The Chimeric Chaoyang-Zika Vaccine Candidate Is Safe and Protective in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Animal Experiments
2.3. Measurement of Viral Genome by qRT-PCR
2.4. Neutralizing Antibody Response Determination
2.5. Data Analysis
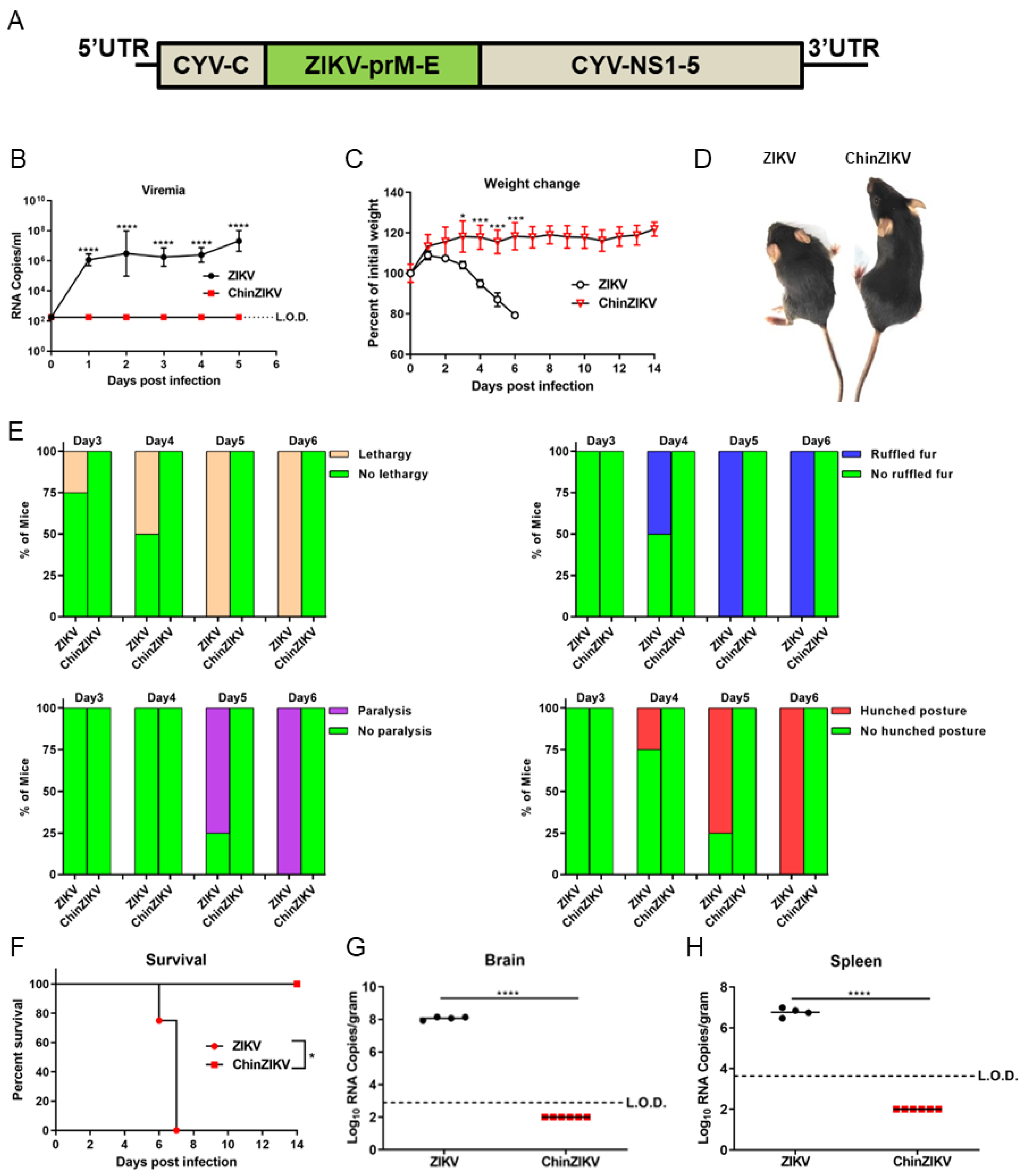
3. Results
3.1. ChinZIKV Is Safe in Immunodeficient Mice
3.2. Safety of ChinZIKV in Suckling Mice
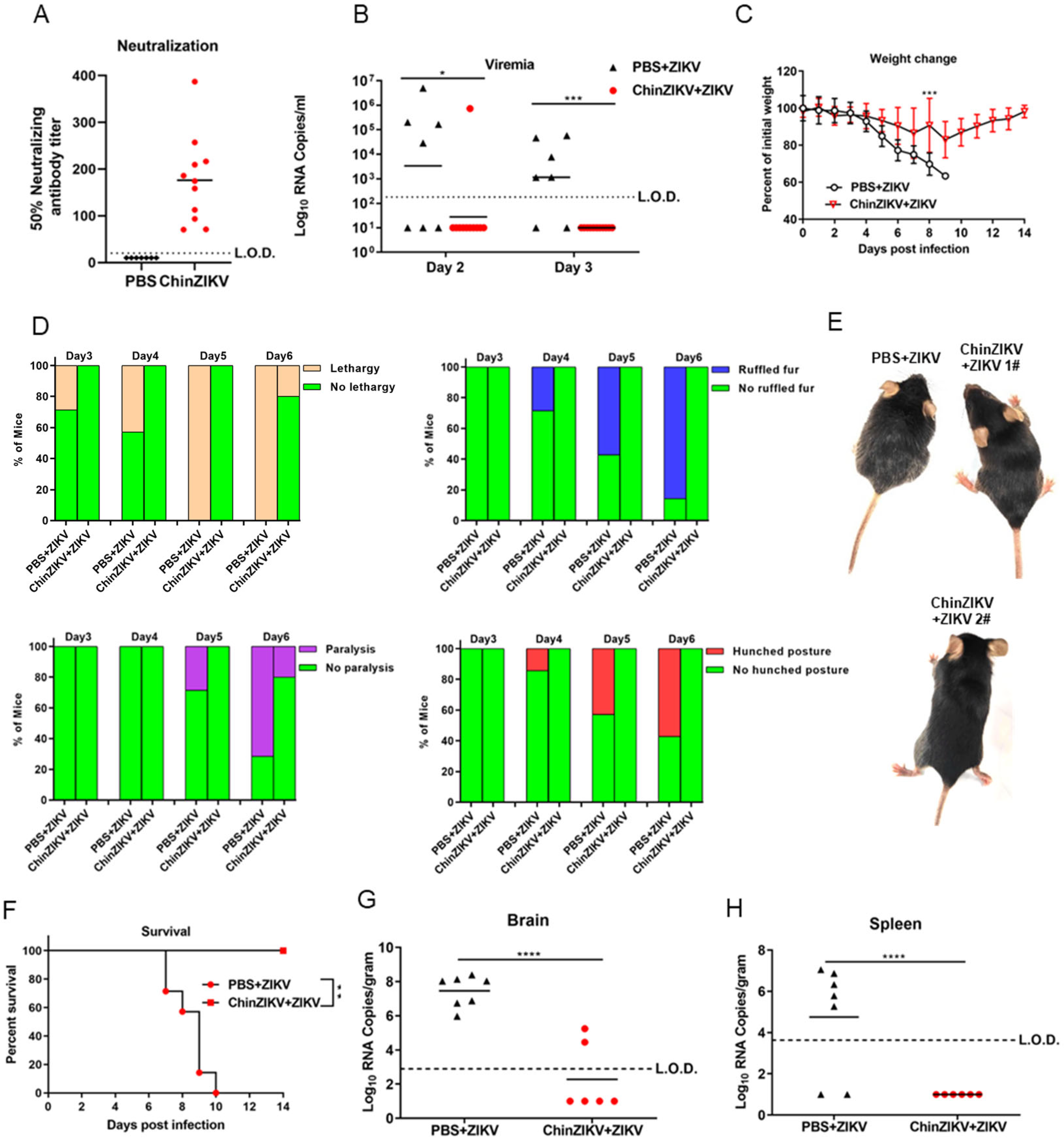
3.3. Immunogenicity and Efficacy of ChinZIKV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.G.; Ksiazek, T.G.; Suhandiman; Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Fagbami, A.H. Zika virus infections in Nigeria: Virological and seroepidemiological investigations in Oyo State. Epidemiol. Infect. 1979, 83, 213–219. [Google Scholar] [CrossRef]
- Simpson, D.I. Zika Virus Infection in Man. Trans. R. Soc. Trop. Med. Hyg. 1964, 58, 335–338. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- De Oliveira, W.K.; Carmo, E.H.; Henriques, C.M.; Coelho, G.; Vazquez, E.; Cortez-Escalante, J.; Molina, J.; Aldighieri, S.; Espinal, M.A.; Dye, C. Zika Virus Infection and Associated Neurologic Disorders in Brazil. N. Engl. J. Med. 2017, 376, 1591–1593. [Google Scholar] [CrossRef]
- Hazin, A.N.; Poretti, A.; Di Cavalcanti Souza Cruz, D.; Tenorio, M.; van der Linden, A.; Pena, L.J.; Brito, C.; Gil, L.H.; de Barros Miranda-Filho, D.; Marques, E.T.; et al. Computed Tomographic Findings in Microcephaly Associated with Zika Virus. N. Engl. J. Med. 2016, 374, 2193–2195. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Hills, S.L.; Fischer, M.; Petersen, L.R. Epidemiology of Zika Virus Infection. J. Infect. Dis. 2017, 216, S868–S874. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, M.Y.; Li, R.T.; Qi, Y.N.; Yang, G.; Deng, Y.Q.; Li, X.F.; Li, L.; Yang, X.; Liu, J.F.; et al. Zika virus leads to olfactory disorders in mice by targeting olfactory ensheathing cells. EBioMedicine 2023, 89, 104457. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, S.; Ma, S.; Jia, L.; Zhang, F.; Zhang, Y.; Zhang, J.; Wong, G.; Zhang, S.; Lu, X.; et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 2016, 167, 1511–1524.e10. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B.; Schaub, B.; Funk, A.L.; Ardillon, V.; Boullard, M.; Cabié, A.; Callier, C.; Carles, G.; Cassadou, S.; Césaire, R.; et al. Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N. Engl. J. Med. 2018, 378, 985–994. [Google Scholar] [CrossRef]
- Aliota, M.T.; Bassit, L.; Bradrick, S.S.; Cox, B.; Garcia-Blanco, M.A.; Gavegnano, C.; Friedrich, T.C.; Golos, T.G.; Griffin, D.E.; Haddow, A.D.; et al. Zika in the Americas, year 2: What have we learned? What gaps remain? A report from the Global Virus Network. Antivir. Res. 2017, 144, 223–246. [Google Scholar] [CrossRef]
- Setoh, Y.X.; Prow, N.A.; Peng, N.; Hugo, L.E.; Devine, G.; Hazlewood, J.E.; Suhrbier, A.; Khromykh, A.A. De Novo Generation and Characterization of New Zika Virus Isolate Using Sequence Data from a Microcephaly Case. mSphere 2017, 2, e00190-17. [Google Scholar] [CrossRef]
- Ospina, M.L.; Tong, V.T.; Gonzalez, M.; Valencia, D.; Mercado, M.; Gilboa, S.M.; Rodriguez, A.J.; Tinker, S.C.; Rico, A.; Winfield, C.M.; et al. Zika Virus Disease and Pregnancy Outcomes in Colombia. N. Engl. J. Med. 2020, 383, 537–545. [Google Scholar] [CrossRef]
- Blackman, M.A.; Kim, I.J.; Lin, J.S.; Thomas, S.J. Challenges of Vaccine Development for Zika Virus. Viral Immunol. 2018, 31, 117–123. [Google Scholar] [CrossRef]
- Yeasmin, M.; Molla, M.M.A.; Masud, H.; Saif-Ur-Rahman, K.M. Safety and immunogenicity of Zika virus vaccine: A systematic review of clinical trials. Rev. Med. Virol. 2023, 33, e2385. [Google Scholar] [CrossRef]
- Diamond, M.S.; Ledgerwood, J.E.; Pierson, T.C. Zika Virus Vaccine Development: Progress in the Face of New Challenges. Annu. Rev. Med. 2019, 70, 121–135. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, L.; Zhang, Z.; Marin-Lopez, A. Current Advances in Zika Vaccine Development. Vaccines 2022, 10, 1816. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Xie, X.; Shi, P.Y. Zika Virus Vaccine: Progress and Challenges. Cell Host Microbe 2018, 24, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Langenburg, T. A Perspective on Current Flavivirus Vaccine Development: A Brief Review. Viruses 2023, 15, 860. [Google Scholar] [CrossRef]
- Gaythorpe, K.A.; Hamlet, A.; Jean, K.; Garkauskas Ramos, D.; Cibrelus, L.; Garske, T.; Ferguson, N. The global burden of yellow fever. Elife 2021, 10, e64670. [Google Scholar] [CrossRef]
- Fernandes, N.; Cunha, M.S.; Suarez, P.E.N.; Machado, E.F.; Garcia, J.M.; De Carvalho, A.; Figueiredo, K.B.; Ressio, R.A.; Matsumoto, P.S.S.; Saad, L.D.C.; et al. Phylogenetic analysis reveals a new introduction of Yellow Fever virus in São Paulo State, Brazil, 2023. Acta Trop. 2023, 251, 107110. [Google Scholar] [CrossRef] [PubMed]
- Le Hir, A.; Durand, G.A.; Boucraut, J.; Garnier, A.; Mura, M.; Diamantis, S.; Carles, M.; Durand, C.; Schweitzer, C.; Audouard, C.; et al. Yellow fever vaccine-associated neurologic and viscerotropic disease: A 10-year case series of the French National Reference Center for arboviruses with clinical and immunological insights. J. Travel Med. 2023. [Google Scholar] [CrossRef]
- Roukens, A.H.E.; Visser, L.G. Fractional-dose yellow fever vaccination: An expert review. J. Travel. Med. 2019, 26, taz024. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Li, X.F.; Zhao, H.; Deng, Y.Q.; Xu, Y.P.; Wang, H.Y.; Liang, G.D.; Qin, C.F. Reduction of neutralization antibody against heterologous circulating strains in adults immunized with Japanese encephalitis live vaccine. Hum. Vaccines Immunother. 2014, 10, 2704–2705. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.S.; Li, X.M.; Zhu, Q.Y.; Wang, D.D.; Chen, H.C.; Qian, P. Isolation and molecular characterization of genotype 1 Japanese encephalitis virus, SX09S-01, from pigs in China. Virol. J. 2011, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wu, Z.; Chen, H.; Li, C.; Guo, X.; Liu, R.; Wang, G.; Zhou, M.; Zhao, T. Japanese Encephalitis Virus Infection Rate and Detection of Genotype I From Culex tritaeniorhynchus Collected From Jiangsu, China. Vector Borne Zoonotic Dis. 2017, 17, 503–509. [Google Scholar] [CrossRef]
- Fan, Y.C.; Chen, Y.Y.; Chen, J.M.; Huang, C.; Huang, M.; Chiou, S.S. Effectiveness of Live-Attenuated Genotype III Japanese Encephalitis Viral Vaccine against Circulating Genotype I Viruses in Swine. Viruses 2022, 14, 114. [Google Scholar] [CrossRef]
- Hameed, M.; Wahaab, A.; Nawaz, M.; Khan, S.; Nazir, J.; Liu, K.; Wei, J.; Ma, Z. Potential Role of Birds in Japanese Encephalitis Virus Zoonotic Transmission and Genotype Shift. Viruses 2021, 13, 357. [Google Scholar] [CrossRef]
- Li, F.; Feng, Y.; Wang, G.; Zhang, W.; Fu, S.; Wang, Z.; Yin, Q.; Nie, K.; Yan, J.; Deng, X.; et al. Tracing the spatiotemporal phylodynamics of Japanese encephalitis virus genotype I throughout Asia and the western Pacific. PLoS Negl. Trop. Dis. 2023, 17, e0011192. [Google Scholar] [CrossRef]
- Pan, X.L.; Liu, H.; Wang, H.Y.; Fu, S.H.; Liu, H.Z.; Zhang, H.L.; Li, M.H.; Gao, X.Y.; Wang, J.L.; Sun, X.H.; et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J. Virol. 2011, 85, 9847–9853. [Google Scholar] [CrossRef]
- Xiao, C.; Li, C.; Di, D.; Cappelle, J.; Liu, L.; Wang, X.; Pang, L.; Xu, J.; Liu, K.; Li, B.; et al. Differential replication efficiencies between Japanese encephalitis virus genotype I and III in avian cultured cells and young domestic ducklings. PLoS Negl. Trop. Dis. 2018, 12, e0007046. [Google Scholar] [CrossRef]
- Irwin, A. Polio is on the brink of eradication. Here’s how to keep it from coming back. Nature 2023, 623, 680–682. [Google Scholar] [CrossRef]
- Microbe, T.L. Polio eradication, elusive but achievable. Lancet Microbe 2023, 4, e963. [Google Scholar] [CrossRef]
- Fenner, F. The Global Eradication of Smallpox. Med. J. Aust. 1980, 1, 455–456. [Google Scholar] [CrossRef]
- Yu, Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine 2010, 28, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Advisory Committee on Vaccine Safety, 9–10 June 2005. Wkly. Epidemiol. Rec. 2005, 80, 242–247. [Google Scholar]
- He, W.T.; Hou, X.; Zhao, J.; Sun, J.; He, H.; Si, W.; Wang, J.; Jiang, Z.; Yan, Z.; Xing, G.; et al. Virome characterization of game animals in China reveals a spectrum of emerging pathogens. Cell 2022, 185, 1117–1129.e8. [Google Scholar] [CrossRef]
- Weaver, S.C.; Barrett, A.D.T. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Roby, J.A.; Setoh, Y.X.; Hall, R.A.; Khromykh, A.A. Post-translational regulation and modifications of flavivirus structural proteins. J. Gen. Virol. 2015, 96, 1551–1569. [Google Scholar] [CrossRef]
- Hazlewood, J.E.; Tang, B.; Yan, K.; Rawle, D.J.; Harrison, J.J.; Hall, R.A.; Hobson-Peters, J.; Suhrbier, A. The Chimeric Binjari-Zika Vaccine Provides Long-Term Protection against ZIKA Virus Challenge. Vaccines 2022, 10, 85. [Google Scholar] [CrossRef]
- Touret, F.; Gilles, M.; Klitting, R.; Aubry, F.; de Lamballerie, X.; Nougairède, A. Live Zika virus chimeric vaccine candidate based on a yellow fever 17-D attenuated backbone. Emerg. Microbes Infect. 2018, 7, 161. [Google Scholar] [CrossRef]
- Kum, D.B.; Boudewijns, R.; Ma, J.; Mishra, N.; Schols, D.; Neyts, J.; Dallmeier, K. A chimeric yellow fever-Zika virus vaccine candidate fully protects against yellow fever virus infection in mice. Emerg. Microbes Infect. 2020, 9, 520–533. [Google Scholar] [CrossRef]
- Piyasena, T.B.H.; Newton, N.D.; Hobson-Peters, J.; Vet, L.J.; Setoh, Y.X.; Bielefeldt-Ohmann, H.; Khromykh, A.A.; Hall, R.A. Chimeric viruses of the insect-specific flavivirus Palm Creek with structural proteins of vertebrate-infecting flaviviruses identify barriers to replication of insect-specific flaviviruses in vertebrate cells. J. Gen. Virol. 2019, 100, 1580–1586. [Google Scholar] [CrossRef]
- Wang, H.J.; Li, X.F.; Ye, Q.; Li, S.H.; Deng, Y.Q.; Zhao, H.; Xu, Y.P.; Ma, J.; Qin, E.D.; Qin, C.F. Recombinant chimeric Japanese encephalitis virus/tick-borne encephalitis virus is attenuated and protective in mice. Vaccine 2014, 32, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Firth, A.E. Insect-specific flaviviruses: A systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, M.; Ze-Ze, L.; Ruzek, D.; Vazquez, A.; Jeffries, C.; Defilippo, F.; Osorio, H.C.; Kilian, P.; Ruiz, S.; Fooks, A.R.; et al. Detection of mosquito-only flaviviruses in Europe. J. Gen. Virol. 2012, 93, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Moureau, G.; Harbach, R.E.; Mukwaya, L.; Goodger, K.; Ssenfuka, F.; Gould, E.; Holmes, E.C.; de Lamballerie, X. Isolation of a novel species of flavivirus and a new strain of Culex flavivirus (Flaviviridae) from a natural mosquito population in Uganda. J. Gen. Virol. 2009, 90, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.B.; Nga, P.T.; Miller, B.R. Isolation and characterization of a new mosquito flavivirus, Quang Binh virus, from Vietnam. Arch. Virol. 2009, 154, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Grubaugh, N.D.; Kondig, J.P.; Turell, M.J.; Kim, H.C.; Klein, T.A.; O’Guinn, M.L. Isolation and genomic characterization of Chaoyang virus strain ROK144 from Aedes vexans nipponii from the Republic of Korea. Virology 2013, 435, 220–224. [Google Scholar] [CrossRef]
- Wang, Z.-s.; An, S.-y.; Wang, Y. A new virus of flavivirus: Chaoyang viru sisolated in Liaoning province. Chin. J. Public Health 2009, 25, 769–772. [Google Scholar]
- Wen, D.; Ding, L.S.; Zhang, Y.; Li, X.; Zhang, X.; Yuan, F.; Zhao, T.; Zheng, A. Suppression of flavivirus transmission from animal hosts to mosquitoes with a mosquito-delivered vaccine. Nat. Commun. 2022, 13, 7780. [Google Scholar] [CrossRef]
- Dong, H.-L.; He, M.-J.; Wang, Q.-Y.; Cui, J.-Z.; Chen, Z.-L.; Xiong, X.-H.; Zhang, L.-C.; Cheng, H.; Xiong, G.-Q.; Hu, A.; et al. Rapid Generation of Recombinant Flaviviruses Using Circular Polymerase Extension Reaction. Vaccines 2023, 11, 1250. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-C.; Li, X.-F.; Deng, Y.-Q.; Tong, Y.-G.; Qin, C.-F. Excretion of infectious Zika virus in urine. Lancet Infect. Dis. 2016, 16, 641–642. [Google Scholar] [CrossRef]
- Li, X.F.; Dong, H.L.; Huang, X.Y.; Qiu, Y.F.; Wang, H.J.; Deng, Y.Q.; Zhang, N.N.; Ye, Q.; Zhao, H.; Liu, Z.Y.; et al. Characterization of a 2016 Clinical Isolate of Zika Virus in Non-human Primates. EBioMedicine 2016, 12, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-I.; Lee, Y.-M. Japanese encephalitis. Hum. Vaccines Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-L.; Wang, H.-J.; Liu, Z.-Y.; Ye, Q.; Qin, X.-L.; Li, D.; Deng, Y.-Q.; Jiang, T.; Li, X.-F.; Qin, C.-F. Visualization of yellow fever virus infection in mice using a bioluminescent reporter virus. Emerg. Microbes Infect. 2021, 10, 1739–1750. [Google Scholar] [CrossRef]
- Alves Dos Santos, E.; Fink, K. Animal Models for Dengue and Zika Vaccine Development. Adv. Exp. Med. Biol. 2018, 1062, 215–239. [Google Scholar]
- Harrison, J.J.; Hobson-Peters, J.; Bielefeldt-Ohmann, H.; Hall, R.A. Chimeric Vaccines Based on Novel Insect-Specific Flaviviruses. Vaccines 2021, 9, 1230. [Google Scholar] [CrossRef]
- Zhou, N.; Huang, E.; Guo, X.; Xiong, Y.; Xie, J.; Cai, T.; Du, Y.; Wu, Q.; Guo, S.; Han, W.; et al. Cell fusing agent virus isolated from Aag2 cells does not vertically transmit in Aedes aegypti via artificial infection. Parasites Vectors 2023, 16, 402. [Google Scholar] [CrossRef]
- Torres, F.J.; Parry, R.; Hugo, L.E.; Slonchak, A.; Newton, N.D.; Vet, L.J.; Modhiran, N.; Pullinger, B.; Wang, X.; Potter, J.; et al. Reporter Flaviviruses as Tools to Demonstrate Homologous and Heterologous Superinfection Exclusion. Viruses 2022, 14, 1501. [Google Scholar] [CrossRef]
- Guirakhoo, F.; Zhang, Z.; Myers, G.; Johnson, B.W.; Pugachev, K.; Nichols, R.; Brown, N.; Levenbook, I.; Draper, K.; Cyrek, S.; et al. A single amino acid substitution in the envelope protein of chimeric yellow fever-dengue 1 vaccine virus reduces neurovirulence for suckling mice and viremia/viscerotropism for monkeys. J. Virol. 2004, 78, 9998–10008. [Google Scholar] [CrossRef]
- Song, G.Y.; Huang, X.Y.; He, M.J.; Zhou, H.Y.; Li, R.T.; Tian, Y.; Wang, Y.; Cheng, M.L.; Chen, X.; Zhang, R.R.; et al. A single amino acid substitution in the capsid protein of Zika virus contributes to a neurovirulent phenotype. Nat. Commun. 2023, 14, 6832. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Shi, Y.; Wang, H.; Li, G.; Li, X.; Wang, B.; Su, X.; Wang, J.; Teng, Q.; Yang, J.; et al. A Single Mutation at Position 156 in the Envelope Protein of Tembusu Virus Is Responsible for Virus Tissue Tropism and Transmissibility in Ducks. J. Virol. 2018, 92, e00427-18. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Li, X.F.; Zhao, H.; Li, S.H.; Deng, Y.Q.; Cao, R.Y.; Song, K.Y.; Wang, H.J.; Hua, R.H.; Yu, Y.X.; et al. A single nucleotide mutation in NS2A of Japanese encephalitis-live vaccine virus (SA14-14-2) ablates NS1’ formation and contributes to attenuation. J. Gen. Virol. 2012, 93, 1959–1964. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.-L.; Chen, Z.-L.; He, M.-J.; Cui, J.-Z.; Cheng, H.; Wang, Q.-Y.; Xiong, X.-H.; Liu, G.; Chen, H.-P. The Chimeric Chaoyang-Zika Vaccine Candidate Is Safe and Protective in Mice. Vaccines 2024, 12, 215. https://doi.org/10.3390/vaccines12020215
Dong H-L, Chen Z-L, He M-J, Cui J-Z, Cheng H, Wang Q-Y, Xiong X-H, Liu G, Chen H-P. The Chimeric Chaoyang-Zika Vaccine Candidate Is Safe and Protective in Mice. Vaccines. 2024; 12(2):215. https://doi.org/10.3390/vaccines12020215
Chicago/Turabian StyleDong, Hao-Long, Zhi-Li Chen, Mei-Juan He, Jia-Zhen Cui, Hao Cheng, Qing-Yang Wang, Xiang-Hua Xiong, Gang Liu, and Hui-Peng Chen. 2024. "The Chimeric Chaoyang-Zika Vaccine Candidate Is Safe and Protective in Mice" Vaccines 12, no. 2: 215. https://doi.org/10.3390/vaccines12020215
APA StyleDong, H.-L., Chen, Z.-L., He, M.-J., Cui, J.-Z., Cheng, H., Wang, Q.-Y., Xiong, X.-H., Liu, G., & Chen, H.-P. (2024). The Chimeric Chaoyang-Zika Vaccine Candidate Is Safe and Protective in Mice. Vaccines, 12(2), 215. https://doi.org/10.3390/vaccines12020215





