prevention |
| CIES | Carrier-Induced Epitopic Suppression |
| CRM197 | Cross-Reactive Material 197 |
| DT | Diphtheria Toxoid |
| MCC | Meningococcal Serogroup C Conjugate |
| MCC | Meningococcal Conjugate Vaccine |
| MenC | Meningitis C |
| OMPC | Outer Membrane Protein Complex of Meningococcus |
| PD | Haemophilus protein D |
| PCovac | PastoCovac vaccine |
| PCV13 | Pneumococcal Conjugate Vaccine |
| SmPCs | Summaries of Product Characteristics |
| Td | Tetanus–Diphtheria vaccine |
| Tdap | Tetanus, Diphtheria, and Pertussis Vaccine |
| TT | Tetanus Toxoid |
| VLPs | Virus-like Particles |
| WHO | World Health Organization |
References
- Gilbert, S.C. T-cell-inducing vaccines—What’s the future. Immunology 2012, 135, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Masum, H.U.; Wajed, S.; Talukder, A. A comprehensive review on COVID-19 vaccines: Development, effectiveness, adverse effects, distribution and challenges. Virusdisease 2022, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Larijani, M.S.; Pouriayevali, M.H.; Sadat, S.M.; Ramezani, A. Production of Recombinant HIV-1 p24-Nef Protein in Two Forms as Potential Candidate Vaccines in Three Vehicles. Curr. Drug Deliv. 2020, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Larijani, M.S.; Ramezani, A.; Shirazi, M.M.A.; Bolhassani, A.; Pouriayevali, M.H.; Shahbazi, S.; Sadat, S.M. Evaluation of transduced dendritic cells expressing HIV-1 p24-Nef antigens in HIV-specific cytotoxic T cells induction as a therapeutic candidate vaccine. Virus Res. 2021, 298, 198403. [Google Scholar] [CrossRef]
- Chang, M.-J.; Ollivault-Shiflett, M.; Schuman, R.; Nguyen, S.N.; Kaltashov, I.A.; Bobst, C.; Rajagopal, S.P.; Przedpelski, A.; Barbieri, J.T.; Lees, A. Genetically detoxified tetanus toxin as a vaccine and conjugate carrier protein. Vaccine 2022, 40, 5103–5113. [Google Scholar] [CrossRef]
- Micoli, F.; Adamo, R.; Costantino, P. Protein Carriers for Glycoconjugate Vaccines: History, Selection Criteria, Characterization and New Trends. Molecules 2018, 23, 1451. [Google Scholar] [CrossRef]
- Pichichero, M.E. Protein carriers of conjugate vaccines. Hum. Vaccines Immunother. 2013, 9, 2505–2523. [Google Scholar] [CrossRef]
- Wantuch, P.L.; Sun, L.; LoPilato, R.K.; Mousa, J.J.; Haltiwanger, R.S.; Avci, F.Y. Isolation and characterization of new human carrier peptides from two important vaccine immunogens. Vaccine 2020, 38, 2315–2325. [Google Scholar] [CrossRef]
- Ramezani, A.; Sorouri, R.; Maghsoudi, S.H.; Dahmardeh, S.; Doroud, D.; Larijani, M.S.; Eybpoosh, S.; Mostafavi, E.; Olyaeemanesh, A.; Salehi-Vaziri, M.; et al. PastoCovac and PastoCovac Plus as protein subunit COVID-19 vaccines led to great humoral immune responses in BBIP-CorV immunized individuals. Sci. Rep. 2023, 13, 8065. [Google Scholar] [CrossRef]
- Valdes-Balbin, Y.; Santana-Mederos, D.; Paquet, F.; Fernandez, S.; Climent, Y.; Chiodo, F.; Rodríguez, L.; Ramirez, B.S.; Leon, K.; Hernandez, T.; et al. Molecular Aspects Concerning the Use of the SARS-CoV-2 Receptor Binding Domain as a Target for Preventive Vaccines. ACS Cent. Sci. 2021, 7, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Eugenia-Toledo-Romaní, M.; Verdecia-Sánchez, L.; Rodríguez-González, M.; Rodríguez-Noda, L.; Valenzuela-Silva, C.; Paredes-Moreno, B.; Sánchez-Ramírez, B.; Pérez-Nicado, R.; González-Mugica, R.; Hernández-García, T.; et al. Safety and immunogenicity of anti-SARS CoV-2 vaccine SOBERANA 02 in homologous or heterologous scheme: Open label phase I and phase IIa clinical trials. Vaccine 2022, 40, 4220–4230. [Google Scholar] [CrossRef]
- Bröker, M. Potential protective immunogenicity of tetanus toxoid, diphtheria toxoid and Cross Reacting Material 197 (CRM197) when used as carrier proteins in glycoconjugates. Hum. Vaccines Immunother. 2016, 12, 664–667. [Google Scholar] [CrossRef]
- Slifka, A.M.; Park, B.; Gao, L.; Slifka, M.K. Incidence of Tetanus and Diphtheria in Relation to Adult Vaccination Schedules. Clin. Infect. Dis. 2021, 72, 285–292. [Google Scholar] [CrossRef]
- Desai, S.; Scobie, H.M.; Cherian, T.; Goodman, T. Use of tetanus-diphtheria (Td) vaccine in children 4–7 years of age: World Health Organization consultation of experts. Vaccine 2020, 38, 3800–3807. [Google Scholar] [CrossRef]
- Liu, S.-W.; Huang, L.-C.; Chung, W.-F.; Wu, J.; Chen, L.-F.; Chen, Y.-C. Tetanus Vaccination and Extra-Immunization among Adult Populations: Eight-Year Follow Up Cohort Study of 771,443 Adults in Taiwan, 2006–2013. Int. J. Environ. Res. Public Health 2018, 15, 1622. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.T.; Limbo, O.; Smith, C.; Woehl, J.; Yang, L.; et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, P.J.M.; Caniels, T.G.; van der Straten, K.; Snitselaar, J.L.; Aldon, Y.; Bangaru, S.; Torres, J.L.; Okba, N.M.A.; Claireaux, M.; Kerster, G.; et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 2020, 369, 643–650. [Google Scholar] [CrossRef]
- Valdes-Balbin, Y.; Santana-Mederos, D.; Quintero, L.; Fernández, S.; Rodriguez, L.; Ramirez, B.S.; Perez-Nicado, R.; Acosta, C.; Méndez, Y.; Ricardo, M.G.; et al. SARS-CoV-2 RBD-Tetanus Toxoid Conjugate Vaccine Induces a Strong Neutralizing Immunity in Preclinical Studies. ACS Chem. Biol. 2021, 16, 1223–1233. [Google Scholar] [CrossRef]
- Jegerlehner, A.; Wiesel, M.; Dietmeier, K.; Zabel, F.; Gatto, D.; Saudan, P.; Bachmann, M.F. Carrier induced epitopic suppression of antibody responses induced by virus-like particles is a dynamic phenomenon caused by carrier-specific antibodies. Vaccine 2010, 28, 5503–5512. [Google Scholar] [CrossRef] [PubMed]
- van der Put, R.M.F.; Metz, B.; Pieters, R.J. Carriers and Antigens: New Developments in Glycoconjugate Vaccines. Vaccines 2023, 11, 219. [Google Scholar] [CrossRef]
- Findlow, H.; Borrow, R. Interactions of conjugate vaccines and co-administered vaccines. Hum. Vaccines Immunother. 2016, 12, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Tashani, M.; Heron, L.; Wong, M.; Rashid, H.; Booy, R. Tetanus–diphtheria–pertussis vaccine may suppress the immune response to subsequent immunization with pneumococcal CRM197-conjugate vaccine (coadministered with quadrivalent meningococcal TT-conjugate vaccine): A randomized, controlled trial. J. Travel Med. 2017, 24, tax006. [Google Scholar] [CrossRef] [PubMed]
- Eybpoosh, S.; Biglari, A.; Sorouri, R.; Ashrafian, F.; Sadat Larijani, M.; Verez-Bencomo, V.; Toledo-Romani, M.E.; Valenzuela Silva, C.; Salehi-Vaziri, M.; Dahmardeh, S.; et al. Immunogenicity and safety of heterologous boost immunization with PastoCovac Plus against COVID-19 in ChAdOx1-S or BBIBP-CorV primed individuals. PLoS Pathog. 2023, 19, e1011744. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, B.; Larijani, M.S.; Fotouhi, F.; Biglari, A.; Sorouri, R.; Amiri, F.B.; Eslamifar, A.; Jalali, T.; Salehi-Vaziri, M.; Banifazl, M.; et al. Evaluation of PastoCovac plus vaccine as a booster dose on vaccinated individuals with inactivated COVID-19 vaccine. Heliyon 2023, 9, e20555. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Cheong, H.J.; Noh, J.Y.; Choi, M.J.; Yoon, J.G.; Na Lee, S.; Kang, S.H.; Jeong, E.J.; Jo, Y.M.; Kim, W.J. Immunogenicity and safety of a tetanus-diphtheria vaccine and a 13-valent pneumococcal conjugate vaccine after concomitant vaccination in ≥50-year-old adults. BMC Infect. Dis. 2018, 18, 628. [Google Scholar] [CrossRef]

| Study Groups | N | Mean ± SD | Median [IQR] | (Min–Max) |
|---|---|---|---|---|
| PCovac-1D | 15 | 42.3 ± 31.2 | 26.8 [82.4–15.8] | (4–91.8) |
| PCovac-2D | 15 | 74.3 ± 51 | 62.1 [109.6–33.5] | (7.6–167.5) |
| PCovac-3D | 19 | 87 ± 28.9 | 97.1 [114.5–61.8] | (39.1–129.4) |
| CT | 10 | 67.8 ± 26.4 | 62.1 [89.7–48.9] | (27.6–108.7) |
| TC | 11 | 88.9 ± 37.6 | 99.2 [110.1–62.2] | (30.9–163.7) |
| Tetanus | 15 | 52 ± 20.7 | 52.5 [72.1–32.2] | (14.2–79.3) |
| Control | 21 | 29.9 ± 27.2 | 24.7 [38.3–9.6] | (4.2–107.8) |
| Group | Group | p Value |
|---|---|---|
| Tetanus | CT | p = 0.109 |
| Tetanus | TC | p = 0.004 * |
| Tetanus | CT + TC | p = 0.006 |
| CT | TC | p = 0.157 |
| PCovac-1D | PCovac-2D | p = 0.061 |
| PCovac-1D | PCovac-3D | p = 0.003 |
| PCovac-1D | Tetanus | p = 0.326 |
| PCovac-2D | Tetanus | p = 0.128 |
| PCovac-3D | Tetanus | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroud, D.; Ashrafian, F.; Javadi, A.; Dahmardeh, S.; Banifazl, M.; Bavand, A.; Sadat Larijani, M.; Ramezani, A. Potential Protectivity of a Conjugated COVID-19 Vaccine against Tetanus. Vaccines 2024, 12, 243. https://doi.org/10.3390/vaccines12030243
Doroud D, Ashrafian F, Javadi A, Dahmardeh S, Banifazl M, Bavand A, Sadat Larijani M, Ramezani A. Potential Protectivity of a Conjugated COVID-19 Vaccine against Tetanus. Vaccines. 2024; 12(3):243. https://doi.org/10.3390/vaccines12030243
Chicago/Turabian StyleDoroud, Delaram, Fatemeh Ashrafian, Amir Javadi, Sarah Dahmardeh, Mohammad Banifazl, Anahita Bavand, Mona Sadat Larijani, and Amitis Ramezani. 2024. "Potential Protectivity of a Conjugated COVID-19 Vaccine against Tetanus" Vaccines 12, no. 3: 243. https://doi.org/10.3390/vaccines12030243
APA StyleDoroud, D., Ashrafian, F., Javadi, A., Dahmardeh, S., Banifazl, M., Bavand, A., Sadat Larijani, M., & Ramezani, A. (2024). Potential Protectivity of a Conjugated COVID-19 Vaccine against Tetanus. Vaccines, 12(3), 243. https://doi.org/10.3390/vaccines12030243






