Effectiveness of BNT162b2 BA.4/5 Bivalent COVID-19 Vaccine against Long COVID Symptoms: A US Nationwide Study
Abstract
1. Background
2. Methods
2.1. Study Design and Cohorts
2.2. Baseline Characteristics and Symptoms
2.3. Long COVID Symptoms
2.4. Statistical Methods
3. Results
3.1. Study Population
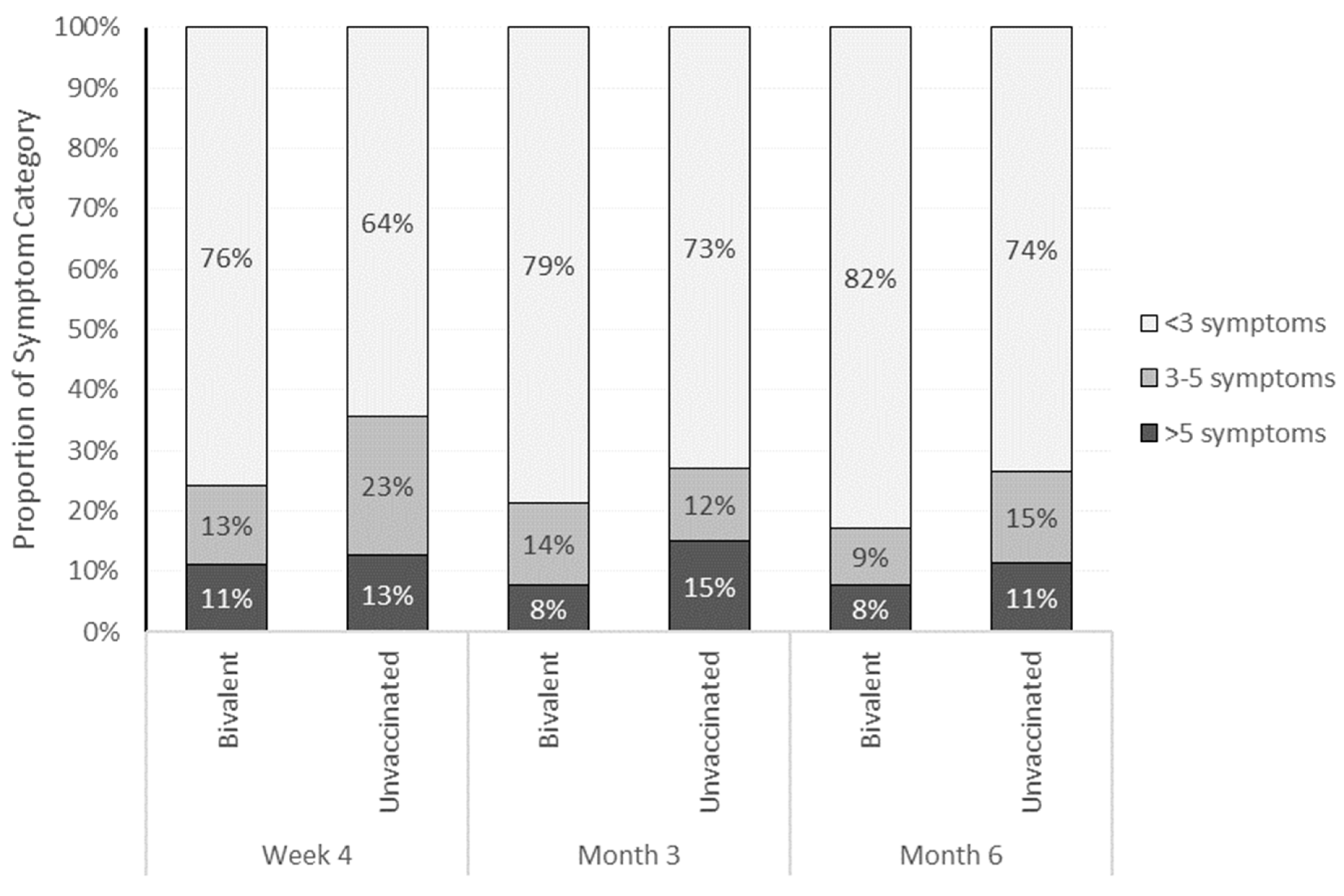
3.2. Long COVID-19 Risk and Symptoms
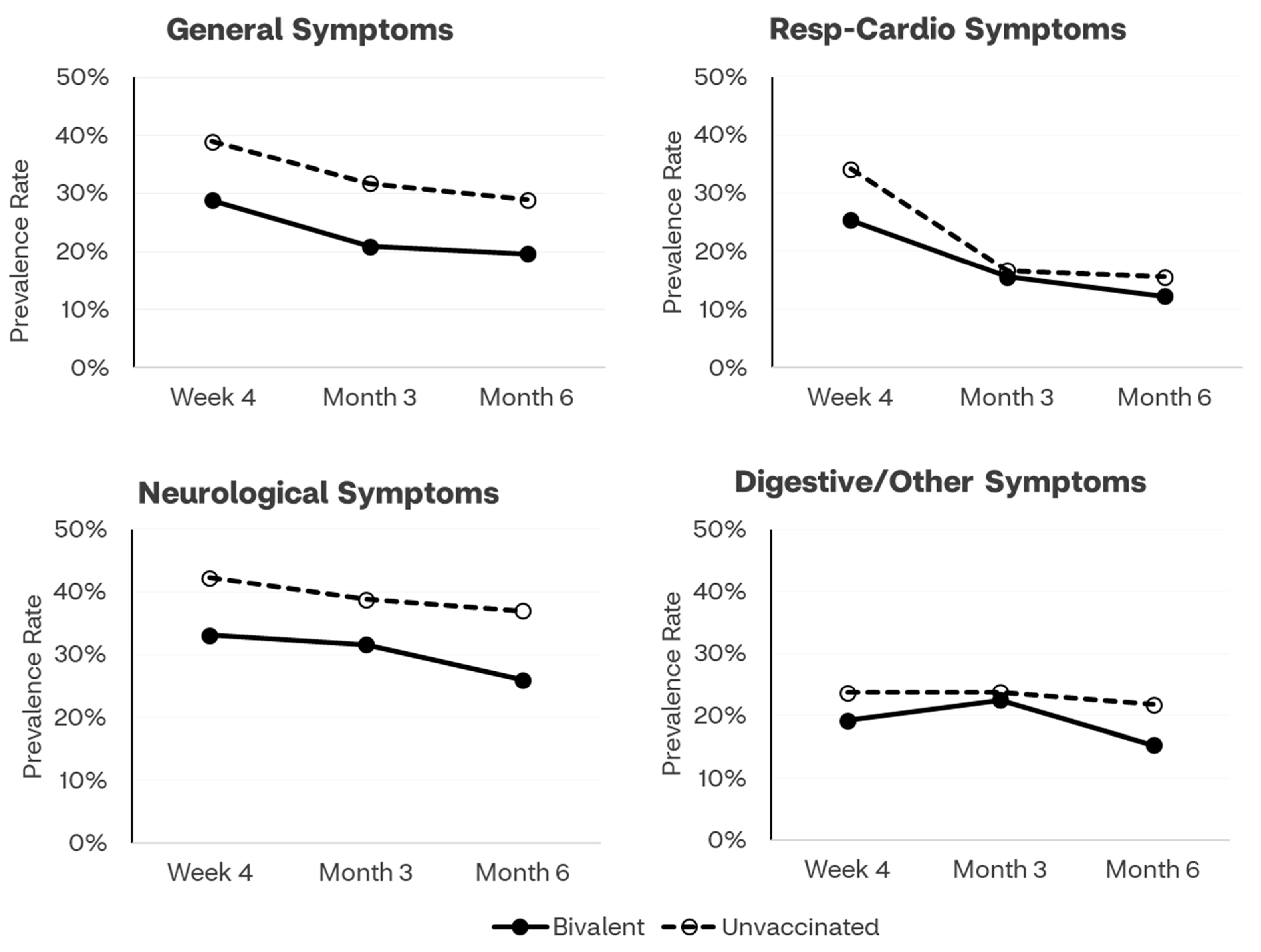
3.2.1. Time Trends through Month 6
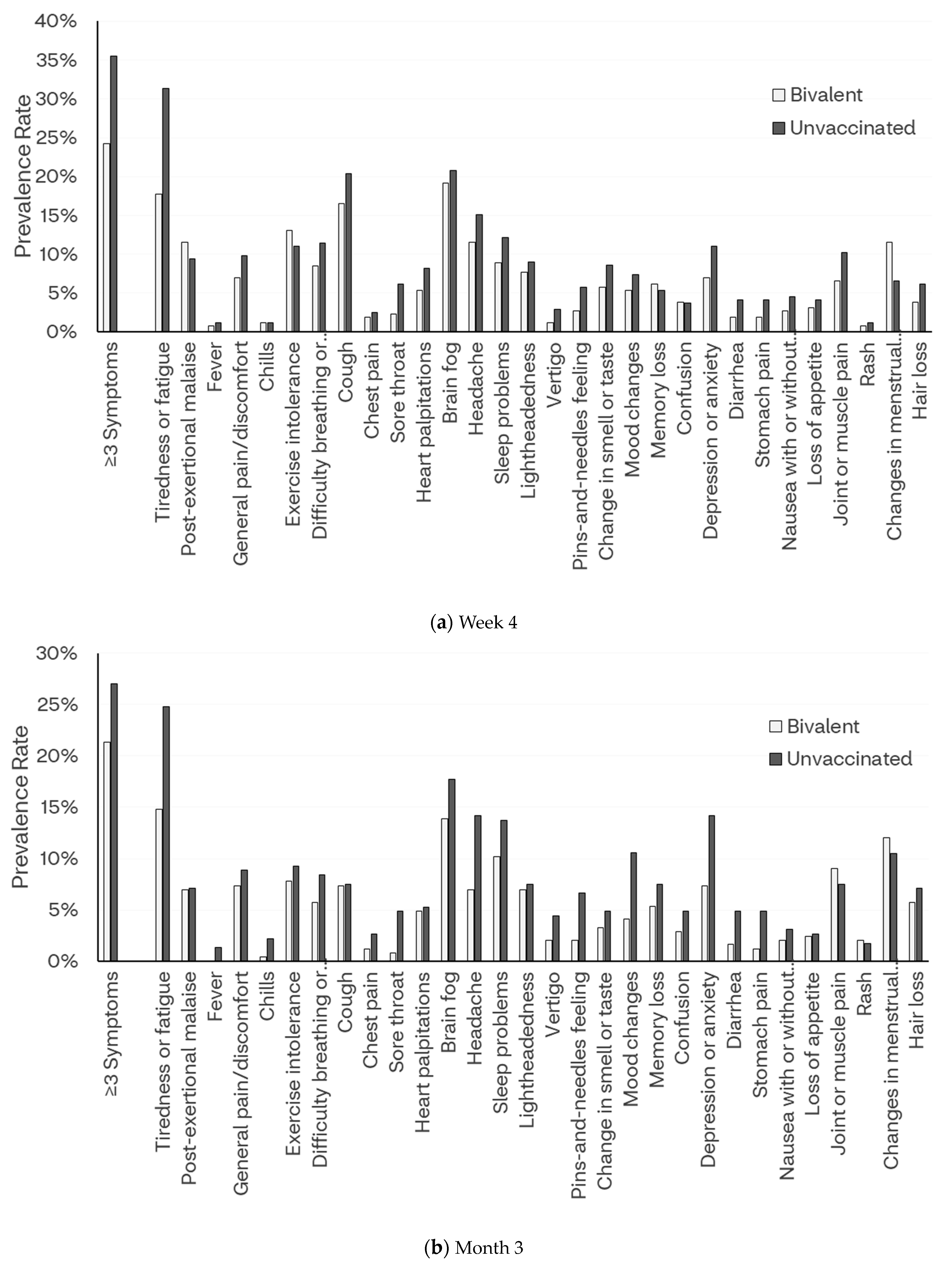
3.2.2. Week 4
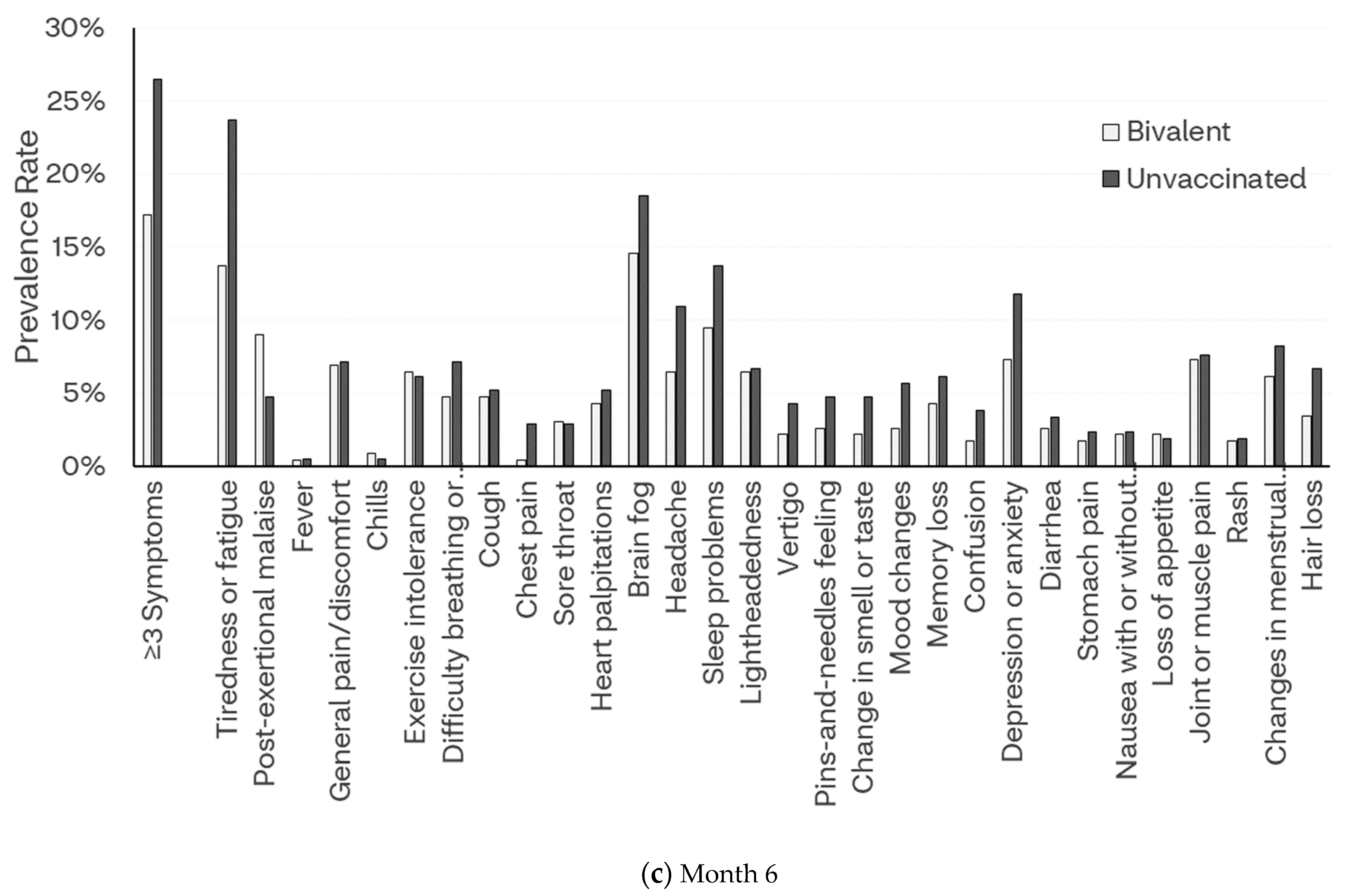
3.2.3. Month 3
3.2.4. Month 6
3.3. Relationship between Vaccination Status and Risk of Long COVID
3.4. Frequency of Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention; |
| CI | confidence interval; |
| SD | standard deviation; |
| SVI | social vulnerability index. |
References
- Centers for Disease Control and Prevention. Long COVID or Post-COVID Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 11 December 2023).
- Landry, M.; Bornstein, S.; Nagaraj, N.; Sardon, G.A., Jr.; Castel, A.; Vyas, A.; McDonnell, K.; Agneshwar, M.; Wilkinson, A.; Goldman, L. Postacute Sequelae of SARS-CoV-2 in University Setting. Emerg. Infect. Dis. 2023, 29, 519. [Google Scholar] [CrossRef]
- Perlis, R.H.; Santillana, M.; Ognyanova, K.; Safarpour, A.; Trujillo, K.L.; Simonson, M.D.; Green, J.; Quintana, A.; Druckman, J.; Baum, M.A. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw. Open 2022, 5, e2238804. [Google Scholar] [CrossRef]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Wang, R.; Yu, H.; Spatz, E.S.; Montoy, J.C.; Rodriguez, R.; Chang, A.M.; Elmore, J.G.; Hannikainen, P.A.; Hill, M. Severe Fatigue and Persistent Symptoms at Three Months Following SARS-CoV-2 Infections During the Pre-Delta, Delta, and Omicron Time Periods: A Multicenter Prospective Cohort Study. Clin. Infect. Dis. 2023, 76, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Spatz, E.S.; Gottlieb, M.; Wisk, L.E.; Anderson, J.; Chang, A.M.; Gentile, N.L.; Hill, M.J.; Huebinger, R.M.; Idris, A.H.; Kinsman, J. Three-Month Symptom Profiles Among Symptomatic Adults with Positive and Negative Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Tests: A Prospective Cohort Study from the INSPIRE Group. Clin. Infect. Dis. 2022, 76, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Ford, N.D.; Slaughter, D.; Edwards, D.; Dalton, A.; Perrine, C.; Vahratian, A.; Saydah, S. Long COVID and Significant Activity Limitation Among Adults, by Age—United States, June 1–13, 2022, to June 7–19, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tebbutt, S.J. Long COVID: The next public health crisis is already on its way. Lancet Reg. Health–Eur. 2023, 28, 100612. [Google Scholar] [CrossRef]
- Nittas, V.; Gao, M.; West, E.A.; Ballouz, T.; Menges, D.; Wulf Hanson, S.; Puhan, M.A. Long COVID Through a Public Health Lens: An Umbrella Review. Public. Health Rev. 2022, 43, 1604501. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, O.; Stehlik, P.; Clark, J. Effect of covid-19 vaccination on long covid: Systematic review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Baraff, A.; Fox, A.; Shahoumian, T.; Hickok, A.; O’Hare, A.M.; Bohnert, A.S.; Boyko, E.J.; Maciejewski, M.L.; Bowling, C.B. Rates and Factors Associated with Documentation of Diagnostic Codes for Long COVID in the National Veterans Affairs Health Care System. JAMA Netw. Open 2022, 5, e2224359. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use (accessed on 9 September 2023).
- Di Fusco, M.; Sun, X.; Anatale-Tardiff, L.; Yehoshua, A.; Coetzer, H.; Alvarez, M.B.; Allen, K.E.; Porter, T.M.; Puzniak, L.; Lopez, S.M.; et al. Impact of bivalent BA. 4/5 BNT162b2 COVID-19 vaccine on acute symptoms, quality of life, work productivity and activity levels among symptomatic US adults testing positive for SARS-CoV-2 at a national retail pharmacy. Vaccines 2023, 11, 1669. [Google Scholar] [CrossRef]
- Menegale, F.; Manica, M.; Zardini, A.; Guzzetta, G.; Marziano, V.; d’Andrea, V.; Trentini, F.; Ajelli, M.; Poletti, P.; Merler, S. Evaluation of Waning of SARS-CoV-2 Vaccine–Induced Immunity: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2310650. [Google Scholar] [CrossRef] [PubMed]
- CDC/ATSDR Social Vulnerability Index. Available online: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html (accessed on 19 December 2023).
- Centers for Disease Control and Prevention. Symptoms of COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 22 August 2023).
- Rosner, B. Fundamentals of Biostatistics, 8th ed.; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Freeman, G.; Halton, J.H. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 1951, 38, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, G.M.; Laird, N.M.; Ware, J.H. Applied Longitudinal Analysis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 998. [Google Scholar]
- STROBE Statement—Checklist of Items That Should Be Included in Reports of Cohort Studies. Available online: https://www.strobe-statement.org/download/strobe-checklist-cohort-studies-pdf (accessed on 9 September 2023).
- Maier, H.E.; Kowalski-Dobson, T.; Eckard, A.; Gherasim, C.; Manthei, D.; Meyers, A.; Davis, D.; Bakker, K.; Lindsey, K.; Chu, Z.; et al. Reduction in long-COVID symptoms and symptom severity in vaccinated compared to unvaccinated adults. Open Forum Infect. Dis. 2024, 11, ofae039. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, M.; Sun, X.; Moran, M.M.; Coetzer, H.; Zamparo, J.M.; Alvarez, M.B.; Puzniak, L.; Tabak, Y.P.; Cappelleri, J.C. Impact of COVID-19 and effects of booster vaccination with BNT162b2 on six-month long COVID symptoms, quality of life, work productivity and activity impairment during Omicron. J. Patient-Rep. Outcomes 2023, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk factors associated with Post− COVID-19 condition: A systematic review and meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Xie, F.; Takhar, H.; Ogun, O.A.; Simmons, S.; et al. Effectiveness of BNT162b2 BA. 4/5 bivalent mRNA vaccine against a range of COVID-19 outcomes in a large health system in the USA: A test-negative case–control study. Lancet Respir. Med. 2023, 11, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
| All | BNT162b2 | Unvaccinated | p a | |
|---|---|---|---|---|
| Total, n (%) | 505 | 260 | 245 | |
| Age, years | ||||
| Mean, SD | 46.3 (15.5) | 50.0 (16.0) | 42.4 (14.0) | <0.001 |
| 18–29 | 76 (15.0%) | 28 (10.8%) | 48 (19.6%) | <0.001 |
| 30–49 | 220 (43.6%) | 99 (38.1%) | 121 (49.4%) | |
| 50–64 | 125 (24.8%) | 69 (26.5%) | 56 (22.9%) | |
| ≥65 | 84 (16.7%) | 64 (24.6%) | 20 (8.2%) | |
| Gender | 0.086 | |||
| Female | 357 (70.7%) | 173 (66.5%) | 184 (75.1%) | |
| Male | 144 (28.5%) | 84 (32.3%) | 60 (24.5%) | |
| Unknown | 4 (0.8%) | 3 (1.2%) | 1 (0.4%) | |
| Race/Ethnicity | 0.006 | |||
| White or Caucasian | 305 (60.4%) | 168 (64.6%) | 137 (55.9%) | |
| Black or African American | 40 (7.9%) | 15 (5.8%) | 25 (10.2%) | |
| Hispanic | 73 (14.5%) | 26 (10.0%) | 47 (19.2%) | |
| Asian | 49 (9.7%) | 31 (11.9%) | 18 (7.4%) | |
| Other | 38 (7.5%) | 20 (7.7%) | 18 (7.4%) | |
| US Geographic Region | 0.053 | |||
| Northeast | 68 (13.5%) | 38 (14.6%) | 30 (12.2%) | |
| South | 201 (39.8%) | 89 (34.2%) | 112 (45.7%) | |
| Midwest | 113 (22.4%) | 67 (25.8%) | 46 (18.8%) | |
| West | 123 (24.4%) | 66 (25.4%) | 57 (23.3%) | |
| Social vulnerability index, Mean (SD) b | 0.44 (0.23) | 0.39 (0.22) | 0.49 (0.23) | <0.001 |
| Previously tested positive | 204 (40.4%) | 98 (37.7%) | 106 (43.3%) | 0.202 |
| Live or work in high-risk setting | 25 (5.0%) | 10 (3.9%) | 15 (6.1%) | 0.239 |
| Work in healthcare | 75 (14.9%) | 33 (12.7%) | 42 (17.1%) | 0.160 |
| Self-reported comorbidity | ||||
| Number of comorbidities, Mean (SD) | 0.38 (0.75) | 0.46 (0.79) | 0.30 (0.70) | 0.015 |
| Asthma or chronic lung disease | 25 (5.0%) | 17 (6.5%) | 8 (3.3%) | 0.090 |
| Immunocompromised conditions or weakened immune system c | 2 (0.4%) | 1 (0.4%) | 1 (0.4%) | 0.966 |
| Diabetes | 27 (5.4%) | 17 (6.5%) | 10 (4.1%) | 0.220 |
| Heart conditions or hypertension | 79 (15.6%) | 49 (18.8%) | 30 (12.2%) | 0.041 |
| Overweight or obesity | 60 (11.9%) | 36 (13.8%) | 24 (9.8%) | 0.160 |
| At least 1 comorbidity | 127 (25.1%) | 81 (31.2%) | 46 (18.8%) | 0.001 |
| Mean days since last vaccine dose, SD | 337 (209) | 165 (46) | 546 (121) | <0.001 |
| Nirmatrelvir/Ritonavir use, n (%) | 116 (23.0%) | 74 (28.5%) | 42 (17.1%) | 0.003 |
| Acute COVID-19 symptoms on index day d | 505 (100%) | 260 (100%) | 245 (100%) | |
| Mean number of symptoms, SD | 5.3 (2.3) | 5.0 (2.3) | 5.7 (2.3) | 0.001 |
| All (n = 444) | BNT162b2 (n = 233) | Unvaccinated (n = 211) | Observed | Model-Based | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||||
| Long COVID | |||||||
| ≥2 symptoms | 138 (31.1%) | 60 (25.8%) | 78 (37.0%) | 0.59 (0.39, 0.89) | 0.011 | 0.63 (0.41, 0.96) | 0.030 |
| ≥3 symptoms | 96 (21.6%) | 40 (17.2%) | 56 (26.5%) | 0.57 (0.36, 0.91) | 0.017 | 0.59 (0.36, 0.96) | 0.034 |
| Symptom category | |||||||
| General symptoms | 107 (24.0%) | 46 (19.6%) | 61 (28.9%) | 0.60 (0.39, 0.94) | 0.021 | 0.63 (0.40, 0.99) | 0.048 |
| Respiratory and cardio symptoms | 62 (13.9%) | 29 (12.3%) | 33 (15.6%) | 0.77 (0.45, 1.31) | 0.315 | 0.80 (0.47, 1.38) | 0.424 |
| Neurologic symptoms | 139 (31.2%) | 61 (26.0%) | 78 (37.0%) | 0.60 (0.40, 0.91) | 0.012 | 0.60 (0.39, 0.92) | 0.018 |
| Digestive/other symptoms | 82 (18.4%) | 36 (15.3%) | 46 (21.8%) | 0.66 (0.40, 1.06) | 0.078 | 0.69 (0.42, 1.13) | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fusco, M.; Sun, X.; Allen, K.E.; Yehoshua, A.; Berk, A.; Alvarez, M.B.; Porter, T.M.; Ren, J.; Puzniak, L.; Lopez, S.M.C.; et al. Effectiveness of BNT162b2 BA.4/5 Bivalent COVID-19 Vaccine against Long COVID Symptoms: A US Nationwide Study. Vaccines 2024, 12, 183. https://doi.org/10.3390/vaccines12020183
Di Fusco M, Sun X, Allen KE, Yehoshua A, Berk A, Alvarez MB, Porter TM, Ren J, Puzniak L, Lopez SMC, et al. Effectiveness of BNT162b2 BA.4/5 Bivalent COVID-19 Vaccine against Long COVID Symptoms: A US Nationwide Study. Vaccines. 2024; 12(2):183. https://doi.org/10.3390/vaccines12020183
Chicago/Turabian StyleDi Fusco, Manuela, Xiaowu Sun, Kristen E. Allen, Alon Yehoshua, Alexandra Berk, Mary B. Alvarez, Thomas M. Porter, Jinma Ren, Laura Puzniak, Santiago M. C. Lopez, and et al. 2024. "Effectiveness of BNT162b2 BA.4/5 Bivalent COVID-19 Vaccine against Long COVID Symptoms: A US Nationwide Study" Vaccines 12, no. 2: 183. https://doi.org/10.3390/vaccines12020183
APA StyleDi Fusco, M., Sun, X., Allen, K. E., Yehoshua, A., Berk, A., Alvarez, M. B., Porter, T. M., Ren, J., Puzniak, L., Lopez, S. M. C., & Cappelleri, J. C. (2024). Effectiveness of BNT162b2 BA.4/5 Bivalent COVID-19 Vaccine against Long COVID Symptoms: A US Nationwide Study. Vaccines, 12(2), 183. https://doi.org/10.3390/vaccines12020183






