Evaluating Antigen- and Vector-Specific Immune Responses of a Recombinant Pichinde Virus-Based Vaccine Expressing the Lymphocytic Choriomeningitis Virus Nucleoprotein
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Mammalian Cells, Plasmids, and Viruses
2.3. Tetramers and Antibodies
2.4. UV Inactivation of LCMV and rPICV Virions
2.5. Generation of Recombinant rP18tri Vaccine Encoding LCMN NP Gene (rP18tri-NPLCMV)
2.6. Viral Plaque Assay
2.7. Mouse Immunizations, Infection, and Tissue Collection
2.8. LCMV RT-qPCR
2.9. Evaluation of Antigen-Specific CD8+ T Cells by MHC-I Tetramer Analysis
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. PICV Neutralization Assays
2.12. Statistical Analysis
3. Results
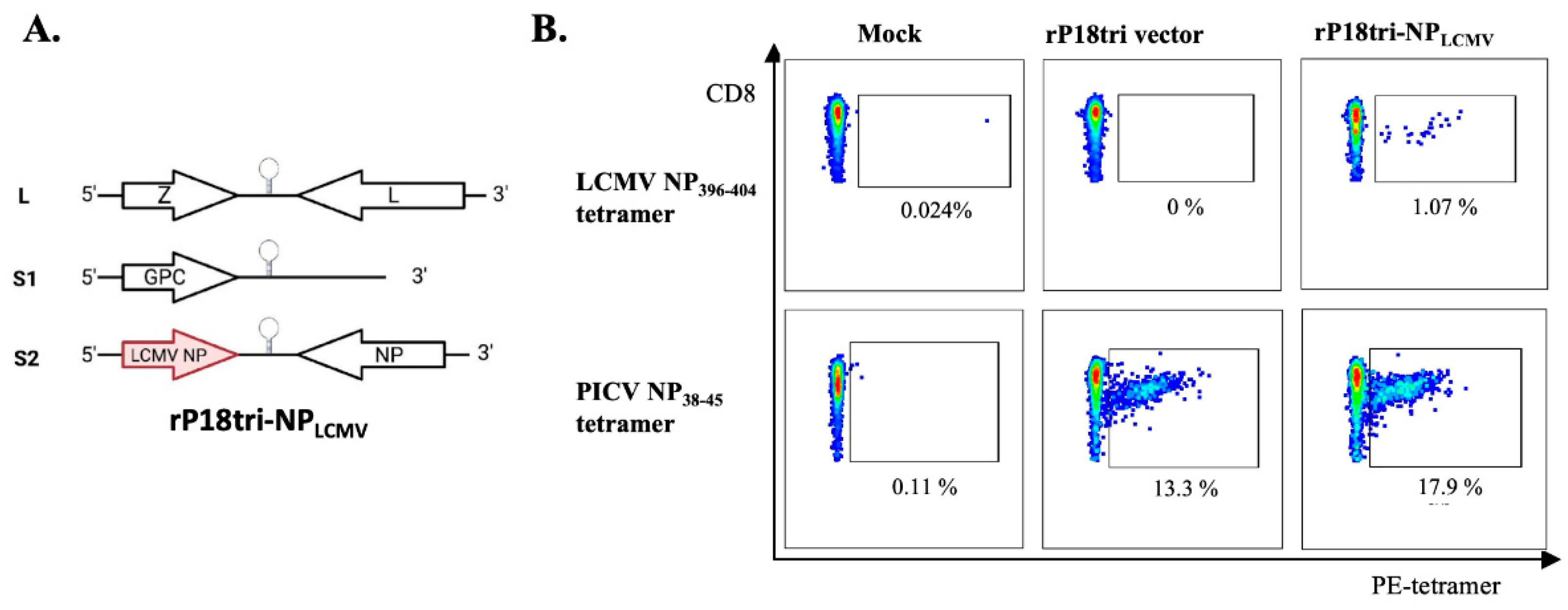
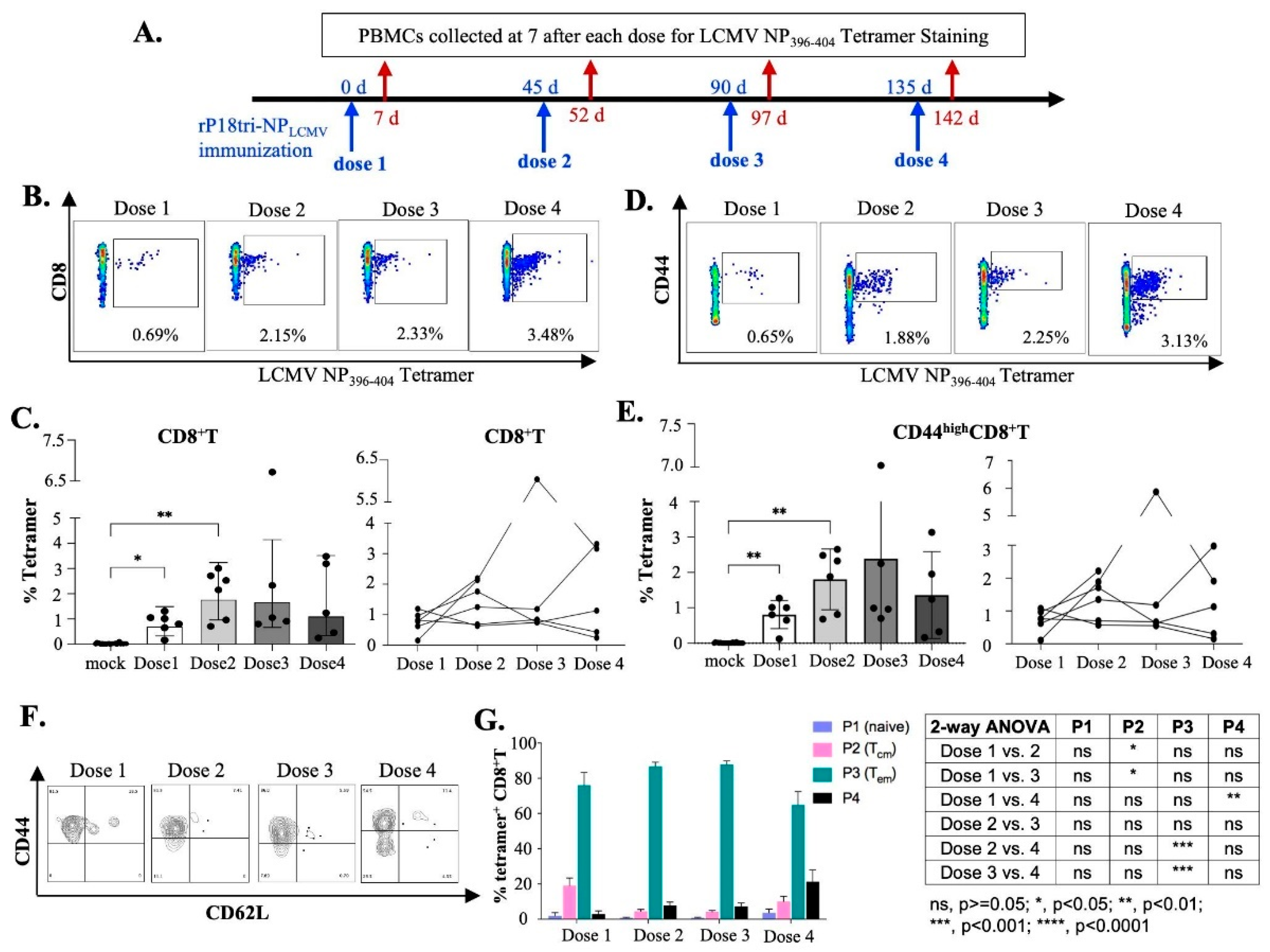
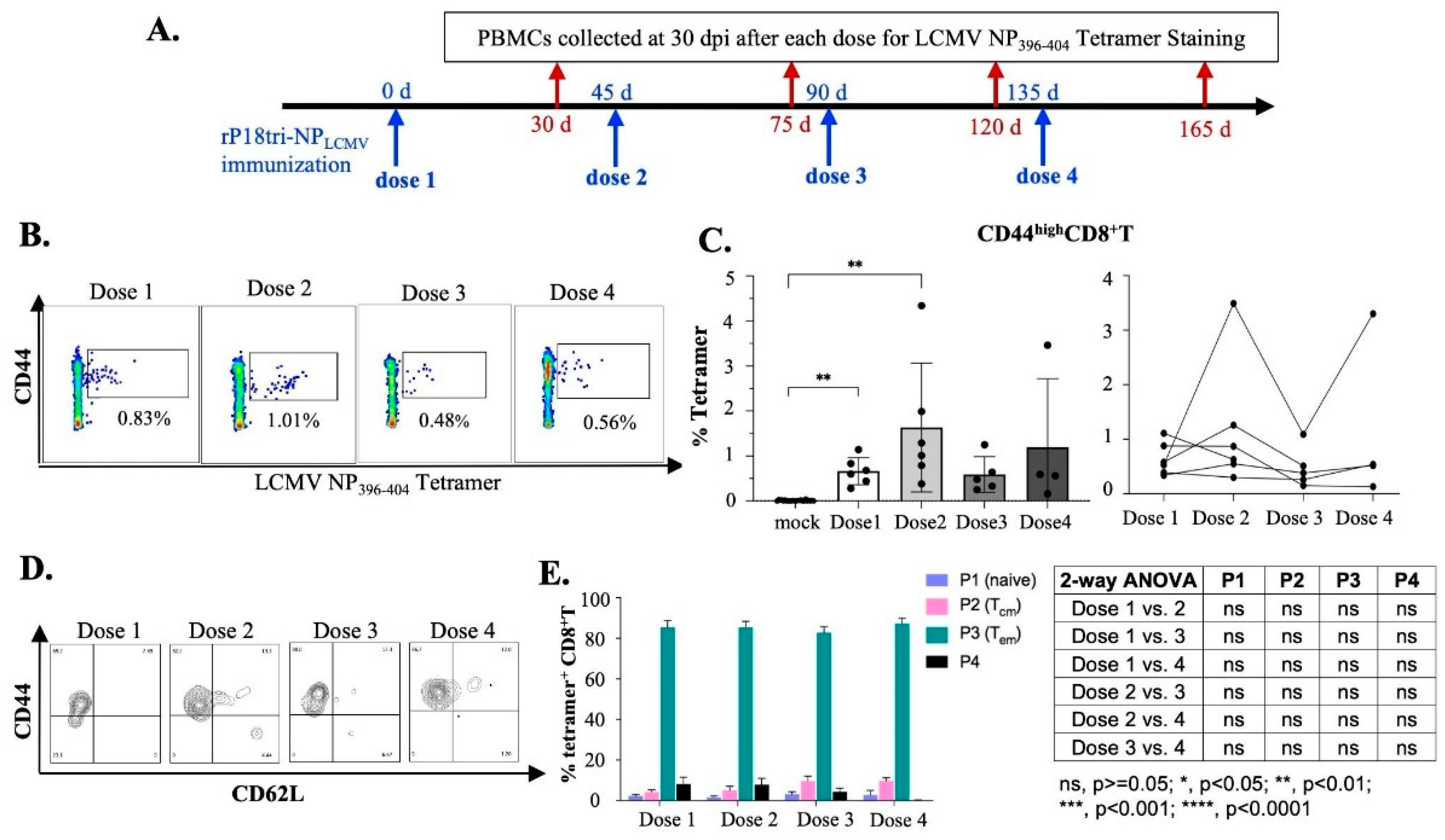
3.1. Detection of Antigen- and Vector-Specific CD8+ T Cells by Tetramer Staining in Mice Immunized with rP18tri-NPLCMV Vaccine
3.2. Evaluation of Antigen-Specific Effector CD8+ T Cells After Multiple-Dose Immunization
3.3. Evaluation of Antigen-Specific Memory CD8+ T Cells After Repeated Vaccination Doses
3.4. Evaluation of Vector-Specific CD8+ T Cells After Repeated Vaccinations
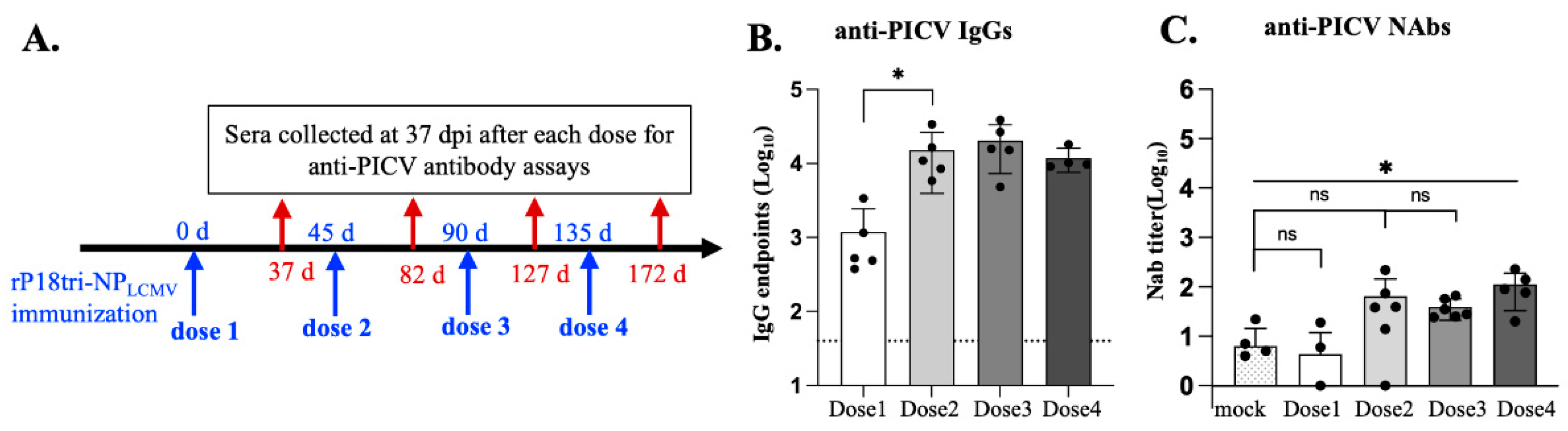
3.5. Evaluation of Vector-Specific Antibodies After Repeated Vaccination
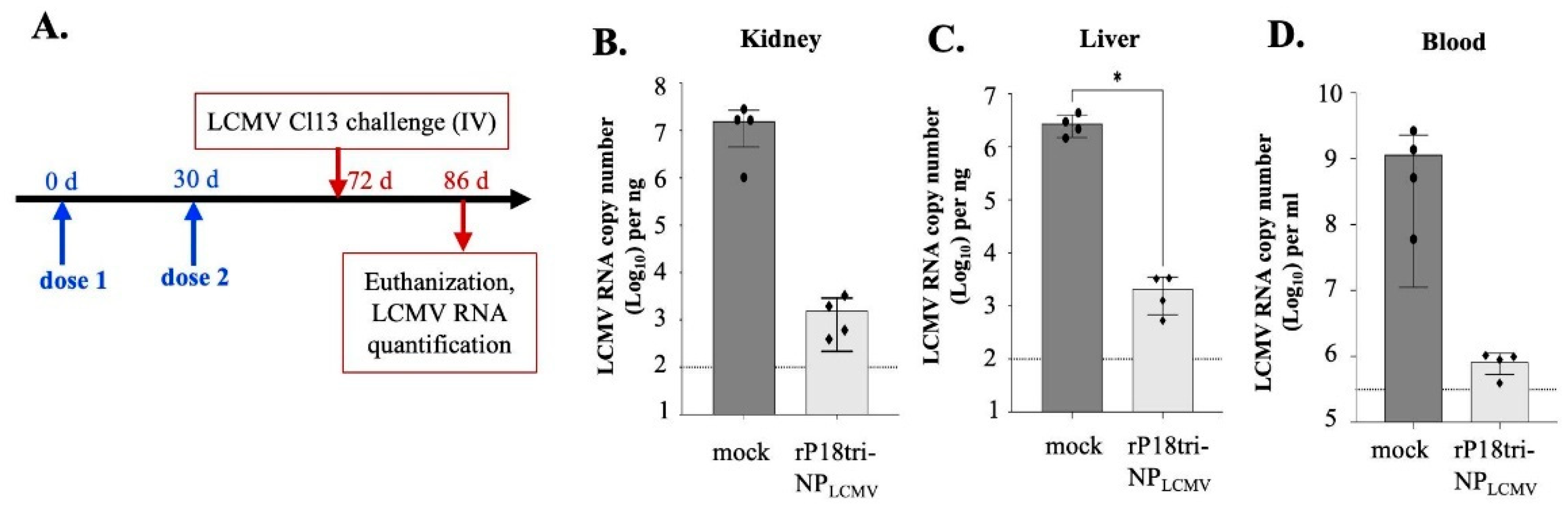
3.6. The rP18tri-NPLCMV Vaccine Reduced the LCMV Load in a Mouse Model of Chronic Infection
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bull, J.J.; Nuismer, S.L.; Antia, R. Recombinant Vector Vaccine Evolution. PLoS Comput. Biol. 2019, 15, e1006857. [Google Scholar] [CrossRef] [PubMed]
- Draper, S.J.; Heeney, J.L. Viruses as Vaccine Vectors for Infectious Diseases and Cancer. Nat. Rev. Microbiol. 2010, 8, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Liang, H.; Chen, P.; Li, Y.; Li, Z.; Fan, S.; Wu, K.; Li, X.; Chen, W.; Qin, Y.; et al. Viral Vector Vaccine Development and Application during the COVID-19 Pandemic. Microorganisms 2022, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. NPJ Vaccines 2022, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral Vectored Vaccines: Design, Development, Preventive and Therapeutic Applications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef]
- Center for Biologics Evaluation and Research. Vaccines Licensed for Use in the United States; FDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- Holman, D.H.; Wang, D.; Woraratanadharm, J.; Dong, J.Y. Chapter 7—Viral Vectors. In Vaccines for Biodefense and Emerging and Neglected Diseases; Barrett, A.D.T., Stanberry, L.R., Eds.; Academic Press: London, UK, 2009; pp. 77–91. ISBN 978-0-12-369408-9. [Google Scholar]
- Altenburg, A.F.; van Trierum, S.E.; de Bruin, E.; de Meulder, D.; van de Sandt, C.E.; van der Klis, F.R.M.; Fouchier, R.A.M.; Koopmans, M.P.G.; Rimmelzwaan, G.F.; de Vries, R.D. Effects of Pre-Existing Orthopoxvirus-Specific Immunity on the Performance of Modified Vaccinia Virus Ankara-Based Influenza Vaccines. Sci. Rep. 2018, 8, 6474. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, B.; Pérez, P.; Marcos-Villar, L.; Albericio, G.; Astorgano, D.; Álvarez, E.; Sin, L.; Gómez, C.E.; García-Arriaza, J.; Esteban, M. Highly Attenuated Poxvirus-Based Vaccines Against Emerging Viral Diseases. J. Mol. Biol. 2023, 435, 168173. [Google Scholar] [CrossRef] [PubMed]
- Dhanwani, R.; Ly, H.; Liang, Y. Recombinant Tri-Segmented Pichinde Virus as a Novel Live Viral Vaccine Platform. Methods Mol. Biol. 2017, 1581, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Dhanwani, R.; Zhou, Y.; Huang, Q.; Verma, V.; Dileepan, M.; Ly, H.; Liang, Y. A Novel Live Pichinde Virus-Based Vaccine Vector Induces Enhanced Humoral and Cellular Immunity after a Booster Dose. J. Virol. 2016, 90, 2551–2560. [Google Scholar] [CrossRef]
- Jahrling, P.B.; Hesse, R.A.; Rhoderick, J.B.; Elwell, M.A.; Moe, J.B. Pathogenesis of a Pichinde Virus Strain Adapted to Produce Lethal Infections in Guinea Pigs. Infect. Immun. 1981, 32, 872–880. [Google Scholar] [CrossRef]
- Trapido, H.; Sanmartín, C. Pichindé Virus: A New Virus of the Tacaribe Group from Colombia. Am. J. Trop. Med. Hyg. 1971, 20, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Buchmeier, M.; Adam, E.; Rawls, W.E. Serological Evidence of Infection by Pichinde Virus Among Laboratory Workers. Infect. Immun. 1974, 9, 821–823. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Kirk, N.M.; Huang, Q.; Vrba, S.; Rahman, M.; Block, A.M.; Murphy, H.; White, D.W.; Namugenyi, S.B.; Ly, H.; Tischler, A.D.; et al. Recombinant Pichinde Viral Vector Expressing Tuberculosis Antigens Elicits Strong T Cell Responses and Protection in Mice. Front. Immunol. 2023, 14, 1127515. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Chaudhari, J.; Huang, Q.; Gauger, P.; De Almeida, M.N.; Ly, H.; Liang, Y.; Vu, H.L.X. Assessment of Immune Responses to a Trivalent Pichinde Virus-Vectored Vaccine Expressing Hemagglutinin Genes from Three Co-Circulating Influenza A Virus Subtypes in Pigs. Vaccines 2023, 11, 1806. [Google Scholar] [CrossRef]
- Kumar, P.; Sharafeldin, T.A.; Kumar, R.; Huang, Q.; Liang, Y.; Goyal, S.M.; Porter, R.E.; Ly, H.; Mor, S.K. Development of a Recombinant Pichinde Virus-Vectored Vaccine against Turkey Arthritis Reovirus and Its Immunological Response Characterization in Vaccinated Animals. Pathogens 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Hallam, S.J.; Koma, T.; Maruyama, J.; Paessler, S. Review of Mammarenavirus Biology and Replication. Front. Microbiol. 2018, 9, 1751. [Google Scholar] [CrossRef]
- LaVergne, S.M.; Sakabe, S.; Momoh, M.; Kanneh, L.; Bond, N.; Garry, R.F.; Grant, D.S.; de la Torre, J.C.; Oldstone, M.B.A.; Schieffelin, J.S.; et al. Expansion of CD8+ T Cell Population in Lassa Virus Survivors with Low T Cell Precursor Frequency Reveals Durable Immune Response in Most Survivors. PLoS Negl. Trop. Dis. 2022, 16, e0010882. [Google Scholar] [CrossRef] [PubMed]
- Lukashevich, I.S.; Paessler, S.; de la Torre, J.C. Lassa Virus Diversity and Feasibility for Universal Prophylactic Vaccine. F1000Research 2019, 8, 134. [Google Scholar] [CrossRef]
- Garry, R.F. Lassa Fever—The Road Ahead. Nat. Rev. Microbiol. 2023, 21, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Reyna, R.A.; Taniguchi, S.; Littlefield, K.; Paessler, S.; Maruyama, J. Vaccine Candidates against Arenavirus Infections. Vaccines 2023, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Radoshitzky, S.R.; de la Torre, J.C. Human Pathogenic Arenaviruses (Arenaviridae). In Encyclopedia of Virology; Academic Press: Cambridge, MA, USA, 2019; pp. 507–517. [Google Scholar] [CrossRef]
- Fisher-Hoch, S.P.; Hutwagner, L.; Brown, B.; McCormick, J.B. Effective Vaccine for Lassa Fever. J. Virol. 2000, 74, 6777–6783. [Google Scholar] [CrossRef]
- Lan, S.; McLay Schelde, L.; Wang, J.; Kumar, N.; Ly, H.; Liang, Y. Development of Infectious Clones for Virulent and Avirulent Pichinde Viruses: A Model Virus To Study Arenavirus-Induced Hemorrhagic Fevers. J. Virol. 2009, 83, 6357–6362. [Google Scholar] [CrossRef]
- Dangi, T.; Chung, Y.R.; Palacio, N.; Penaloza-MacMaster, P. Interrogating Adaptive Immunity Using LCMV. Curr. Protoc. Immunol. 2020, 130, e99. [Google Scholar] [CrossRef] [PubMed]
- Grusdat, M.; Dostert, C.; Brenner, D. Chapter 9—Quantification of Lymphocytic Choriomeningitis Virus Specific T Cells and LCMV Viral Titers. In Methods in Cell Biology; Thomas, C., Galluzzi, L., Eds.; The Immunological Synapse Part A; Academic Press: Cambridge, MA, USA, 2023; Volume 173, pp. 121–131. [Google Scholar]
- Althaus, C.L.; Ganusov, V.V.; De Boer, R.J. Dynamics of CD8+ T Cell Responses during Acute and Chronic Lymphocytic Choriomeningitis Virus Infection. J. Immunol. 2007, 179, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Flatz, L.; Cheng, C.; Wang, L.; Foulds, K.E.; Ko, S.-Y.; Kong, W.-P.; Roychoudhuri, R.; Shi, W.; Bao, S.; Todd, J.-P.; et al. Gene-Based Vaccination with a Mismatched Envelope Protects against Simian Immunodeficiency Virus Infection in Nonhuman Primates. J. Virol. 2012, 86, 7760–7770. [Google Scholar] [CrossRef] [PubMed]
- Obar, J.J.; Lefrançois, L. Memory CD8+ T Cell Differentiation. Ann. N. Y. Acad. Sci. 2010, 1183, 251–266. [Google Scholar] [CrossRef]
- Sommerstein, R.; Flatz, L.; Remy, M.M.; Malinge, P.; Magistrelli, G.; Fischer, N.; Sahin, M.; Bergthaler, A.; Igonet, S.; Ter Meulen, J.; et al. Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection. PLoS Pathog. 2015, 11, e1005276. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, A.V.; Sharma, B.; Nekkalapudi, A.; Wimmer, R.; Gamez-Guerrero, M.; Suthram, S.; Truong, H.; Lee, J.; Li, J.; Martin, R.; et al. Immunogenic Arenavirus Vector SIV Vaccine Reduces Setpoint Viral Load in SIV-Challenged Rhesus Monkeys. npj Vaccines 2023, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Schwendinger, M.; Thiry, G.; De Vos, B.; Leroux-Roels, G.; Bruhwyler, J.; Huygens, A.; Ganeff, C.; Buchinger, H.; Orlinger, K.K.; Pinschewer, D.D.; et al. A Randomized Dose-Escalating Phase I Trial of a Replication-Deficient Lymphocytic Choriomeningitis Virus Vector-Based Vaccine Against Human Cytomegalovirus. J. Infect. Dis. 2020, 225, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Snell, L.M.; MacLeod, B.L.; Law, J.C.; Osokine, I.; Elsaesser, H.J.; Hezaveh, K.; Dickson, R.J.; Gavin, M.A.; Guidos, C.J.; McGaha, T.L.; et al. CD8+ T Cell Priming in Established Chronic Viral Infection Preferentially Directs Differentiation of Memory-like Cells for Sustained Immunity. Immunity 2018, 49, 678. [Google Scholar] [CrossRef] [PubMed]
- Pre-Clinical Data on HOOKIPA’s Alternating 2-Vector Cancer Therapeutics Published in Cell Reports Medicine|Hookipa Pharma Inc. Available online: https://ir.hookipapharma.com/news-releases/news-release-details/pre-clinical-data-hookipas-alternating-2-vector-cancer/ (accessed on 25 September 2024).
- Sharma, B.; Bekerman, E.; Truong, H.; Lee, J.; Gamez-Guerrero, M.; Boopathy, A.; Mital, R.; Huang, K.B.; Ahmadi-Erber, S.; Wimmer, R.; et al. Arenavirus-Based Vectors Generate Robust SIV Immunity in Non-Human Primates. Vaccines 2024, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, H.; Schmidt, S.; Katchar, K.; Qing, X.; Iacobucci, C.; Hwang, A.; Schlienger, K.; Berka, U.; Raguz, J.; Ahmadi-Erber, S.; et al. Development and Characterization of a Novel Non-Lytic Cancer Immunotherapy Using a Recombinant Arenavirus Vector Platform. Front. Oncol. 2021, 11, 732166. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, W.V.; Kirchhammer, N.; Marx, A.-F.; Kallert, S.M.; Krzyzaniak, M.A.; Lu, M.; Darbre, S.; Schmidt, S.; Raguz, J.; Berka, U.; et al. Heterologous Arenavirus Vector Prime-Boost Overrules Self-Tolerance for Efficient Tumor-Specific CD8 T Cell Attack. Cell Rep. Med. 2021, 2, 100209. [Google Scholar] [CrossRef] [PubMed]
- Ibukun, F.I. Inter-Lineage Variation of Lassa Virus Glycoprotein Epitopes: A Challenge to Lassa Virus Vaccine Development. Viruses 2020, 12, 386. [Google Scholar] [CrossRef]
- Oloniniyi, O.K.; Unigwe, U.S.; Okada, S.; Kimura, M.; Koyano, S.; Miyazaki, Y.; Iroezindu, M.O.; Ajayi, N.A.; Chukwubike, C.M.; Chika-Igwenyi, N.M.; et al. Genetic Characterization of Lassa Virus Strains Isolated from 2012 to 2016 in Southeastern Nigeria. PLoS Negl. Trop. Dis. 2018, 12, e0006971. [Google Scholar] [CrossRef] [PubMed]
- Pinschewer, D.D.; Perez, M.; de la Torre, J.C. Role of the Virus Nucleoprotein in the Regulation of Lymphocytic Choriomeningitis Virus Transcription and RNA Replication. J. Virol. 2003, 77, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B.A.; Lewicki, H.; Homann, D.; Nguyen, C.; Julien, S.; Gairin, J.E. Common Antiviral Cytotoxic T-Lymphocyte Epitope for Diverse Arenaviruses. J. Virol. 2001, 75, 6273. [Google Scholar] [CrossRef]
- Kotturi, M.F.; Botten, J.; Maybeno, M.; Sidney, J.; Glenn, J.; Bui, H.-H.; Oseroff, C.; Crotty, S.; Peters, B.; Grey, H.; et al. Polyfunctional CD4+ T Cell Responses to a Set of Pathogenic Arenaviruses Provide Broad Population Coverage. Immunome Res. 2010, 6, 4. [Google Scholar] [CrossRef]
- Sakabe, S.; Hartnett, J.N.; Ngo, N.; Goba, A.; Momoh, M.; Sandi, J.D.; Kanneh, L.; Cubitt, B.; Garcia, S.D.; Ware, B.C.; et al. Identification of Common CD8+ T Cell Epitopes from Lassa Fever Survivors in Nigeria and Sierra Leone. J. Virol. 2020, 94, e00153-20. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cain, M.; Huang, Q.; Sanchez, S.; Ly, H.; Liang, Y. Evaluating Antigen- and Vector-Specific Immune Responses of a Recombinant Pichinde Virus-Based Vaccine Expressing the Lymphocytic Choriomeningitis Virus Nucleoprotein. Vaccines 2024, 12, 1450. https://doi.org/10.3390/vaccines12121450
Cain M, Huang Q, Sanchez S, Ly H, Liang Y. Evaluating Antigen- and Vector-Specific Immune Responses of a Recombinant Pichinde Virus-Based Vaccine Expressing the Lymphocytic Choriomeningitis Virus Nucleoprotein. Vaccines. 2024; 12(12):1450. https://doi.org/10.3390/vaccines12121450
Chicago/Turabian StyleCain, Michaela, Qinfeng Huang, Shania Sanchez, Hinh Ly, and Yuying Liang. 2024. "Evaluating Antigen- and Vector-Specific Immune Responses of a Recombinant Pichinde Virus-Based Vaccine Expressing the Lymphocytic Choriomeningitis Virus Nucleoprotein" Vaccines 12, no. 12: 1450. https://doi.org/10.3390/vaccines12121450
APA StyleCain, M., Huang, Q., Sanchez, S., Ly, H., & Liang, Y. (2024). Evaluating Antigen- and Vector-Specific Immune Responses of a Recombinant Pichinde Virus-Based Vaccine Expressing the Lymphocytic Choriomeningitis Virus Nucleoprotein. Vaccines, 12(12), 1450. https://doi.org/10.3390/vaccines12121450







