A Trivalent Live Vaccine Elicits Cross-Species Protection Against Acute Otitis Media in a Murine Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cultivation
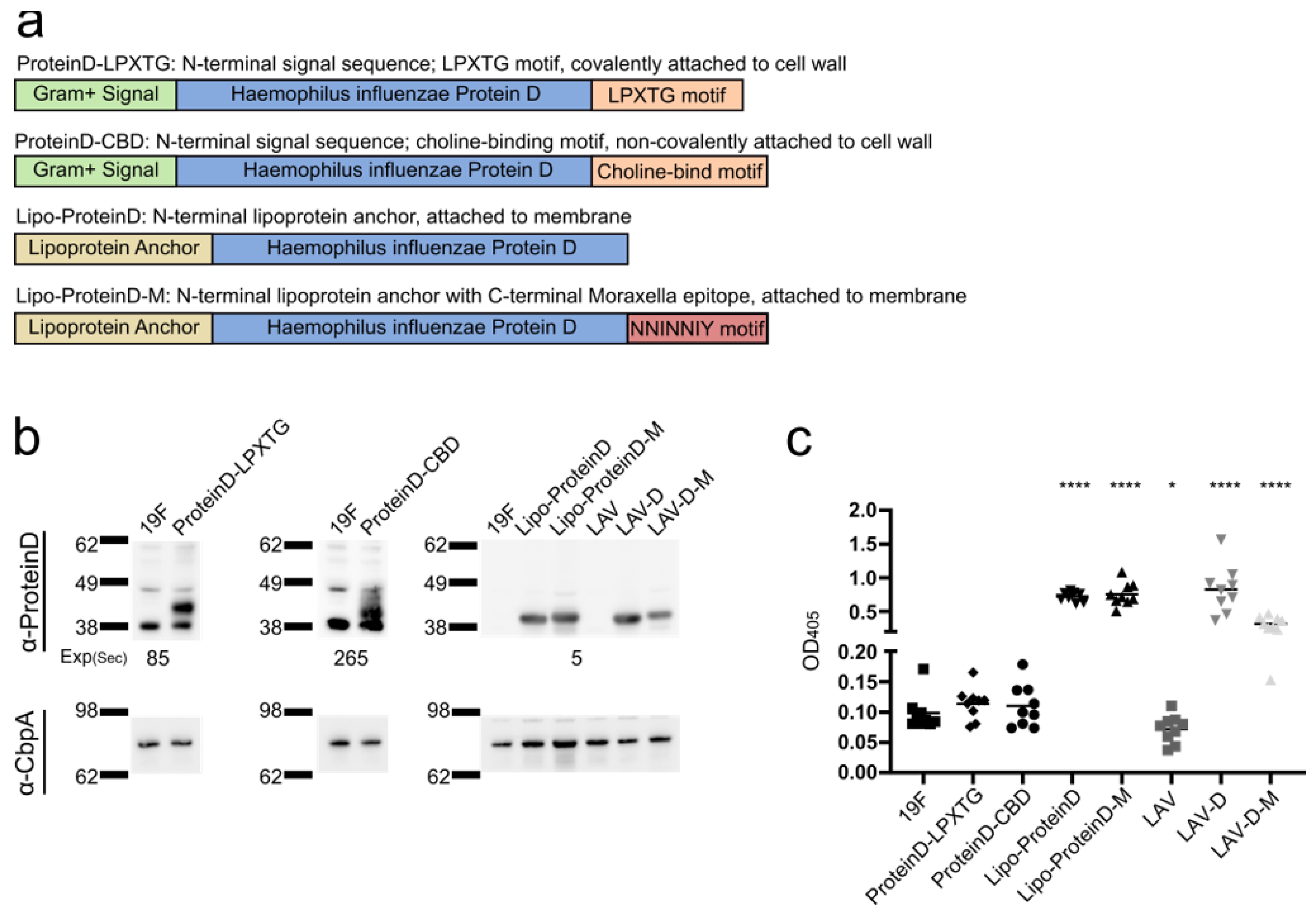
2.2. Genetic Constructs
2.3. Recombinant ProteinD Purification and Antibody Generation
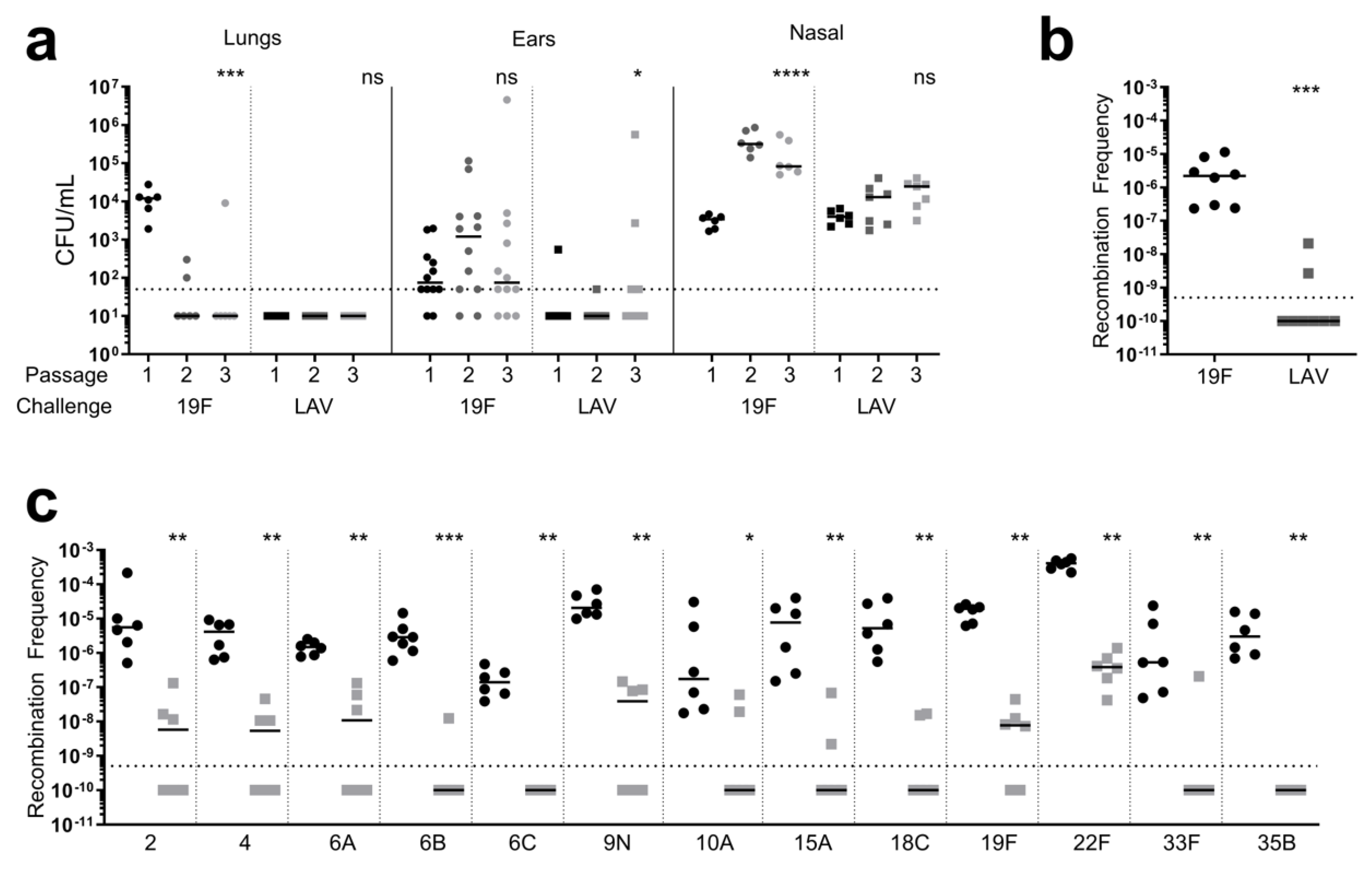
2.4. Recombination Frequency
2.5. Adhesion Assay
2.6. Vaccination Regimens and Protection Efficacy
2.7. Murine Challenge Experiments
2.8. Western Blots
2.9. ELISA Antibody Measurements
3. Results
3.1. Genetic Stability of LAV
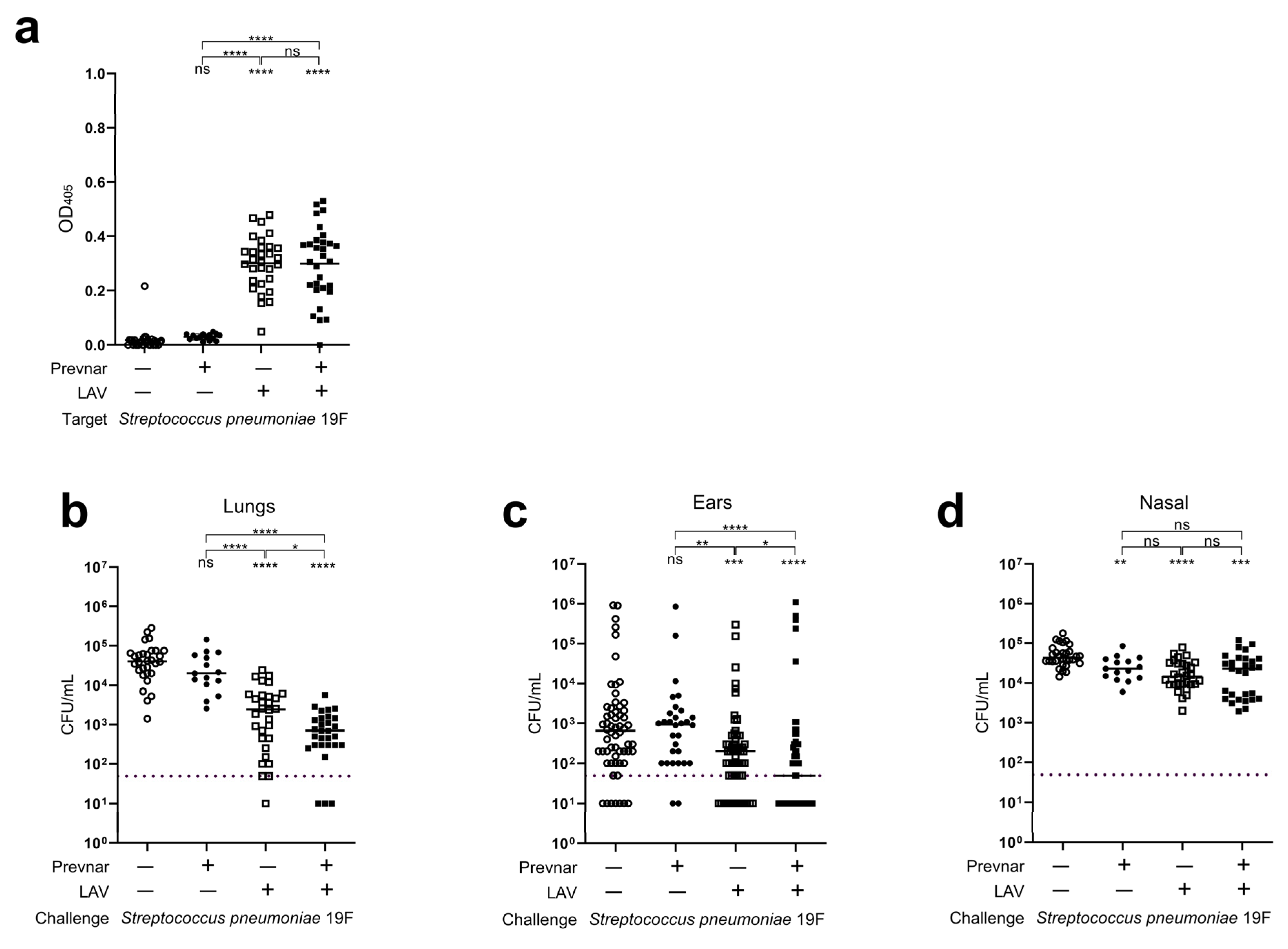
3.2. Impact of Prior Colonization on LAV Efficacy
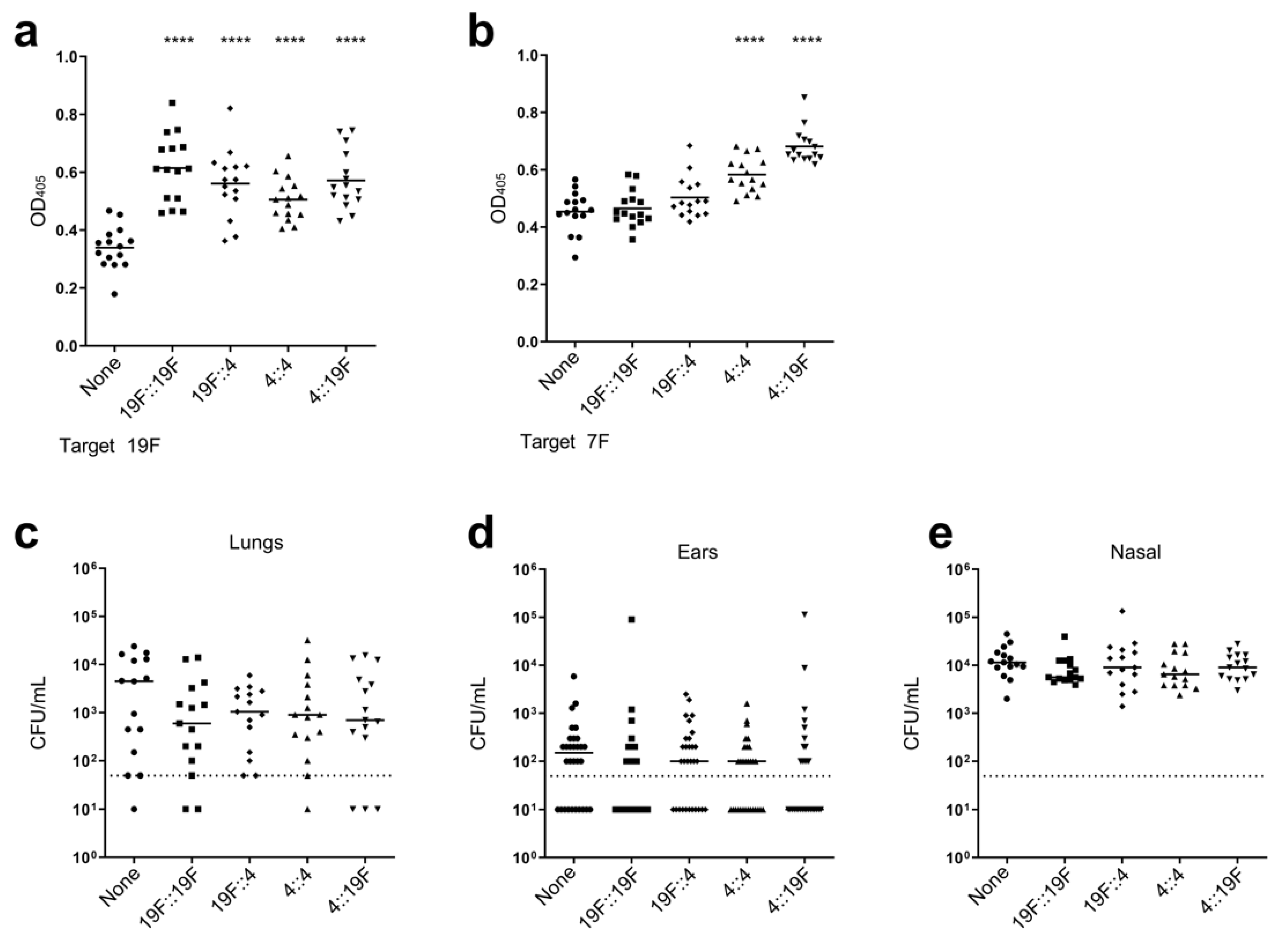
3.3. Impact of Prior Conjugate Vaccine Exposure on LAV Efficacy
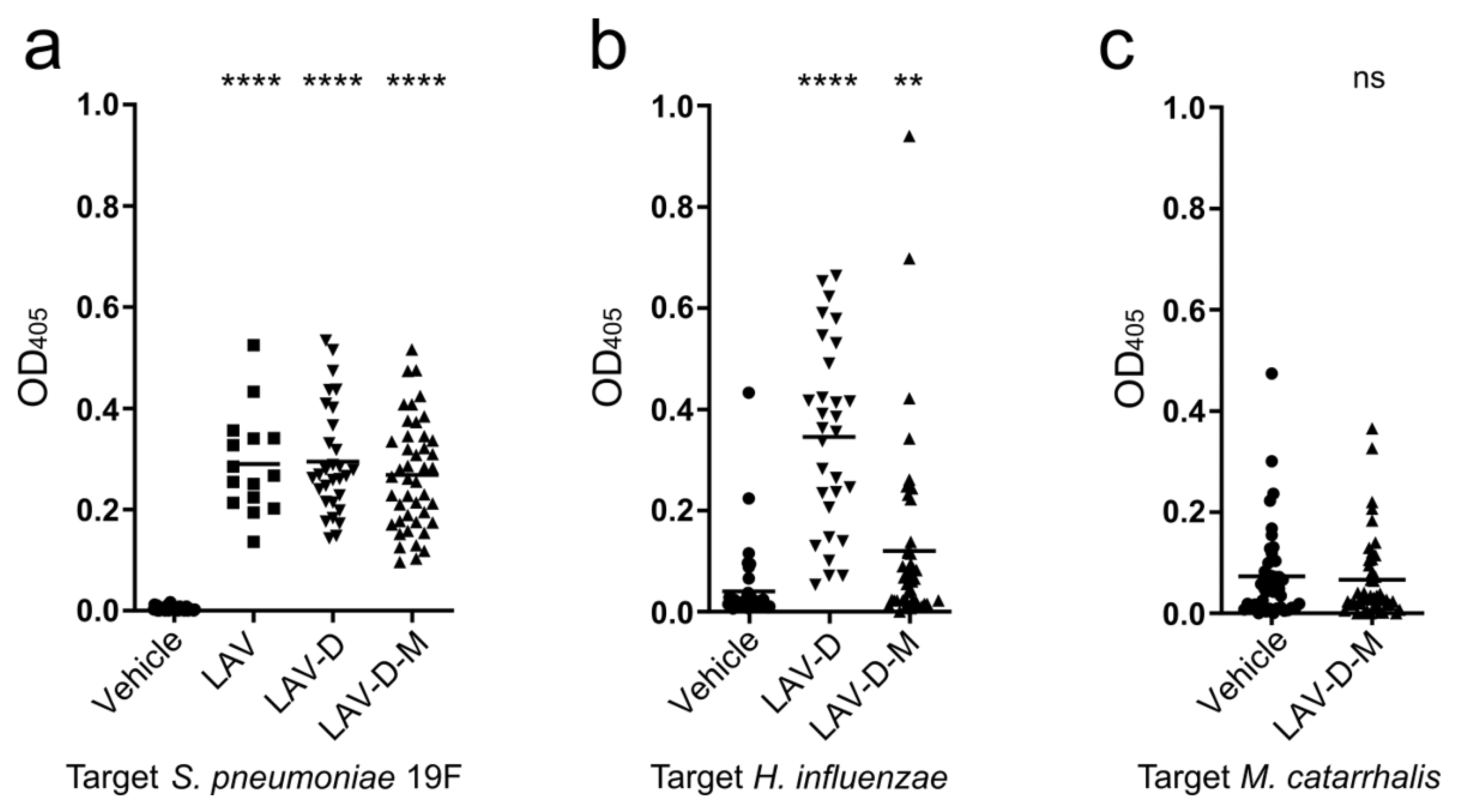
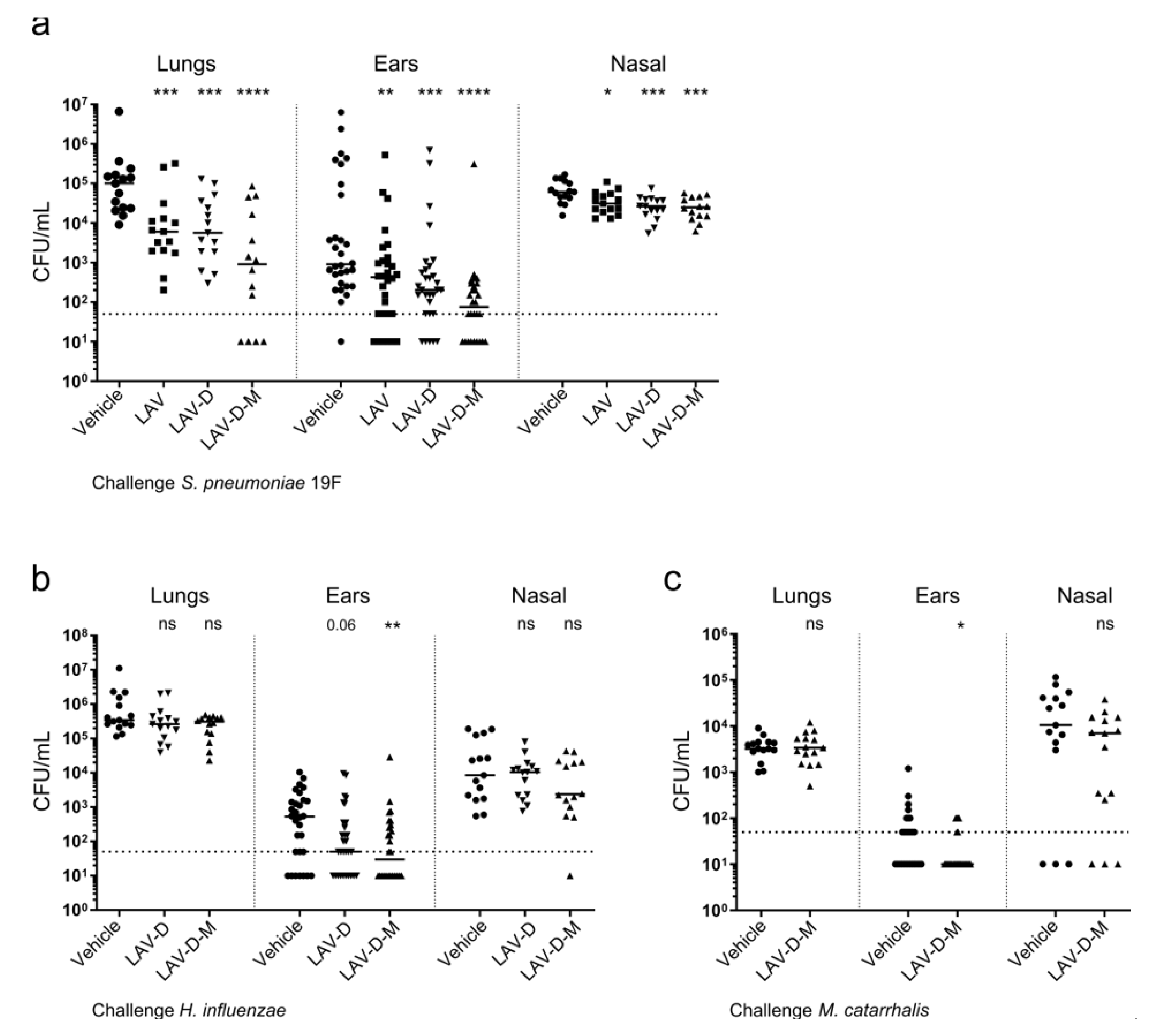
3.4. Efficacy of Polyvalent Vaccines to Protect Against AOM from Multiple Pathogens
3.4.1. Engineering Polyvalent Vaccines
3.4.2. Vaccination Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preventing and Treating Ear Infections. 2017. Available online: https://www.cdc.gov/ear-infection/media/pdfs/Ear-Infection-508.pdf (accessed on 11 December 2024).
- Short, K.R.; Reading, P.C.; Brown, L.E.; Pedersen, J.; Gilbertson, B.; Job, E.R.; Edenborough, K.M.; Habets, M.N.; Zomer, A.; Hermans, P.W.; et al. Influenza-induced inflammation drives pneumococcal otitis media. Infect. Immun. 2013, 81, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Bergenfelz, C.; Hakansson, A.P. Streptococcus pneumoniae Otitis Media Pathogenesis and How It Informs Our Understanding of Vaccine Strategies. Curr. Otorhinolaryngol. Rep. 2017, 5, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimol, S.; Givon-Lavi, N.; Leibovitz, E.; Raiz, S.; Greenberg, D.; Dagan, R. Impact of Widespread Introduction of Pneumococcal Conjugate Vaccines on Pneumococcal and Nonpneumococcal Otitis Media. Clin. Infect. Dis. 2016, 63, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Block, S.L.; Hedrick, J.; Harrison, C.J.; Tyler, R.; Smith, A.; Findlay, R.; Keegan, E. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr. Infect. Dis. J. 2004, 23, 829–833. [Google Scholar] [CrossRef]
- Casey, J.R.; Pichichero, M.E. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr. Infect. Dis. J. 2004, 23, 824–828. [Google Scholar] [CrossRef]
- Jalalvand, F.; Riesbeck, K. Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development. Expert. Rev. Vaccines 2018, 17, 503–512. [Google Scholar] [CrossRef]
- Marchisio, P.; Esposito, S.; Picca, M.; Baggi, E.; Terranova, L.; Orenti, A.; Biganzoli, E.; Principi, N.; Milan AOM Study Group. Prospective evaluation of the aetiology of acute otitis media with spontaneous tympanic membrane perforation. Clin. Microbiol. Infect. 2017, 23, 486.e1–486.e6. [Google Scholar] [CrossRef]
- Ngo, C.C.; Massa, H.M.; Thornton, R.B.; Cripps, A.W. Predominant Bacteria Detected from the Middle Ear Fluid of Children Experiencing Otitis Media: A Systematic Review. PLoS ONE 2016, 11, e0150949. [Google Scholar] [CrossRef]
- Ouldali, N.; Levy, C.; Minodier, P.; Morin, L.; Biscardi, S.; Aurel, M.; Dubos, F.; Dommergues, M.A.; Mezgueldi, E.; Levieux, K.; et al. Long-term Association of 13-Valent Pneumococcal Conjugate Vaccine Implementation with Rates of Community-Acquired Pneumonia in Children. JAMA Pediatr. 2019, 173, 362–370. [Google Scholar] [CrossRef]
- Berger, Y.; Adler, A.; Ariel, T.; Rokney, A.; Averbuch, D.; Grisaru-Soen, G. Paediatric community-acquired bacteraemia, pneumococcal invasive disease and antibiotic resistance fell after the pneumococcal conjugate vaccine was introduced. Acta Paediatr. 2018, 108, 1321–1328. [Google Scholar] [CrossRef]
- Wang, S.; Tafalla, M.; Hanssens, L.; Dolhain, J. A review of Haemophilus influenzae disease in Europe from 2000-2014: Challenges, successes and the contribution of hexavalent combination vaccines. Expert. Rev. Vaccines 2017, 16, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Adil, E.A.; Barrett, D.; Manganella, J.; Kenna, M.A. Ambulatory Visits for Otitis Media before and after the Introduction of Pneumococcal Conjugate Vaccination. J. Pediatr. 2018, 207, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Fortanier, A.C.; Venekamp, R.P.; Hoes, A.W.; Schilder, A.G.M. Does pneumococcal conjugate vaccination affect onset and risk of first acute otitis media and recurrences? A primary care-based cohort study. Vaccine 2019, 37, 1528–1532. [Google Scholar] [CrossRef] [PubMed]
- Keck, J.W.; Wenger, J.D.; Bruden, D.L.; Rudolph, K.M.; Hurlburt, D.A.; Hennessy, T.W.; Bruce, M.G. PCV7-induced changes in pneumococcal carriage and invasive disease burden in Alaskan children. Vaccine 2014, 32, 6478–6484. [Google Scholar] [CrossRef]
- Ramani, R.R.; Hall, W.N.; Boulton, M.; Johnson, D.R.; Zhu, B.P. Impact of PCV7 on invasive pneumococcal disease among children younger than 5 years: A population-based study. Am. J. Public. Health 2004, 94, 958–959. [Google Scholar] [CrossRef]
- Esposito, S.; Lizioli, A.; Lastrico, A.; Begliatti, E.; Rognoni, A.; Tagliabue, C.; Cesati, L.; Carreri, V.; Principi, N. Impact on respiratory tract infections of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months of age. Respir. Res. 2007, 8, 12. [Google Scholar] [CrossRef][Green Version]
- Ubukata, K.; Morozumi, M.; Sakuma, M.; Takata, M.; Mokuno, E.; Tajima, T.; Iwata, S. Etiology of Acute Otitis Media and Characterization of Pneumococcal Isolates After Introduction of 13-Valent Pneumococcal Conjugate Vaccine in Japanese Children. Pediatr. Infect. Dis. J. 2018, 37, 598–604. [Google Scholar] [CrossRef]
- Vesikari, T.; Forsten, A.; Seppa, I.; Kaijalainen, T.; Puumalainen, T.; Soininen, A.; Traskine, M.; Lommel, P.; Schoonbroodt, S.; Hezareh, M.; et al. Effectiveness of the 10-Valent Pneumococcal Nontypeable Haemophilus influenzae Protein D-Conjugated Vaccine (PHiD-CV) Against Carriage and Acute Otitis Media-A Double-Blind Randomized Clinical Trial in Finland. J. Pediatric Infect. Dis. Soc. 2016, 5, 237–248. [Google Scholar] [CrossRef]
- Nurkka, A.; Joensuu, J.; Henckaerts, I.; Peeters, P.; Poolman, J.; Kilpi, T.; Kayhty, H. Immunogenicity and safety of the eleven valent pneumococcal polysaccharide-protein D conjugate vaccine in infants. Pediatr. Infect. Dis. J. 2004, 23, 1008–1014. [Google Scholar] [CrossRef]
- Lee, H.; Choi, E.H.; Lee, H.J. Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines. Korean J. Pediatr. 2014, 57, 55–66. [Google Scholar] [CrossRef]
- Sigurdsson, S.; Eythorsson, E.; Hrafnkelsson, B.; Erlendsdottir, H.; Kristinsson, K.G.; Haraldsson, A. Reduction in All-Cause Acute Otitis Media in Children <3 Years of Age in Primary Care Following Vaccination with 10-Valent Pneumococcal Haemophilus influenzae Protein-D Conjugate Vaccine: A Whole-Population Study. Clin. Infect. Dis. 2018, 67, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Berman-Rosa, M.; O’Donnell, S.; Barker, M.; Quach, C. Efficacy and Effectiveness of the PCV-10 and PCV-13 Vaccines Against Invasive Pneumococcal Disease. Pediatrics 2020, 145, e20190377. [Google Scholar] [CrossRef] [PubMed]
- Rosenblut, A.; Rosenblut, M.; Garcia, K.; Maul, X.; Santolaya, M.E. Frequency of Acute Otitis Media in Children Under 24 Months of Age Before and After the Introduction of the 10-valent Pneumococcal Conjugate Vaccine into the National Immunization Program in Chile. Pediatr. Infect. Dis. J. 2018, 37, 132–134. [Google Scholar] [CrossRef]
- Prymula, R.; Peeters, P.; Chrobok, V.; Kriz, P.; Novakova, E.; Kaliskova, E.; Kohl, I.; Lommel, P.; Poolman, J.; Prieels, J.P.; et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: A randomised double-blind efficacy study. Lancet 2006, 367, 740–748. [Google Scholar] [CrossRef]
- Galgani, I.; Poder, A.; Jogi, R.; Anttila, V.J.; Milleri, S.; Borobia, A.M.; Launay, O.; Testa, M.; Casula, D.; Grassano, L.; et al. Immunogenicity and safety of the non-typable Haemophilus influenzae-Moraxella catarrhalis (NTHi-Mcat) vaccine administered following the recombinant zoster vaccine versus administration alone: Results from a randomized, phase 2a, non-inferiority trial. Hum. Vaccin. Immunother. 2023, 19, 2187194. [Google Scholar] [CrossRef]
- Van Damme, P.; Leroux-Roels, G.; Vandermeulen, C.; De Ryck, I.; Tasciotti, A.; Dozot, M.; Moraschini, L.; Testa, M.; Arora, A.K. Safety and immunogenicity of non-typeable Haemophilus influenzae-Moraxella catarrhalis vaccine. Vaccine 2019, 37, 3113–3122. [Google Scholar] [CrossRef]
- Fortanier, A.C.; Venekamp, R.P.; Boonacker, C.W.; Hak, E.; Schilder, A.G.; Sanders, E.A.; Damoiseaux, R.A. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database Syst. Rev. 2014, 4, CD001480. [Google Scholar] [CrossRef]
- Ochoa-Gondar, O.; Figuerola-Massana, E.; Vila-Corcoles, A.; Aguirre, C.A.; de Diego, C.; Satue, E.; Gomez, F.; Raga, X. Epidemiology of Streptococcus pneumoniae causing acute otitis media among children in Southern Catalonia throughout 2007-2013: Incidence, serotype distribution and vaccine’s effectiveness. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 2104–2108. [Google Scholar] [CrossRef]
- Palmu, A.A.; Lahdenkari, M. Early Vaccine-type Pneumococcal Acute Otitis Media Does not Predispose to Subsequent Otitis When Compared with Early Acute Otitis Media Due to Other Bacterial Etiology. Pediatr. Infect. Dis. J. 2018, 37, 592–594. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Nistico, L.; Sambanthamoorthy, K.; Dice, B.; Nguyen, D.; Mershon, W.J.; Johnson, C.; Hu, F.Z.; Stoodley, P.; Ehrlich, G.D.; et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008, 8, 173. [Google Scholar] [CrossRef]
- Kietzman, C.C.; Gao, G.; Mann, B.; Myers, L.; Tuomanen, E.I. Dynamic capsule restructuring by the main pneumococcal autolysin LytA in response to the epithelium. Nat. Commun. 2016, 7, 10859. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, I.; De Wachter, E.; Crokaert, F.; Verhaegen, J.; Soetens, O.; Pierard, D.; Malfroot, A. Microbiology of bronchoalveolar lavage fluid in children with acute nonresponding or recurrent community-acquired pneumonia: Identification of nontypeable Haemophilus influenzae as a major pathogen. Clin. Infect. Dis. 2011, 52, 1437–1444. [Google Scholar] [CrossRef]
- de Gier, C.; Granland, C.M.; Pickering, J.L.; Walls, T.; Bhuiyan, M.; Mills, N.; Richmond, P.C.; Best, E.J.; Thornton, R.B.; Kirkham, L.S. PCV7- and PCV10-Vaccinated Otitis-Prone Children in New Zealand Have Similar Pneumococcal and Haemophilus influenzae Densities in Their Nasopharynx and Middle Ear. Vaccines 2019, 7, 14. [Google Scholar] [CrossRef]
- Xu, Q.; Kaur, R.; Casey, J.R.; Sabharwal, V.; Pelton, S.; Pichichero, M.E. Nontypeable Streptococcus pneumoniae as an otopathogen. Diagn. Microbiol. Infect. Dis. 2011, 69, 200–204. [Google Scholar] [CrossRef]
- Ren, D.; Almudevar, A.L.; Pichichero, M.E. Synchrony in serum antibody response to conserved proteins of Streptococcus pneumoniae in young children. Hum. Vaccin. Immunother. 2015, 11, 489–497. [Google Scholar] [CrossRef]
- Rowe, H.M.; Mann, B.; Iverson, A.; Poole, A.; Tuomanen, E.; Rosch, J.W. A Cross-Reactive Protein Vaccine Combined with PCV-13 Prevents Streptococcus pneumoniae- and Haemophilus influenzae-Mediated Acute Otitis Media. Infect. Immun. 2019, 87, e00253-19. [Google Scholar] [CrossRef]
- Jang, A.Y.; Ahn, K.B.; Zhi, Y.; Ji, H.J.; Zhang, J.; Han, S.H.; Guo, H.; Lim, S.; Song, J.Y.; Lim, J.H.; et al. Serotype-Independent Protection Against Invasive Pneumococcal Infections Conferred by Live Vaccine with lgt Deletion. Front. Immunol. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Rosch, J.W.; Iverson, A.R.; Humann, J.; Mann, B.; Gao, G.; Vogel, P.; Mina, M.; Murrah, K.A.; Perez, A.C.; Edward Swords, W.; et al. A live-attenuated pneumococcal vaccine elicits CD4+ T-cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol. Med. 2014, 6, 141–154. [Google Scholar] [CrossRef]
- Roche, A.M.; King, S.J.; Weiser, J.N. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect. Immun. 2007, 75, 2469–2475. [Google Scholar] [CrossRef]
- Su, H.; Liu, Q.; Bian, X.; Wang, S.; Curtiss, R., 3rd; Kong, Q. Synthesis and delivery of Streptococcus pneumoniae capsular polysaccharides by recombinant attenuated Salmonella vaccines. Proc. Natl. Acad. Sci. USA 2021, 118, e2013350118. [Google Scholar] [CrossRef]
- Xin, W.; Li, Y.; Mo, H.; Roland, K.L.; Curtiss, R., 3rd. PspA family fusion proteins delivered by attenuated Salmonella enterica serovar Typhimurium extend and enhance protection against Streptococcus pneumoniae. Infect. Immun. 2009, 77, 4518–4528. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Shi, H.; Scarpellini, G.; Torres-Escobar, A.; Roland, K.L.; Curtiss, R., 3rd. Immune responses to recombinant pneumococcal PsaA antigen delivered by a live attenuated Salmonella vaccine. Infect. Immun. 2010, 78, 3258–3271. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.C.; Morona, J.K.; Harrer, S.; Hansman, D.; Morona, R. Immunization of mice with Salmonella typhimurium C5 aroA expressing a genetically toxoided derivative of the pneumococcal toxin pneumolysin. Microb. Pathog. 1993, 14, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.U.; Kim, J.J.; Yang, H.; Kwon, H.J.; Yang, J.Y.; Curtiss Iii, R.; Kweon, M.N. Effective protection against secondary pneumococcal pneumonia by oral vaccination with attenuated Salmonella delivering PspA antigen in mice. Vaccine 2012, 30, 6816–6823. [Google Scholar] [CrossRef]
- Campos, I.B.; Darrieux, M.; Ferreira, D.M.; Miyaji, E.N.; Silva, D.A.; Areas, A.P.; Aires, K.A.; Leite, L.C.; Ho, P.L.; Oliveira, M.L. Nasal immunization of mice with Lactobacillus casei expressing the Pneumococcal Surface Protein A: Induction of antibodies, complement deposition and partial protection against Streptococcus pneumoniae challenge. Microbes Infect. 2008, 10, 481–488. [Google Scholar] [CrossRef]
- Vintini, E.O.; Medina, M.S. Host immunity in the protective response to nasal immunization with a pneumococcal antigen associated to live and heat-killed Lactobacillus casei. BMC Immunol. 2011, 12, 46. [Google Scholar] [CrossRef]
- Hanniffy, S.B.; Carter, A.T.; Hitchin, E.; Wells, J.M. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J. Infect. Dis. 2007, 195, 185–193. [Google Scholar] [CrossRef]
- Hernani Mde, L.; Ferreira, P.C.; Ferreira, D.M.; Miyaji, E.N.; Ho, P.L.; Oliveira, M.L. Nasal immunization of mice with Lactobacillus casei expressing the pneumococcal surface protein C primes the immune system and decreases pneumococcal nasopharyngeal colonization in mice. FEMS Immunol. Med. Microbiol. 2011, 62, 263–272. [Google Scholar] [CrossRef]
- Medina, M.; Villena, J.; Vintini, E.; Hebert, E.M.; Raya, R.; Alvarez, S. Nasal immunization with Lactococcus lactis expressing the pneumococcal protective protein A induces protective immunity in mice. Infect. Immun. 2008, 76, 2696–2705. [Google Scholar] [CrossRef]
- Vintini, E.; Villena, J.; Alvarez, S.; Medina, M. Administration of a probiotic associated with nasal vaccination with inactivated Lactococcus lactis-PppA induces effective protection against pneumoccocal infection in young mice. Clin. Exp. Immunol. 2010, 159, 351–362. [Google Scholar] [CrossRef]
- Langermann, S.; Palaszynski, S.R.; Burlein, J.E.; Koenig, S.; Hanson, M.S.; Briles, D.E.; Stover, C.K. Protective humoral response against pneumococcal infection in mice elicited by recombinant bacille Calmette-Guerin vaccines expressing pneumococcal surface protein A. J. Exp. Med. 1994, 180, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, M.T.; Xu, Q.; Paton, J.C.; Hollingshead, S.K.; Pichichero, M.E.; Briles, D.E.; Girgis, N.; Zeng, M. Mucosal vaccination with a multicomponent adenovirus-vectored vaccine protects against Streptococcus pneumoniae infection in the lung. FEMS Immunol. Med. Microbiol. 2009, 55, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Helms, C.M.; Grizzard, M.B.; Chanock, R.M. Temperature-sensitive mutants of type I Streptococcus pneumoniae: Preparation, characterization, and evidence for attenuation and immunogenicity. J. Infect. Dis. 1977, 136 (Suppl. S1), S208–S215. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Sevillano, E.; Ercoli, G.; Felgner, P.; Ramiro de Assis, R.; Nakajima, R.; Goldblatt, D.; Heyderman, R.S.; Gordon, S.B.; Ferreira, D.M.; Brown, J.S. Preclinical Development of Virulence-attenuated Streptococcus pneumoniae Strains Able to Enhance Protective Immunity against Pneumococcal Infection. Am. J. Respir. Crit. Care Med. 2021, 203, 1037–1041. [Google Scholar] [CrossRef]
- Ramos-Sevillano, E.; Ercoli, G.; Guerra-Assuncao, J.A.; Felgner, P.; Ramiro de Assis, R.; Nakajima, R.; Goldblatt, D.; Tetteh, K.K.A.; Heyderman, R.S.; Gordon, S.B.; et al. Protective Effect of Nasal Colonisation with ∆cps/piaA and ∆cps/proABC Streptococcus pneumoniae Strains against Recolonisation and Invasive Infection. Vaccines 2021, 9, 261. [Google Scholar] [CrossRef]
- Amonov, M.; Simbak, N.; Wan Hassan, W.M.R.; Ismail, S.; NI, A.R.; Clarke, S.C.; Yeo, C.C. Disruption of the cpsE and endA Genes Attenuates Streptococcus pneumoniae Virulence: Towards the Development of a Live Attenuated Vaccine Candidate. Vaccines 2020, 8, 187. [Google Scholar] [CrossRef]
- Kim, S.J.; Seon, S.H.; Luong, T.T.; Ghosh, P.; Pyo, S.; Rhee, D.K. Immunization with attenuated non-transformable pneumococcal pep27 and comD mutant provides serotype-independent protection against pneumococcal infection. Vaccine 2019, 37, 90–98. [Google Scholar] [CrossRef]
- Chimalapati, S.; Cohen, J.M.; Camberlein, E.; MacDonald, N.; Durmort, C.; Vernet, T.; Hermans, P.W.; Mitchell, T.; Brown, J.S. Effects of deletion of the Streptococcus pneumoniae lipoprotein diacylglyceryl transferase gene lgt on ABC transporter function and on growth in vivo. PLoS ONE 2012, 7, e41393. [Google Scholar] [CrossRef]
- Pribyl, T.; Moche, M.; Dreisbach, A.; Bijlsma, J.J.; Saleh, M.; Abdullah, M.R.; Hecker, M.; van Dijl, J.M.; Becher, D.; Hammerschmidt, S. Influence of impaired lipoprotein biogenesis on surface and exoproteome of Streptococcus pneumoniae. J. Proteome Res. 2014, 13, 650–667. [Google Scholar] [CrossRef]
- Chimalapati, S.; Cohen, J.; Camberlein, E.; Durmort, C.; Baxendale, H.; de Vogel, C.; van Belkum, A.; Brown, J.S. Infection with conditionally virulent Streptococcus pneumoniae Deltapab strains induces antibody to conserved protein antigens but does not protect against systemic infection with heterologous strains. Infect. Immun. 2011, 79, 4965–4976. [Google Scholar] [CrossRef]
- Cohen, J.M.; Chimalapati, S.; de Vogel, C.; van Belkum, A.; Baxendale, H.E.; Brown, J.S. Contributions of capsule, lipoproteins and duration of colonisation towards the protective immunity of prior Streptococcus pneumoniae nasopharyngeal colonisation. Vaccine 2012, 30, 4453–4459. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.R.; Papamichail, D.; Yano, M.; Garcia-Suarez Mdel, M.; Pirofski, L.A. Designed reduction of Streptococcus pneumoniae pathogenicity via synthetic changes in virulence factor codon-pair bias. J. Infect. Dis. 2011, 203, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Lacks, S.; Hotchkiss, R.D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim. Biophys. Acta 1960, 39, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Echlin, H.; Rosch, J.W. Advancing Genetic Tools in Streptococcus pneumoniae. Genes. 2020, 11, 965. [Google Scholar] [CrossRef]
- Rowe, H.M.; Livingston, B.; Margolis, E.; Davis, A.; Meliopoulos, V.A.; Echlin, H.; Schultz-Cherry, S.; Rosch, J.W. Respiratory Bacteria Stabilize and Promote Airborne Transmission of Influenza A Virus. mSystems 2020, 5, e00762-20. [Google Scholar] [CrossRef]
- Pozzi, G.; Masala, L.; Iannelli, F.; Manganelli, R.; Havarstein, L.S.; Piccoli, L.; Simon, D.; Morrison, D.A. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: Two allelic variants of the peptide pheromone. J. Bacteriol. 1996, 178, 6087–6090. [Google Scholar] [CrossRef]
- Echlin, H.; Iverson, A.; Sardo, U.; Rosch, J.W. Airway proteolytic control of pneumococcal competence. PLoS Pathog. 2023, 19, e1011421. [Google Scholar] [CrossRef]
- Horton, R.M.; Cai, Z.L.; Ho, S.N.; Pease, L.R. Gene splicing by overlap extension: Tailor-made genes using the polymerase chain reaction. Biotechniques 1990, 8, 528–535. [Google Scholar] [CrossRef]
- Guiral, S.; Henard, V.; Laaberki, M.H.; Granadel, C.; Prudhomme, M.; Martin, B.; Claverys, J.P. Construction and evaluation of a chromosomal expression platform (CEP) for ectopic, maltose-driven gene expression in Streptococcus pneumoniae. Microbiology 2006, 152, 343–349. [Google Scholar] [CrossRef][Green Version]
- Sorg, R.A.; Kuipers, O.P.; Veening, J.W. Gene expression platform for synthetic biology in the human pathogen Streptococcus pneumoniae. ACS Synth. Biol. 2015, 4, 228–239. [Google Scholar] [CrossRef]
- Forsgren, A.; Riesbeck, K.; Janson, H. Protein D of Haemophilus influenzae: A protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin. Infect. Dis. 2008, 46, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.T.; Nordstrom, T.; Forsgren, A.; Riesbeck, K. The respiratory pathogen Moraxella catarrhalis adheres to epithelial cells by interacting with fibronectin through ubiquitous surface proteins A1 and A2. J. Infect. Dis. 2005, 192, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- van Opijnen, T.; Lazinski, D.W.; Camilli, A. Genome-Wide Fitness and Genetic Interactions Determined by Tn-seq, a High-Throughput Massively Parallel Sequencing Method for Microorganisms. Curr. Protoc. Mol. Biol. 2014, 106, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, C.J.; Mahdavi, J.; Thornton, J.; Mann, B.; Wooldridge, K.G.; Abouseada, N.; Oldfield, N.J.; Self, T.; Ala’Aldeen, D.A.; Tuomanen, E.I. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J. Clin. Investig. 2009, 119, 1638–1646. [Google Scholar] [CrossRef]
- Michel, L.V.; Kaur, R.; Zavorin, M.; Pryharski, K.; Khan, M.N.; LaClair, C.; O’Neil, M.; Xu, Q.; Pichichero, M.E. Intranasal coinfection model allows for assessment of protein vaccines against nontypeable Haemophilus influenzae in mice. J. Med. Microbiol. 2018, 67, 1527–1532. [Google Scholar] [CrossRef]
- Stowell, N.C.; Seideman, J.; Raymond, H.A.; Smalley, K.A.; Lamb, R.J.; Egenolf, D.D.; Bugelski, P.J.; Murray, L.A.; Marsters, P.A.; Bunting, R.A.; et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir. Res. 2009, 10, 43. [Google Scholar] [CrossRef]
- Wren, J.T.; Blevins, L.K.; Pang, B.; King, L.B.; Perez, A.C.; Murrah, K.A.; Reimche, J.L.; Alexander-Miller, M.A.; Swords, W.E. Influenza A virus alters pneumococcal nasal colonization and middle ear infection independently of phase variation. Infect. Immun. 2014, 82, 4802–4812. [Google Scholar] [CrossRef]
- Mann, B.; Thornton, J.; Heath, R.; Wade, K.R.; Tweten, R.K.; Gao, G.; El Kasmi, K.; Jordan, J.B.; Mitrea, D.M.; Kriwacki, R.; et al. Broadly protective protein-based pneumococcal vaccine composed of pneumolysin toxoid-CbpA peptide recombinant fusion protein. J. Infect. Dis. 2014, 209, 1116–1125. [Google Scholar] [CrossRef]
- Claverys, J.P.; Havarstein, L.S. Extracellular-peptide control of competence for genetic transformation in Streptococcus pneumoniae. Front. Biosci. 2002, 7, d1798–d1814. [Google Scholar] [CrossRef]
- Cooper, V.S.; Honsa, E.; Rowe, H.; Deitrick, C.; Iverson, A.R.; Whittall, J.J.; Neville, S.L.; McDevitt, C.A.; Kietzman, C.; Rosch, J.W. Experimental Evolution In Vivo To Identify Selective Pressures during Pneumococcal Colonization. mSystems 2020, 5, e00352-20. [Google Scholar] [CrossRef]
- Syrjanen, R.K.; Kilpi, T.M.; Kaijalainen, T.H.; Herva, E.E.; Takala, A.K. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J. Infect. Dis. 2001, 184, 451–459. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tonkin-Hill, G.; Ling, C.; Chaguza, C.; Salter, S.J.; Hinfonthong, P.; Nikolaou, E.; Tate, N.; Pastusiak, A.; Turner, C.; Chewapreecha, C.; et al. Pneumococcal within-host diversity during colonization, transmission and treatment. Nat. Microbiol. 2022, 7, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Croucher, N.J.; Kagedan, L.; Thompson, C.M.; Parkhill, J.; Bentley, S.D.; Finkelstein, J.A.; Lipsitch, M.; Hanage, W.P. Selective and genetic constraints on pneumococcal serotype switching. PLoS Genet. 2015, 11, e1005095. [Google Scholar] [CrossRef] [PubMed]
- Nuorti, J.P.; Whitney, C.G. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine Preventionand 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2010, 59, 1–18. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 521–524. [Google Scholar]
- Johnson, C.N.; Wilde, S.; Tuomanen, E.; Rosch, J.W. Convergent impact of vaccination and antibiotic pressures on pneumococcal populations. Cell Chem. Biol. 2024, 31, 195–206. [Google Scholar] [CrossRef]
- Schilder, A.G.; Chonmaitree, T.; Cripps, A.W.; Rosenfeld, R.M.; Casselbrant, M.L.; Haggard, M.P.; Venekamp, R.P. Otitis media. Nat. Rev. Dis. Primers 2016, 2, 16063. [Google Scholar] [CrossRef]
- Fischetti, V.A. M Protein and Other Surface Proteins on Streptococcus pyogenes. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations, 2nd ed.; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2022. [Google Scholar]
- Bergmann, S.; Hammerschmidt, S. Versatility of pneumococcal surface proteins. Microbiology 2006, 152, 295–303. [Google Scholar] [CrossRef]
- Helminen, M.E.; Maciver, I.; Latimer, J.L.; Klesney-Tait, J.; Cope, L.D.; Paris, M.; McCracken, G.H., Jr.; Hansen, E.J. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J. Infect. Dis. 1994, 170, 867–872. [Google Scholar] [CrossRef]
- Aebi, C.; Maciver, I.; Latimer, J.L.; Cope, L.D.; Stevens, M.K.; Thomas, S.E.; McCracken, G.H., Jr.; Hansen, E.J. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect. Immun. 1997, 65, 4367–4377. [Google Scholar] [CrossRef]
- Henriques-Normark, B.; Tuomanen, E.I. The pneumococcus: Epidemiology, microbiology, and pathogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a010215. [Google Scholar] [CrossRef] [PubMed]
- Kazanjian, P. Changing interest among physicians toward pneumococcal vaccination throughout the twentieth century. J. Hist. Med. Allied Sci. 2004, 59, 555–587. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Madhi, S.A. Review on the immunogenicity and safety of PCV-13 in infants and toddlers. Expert. Rev. Vaccines 2011, 10, 951–980. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. 20-Valent Pneumococcal Conjugate Vaccine: A Review of Its Use in Adults. Drugs 2022, 82, 989–999. [Google Scholar] [CrossRef]
- Mangtani, P.; Cutts, F.; Hall, A.J. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: The state of the evidence. Lancet Infect. Dis. 2003, 3, 71–78. [Google Scholar] [CrossRef]
- Black, S.; Shinefield, H.; Fireman, B.; Lewis, E.; Ray, P.; Hansen, J.R.; Elvin, L.; Ensor, K.M.; Hackell, J.; Siber, G.; et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 2000, 19, 187–195. [Google Scholar] [CrossRef]
- Grijalva, C.G.; Poehling, K.A.; Nuorti, J.P.; Zhu, Y.; Martin, S.W.; Edwards, K.M.; Griffin, M.R. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics 2006, 118, 865–873. [Google Scholar] [CrossRef]
- Shea, K.M.; Weycker, D.; Stevenson, A.E.; Strutton, D.R.; Pelton, S.I. Modeling the decline in pneumococcal acute otitis media following the introduction of pneumococcal conjugate vaccines in the US. Vaccine 2011, 29, 8042–8048. [Google Scholar] [CrossRef]
- Shapiro, D.J.; Gonzales, R.; Cabana, M.D.; Hersh, A.L. National trends in visit rates and antibiotic prescribing for children with acute sinusitis. Pediatrics 2011, 127, 28–34. [Google Scholar] [CrossRef]
- Cutts, F.T.; Zaman, S.M.; Enwere, G.; Jaffar, S.; Levine, O.S.; Okoko, J.B.; Oluwalana, C.; Vaughan, A.; Obaro, S.K.; Leach, A.; et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: Randomised, double-blind, placebo-controlled trial. Lancet 2005, 365, 1139–1146. [Google Scholar] [CrossRef]
- Hwang Fu, Y.H.; Huang, W.Y.C.; Shen, K.; Groves, J.T.; Miller, T.; Shan, S.O. Two-step membrane binding by the bacterial SRP receptor enable efficient and accurate Co-translational protein targeting. eLife 2017, 6, e25885. [Google Scholar] [CrossRef] [PubMed]
- Rosconi, F.; Rudmann, E.; Li, J.; Surujon, D.; Anthony, J.; Frank, M.; Jones, D.S.; Rock, C.; Rosch, J.W.; Johnston, C.D.; et al. A bacterial pan-genome makes gene essentiality strain-dependent and evolvable. Nat. Microbiol. 2022, 7, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Sevillano, E.; Ercoli, G.; Brown, J.S. Mechanisms of Naturally Acquired Immunity to Streptococcus pneumoniae. Front. Immunol. 2019, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Clarke, T.B.; Weiser, J.N. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Investig. 2009, 119, 1899–1909. [Google Scholar] [CrossRef]
- Richards, L.; Ferreira, D.M.; Miyaji, E.N.; Andrew, P.W.; Kadioglu, A. The immunising effect of pneumococcal nasopharyngeal colonisation; protection against future colonisation and fatal invasive disease. Immunobiology 2010, 215, 251–263. [Google Scholar] [CrossRef]
- Malley, R.; Trzcinski, K.; Srivastava, A.; Thompson, C.M.; Anderson, P.W.; Lipsitch, M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA 2005, 102, 4848–4853. [Google Scholar] [CrossRef]
- Wilson, R.; Cohen, J.M.; Jose, R.J.; de Vogel, C.; Baxendale, H.; Brown, J.S. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2015, 8, 627–639. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Neill, D.R.; Bangert, M.; Gritzfeld, J.F.; Green, N.; Wright, A.K.; Pennington, S.H.; Bricio-Moreno, L.; Moreno, A.T.; Miyaji, E.N.; et al. Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am. J. Respir. Crit. Care Med. 2013, 187, 855–864. [Google Scholar] [CrossRef]
- Janson, H.; Heden, L.O.; Forsgren, A. Protein D, the immunoglobulin D-binding protein of Haemophilus influenzae, is a lipoprotein. Infect. Immun. 1992, 60, 1336–1342. [Google Scholar] [CrossRef]
- Fan, X.; Goldfine, H.; Lysenko, E.; Weiser, J.N. The transfer of choline from the host to the bacterial cell surface requires glpQ in Haemophilus influenzae. Mol. Microbiol. 2001, 41, 1029–1036. [Google Scholar] [CrossRef]
- Munson, R.S., Jr.; Sasaki, K. Protein D, a putative immunoglobulin D-binding protein produced by Haemophilus influenzae, is glycerophosphodiester phosphodiesterase. J. Bacteriol. 1993, 175, 4569–4571. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.P.; Peng, Z.R.; Tseng, S.F.; Lin, Y.C.; Sytwu, H.K.; Hsieh, Y.C. Impact of the glpQ2 gene on virulence in a Streptococcus pneumoniae serotype 19A sequence type 320 strain. Infect. Immun. 2015, 83, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Cundell, D.R.; Gerard, N.P.; Gerard, C.; Idanpaan-Heikkila, I.; Tuomanen, E.I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 1995, 377, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Radin, J.N.; Orihuela, C.J.; Murti, G.; Guglielmo, C.; Murray, P.J.; Tuomanen, E.I. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect. Immun. 2005, 73, 7827–7835. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, C.; Ryan, P.; Weiser, J.N.; Johnson, S.; Fontan, P.; Ortqvist, A.; Masure, H.R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 1997, 25, 819–829. [Google Scholar] [CrossRef]
- Orihuela, C.J.; Gao, G.; Francis, K.P.; Yu, J.; Tuomanen, E.I. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 2004, 190, 1661–1669. [Google Scholar] [CrossRef]
- Siggins, M.K.; Gill, S.K.; Langford, P.R.; Li, Y.; Ladhani, S.N.; Tregoning, J.S. PHiD-CV induces anti-Protein D antibodies but does not augment pulmonary clearance of nontypeable Haemophilus influenzae in mice. Vaccine 2015, 33, 4954–4961. [Google Scholar] [CrossRef][Green Version]
- McCullers, J.A.; McAuley, J.L.; Browall, S.; Iverson, A.R.; Boyd, K.L.; Henriques Normark, B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J. Infect. Dis. 2010, 202, 1287–1295. [Google Scholar] [CrossRef]
- McCullers, J.A.; Karlstrom, A.; Iverson, A.R.; Loeffler, J.M.; Fischetti, V.A. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 2007, 3, e28. [Google Scholar] [CrossRef]
- Iverson, A.; Meyer, C.J.; Vogel, P.; Waidyarachchi, S.; Das, N.; Bruhn, D.F.; Poole, A.; Butler, M.M.; Bowlin, T.L.; Lee, R.E.; et al. Efficacy of Aminomethyl Spectinomycins against Complex Upper Respiratory Tract Bacterial Infections. Antimicrob. Agents Chemother. 2019, 63, e02096. [Google Scholar] [CrossRef]
- Rowe, H.M.; Karlsson, E.; Echlin, H.; Chang, T.C.; Wang, L.; van Opijnen, T.; Pounds, S.B.; Schultz-Cherry, S.; Rosch, J.W. Bacterial Factors Required for Transmission of Streptococcus pneumoniae in Mammalian Hosts. Cell Host Microbe 2019, 25, 884–891.e6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echlin, H.; Iverson, A.; McKnight, A.; Rosch, J.W. A Trivalent Live Vaccine Elicits Cross-Species Protection Against Acute Otitis Media in a Murine Model. Vaccines 2024, 12, 1432. https://doi.org/10.3390/vaccines12121432
Echlin H, Iverson A, McKnight A, Rosch JW. A Trivalent Live Vaccine Elicits Cross-Species Protection Against Acute Otitis Media in a Murine Model. Vaccines. 2024; 12(12):1432. https://doi.org/10.3390/vaccines12121432
Chicago/Turabian StyleEchlin, Haley, Amy Iverson, Abigail McKnight, and Jason W. Rosch. 2024. "A Trivalent Live Vaccine Elicits Cross-Species Protection Against Acute Otitis Media in a Murine Model" Vaccines 12, no. 12: 1432. https://doi.org/10.3390/vaccines12121432
APA StyleEchlin, H., Iverson, A., McKnight, A., & Rosch, J. W. (2024). A Trivalent Live Vaccine Elicits Cross-Species Protection Against Acute Otitis Media in a Murine Model. Vaccines, 12(12), 1432. https://doi.org/10.3390/vaccines12121432





