Radiation-Induced Tumor-Derived Extracellular Vesicles Combined with Tyrosine Kinase Inhibitors: An Effective and Safe Therapeutic Approach for Lung Adenocarcinoma with EGFR19Del
Abstract
1. Introduction
2. Materials and Methods
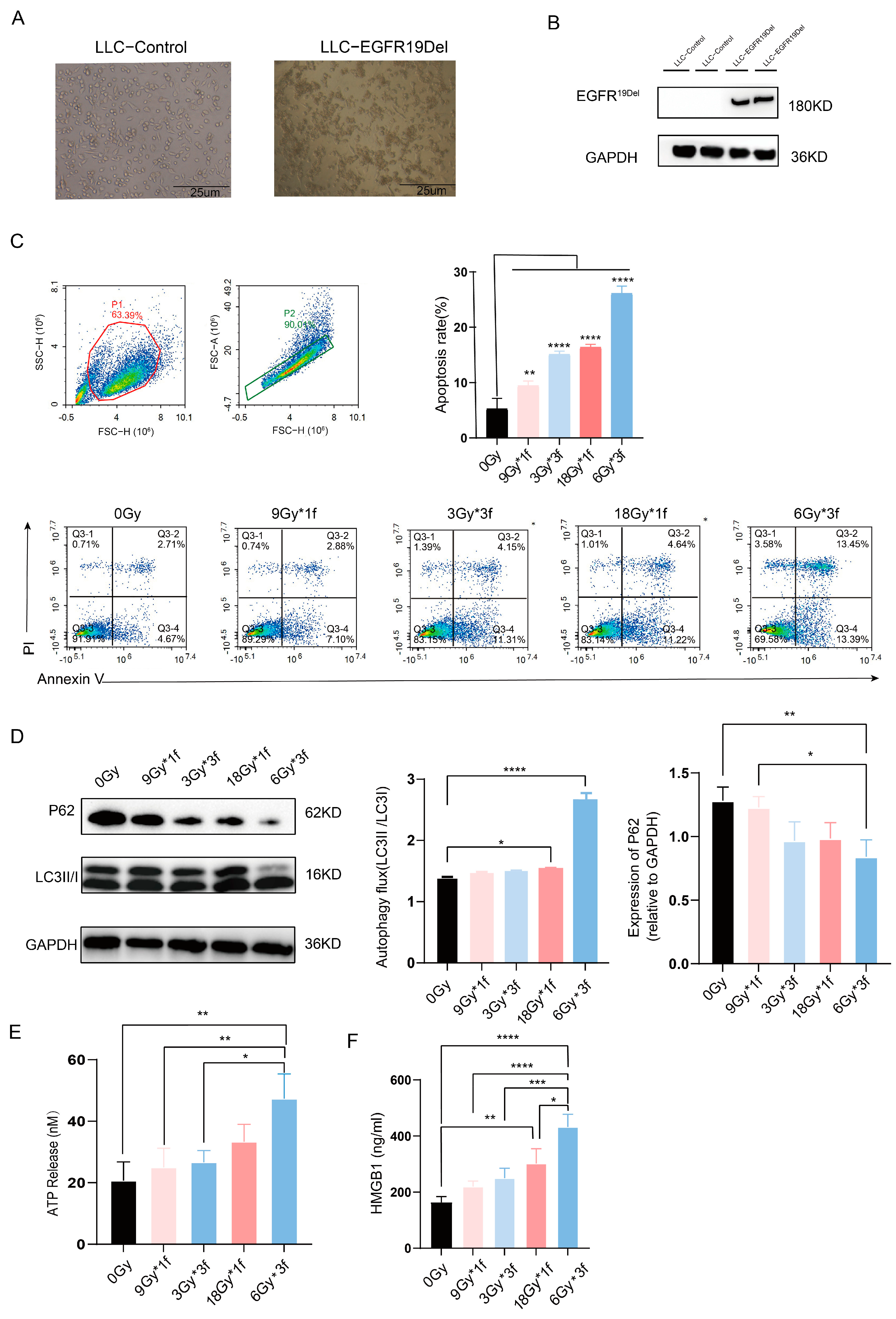
2.1. Construction and Processing of EGFR-19del Encoding LLC Cells
2.2. Apoptosis Detection
2.3. ATP Release
2.4. Bone Marrow-Derived Dendritic Cell (BMDC) Induction and Culture
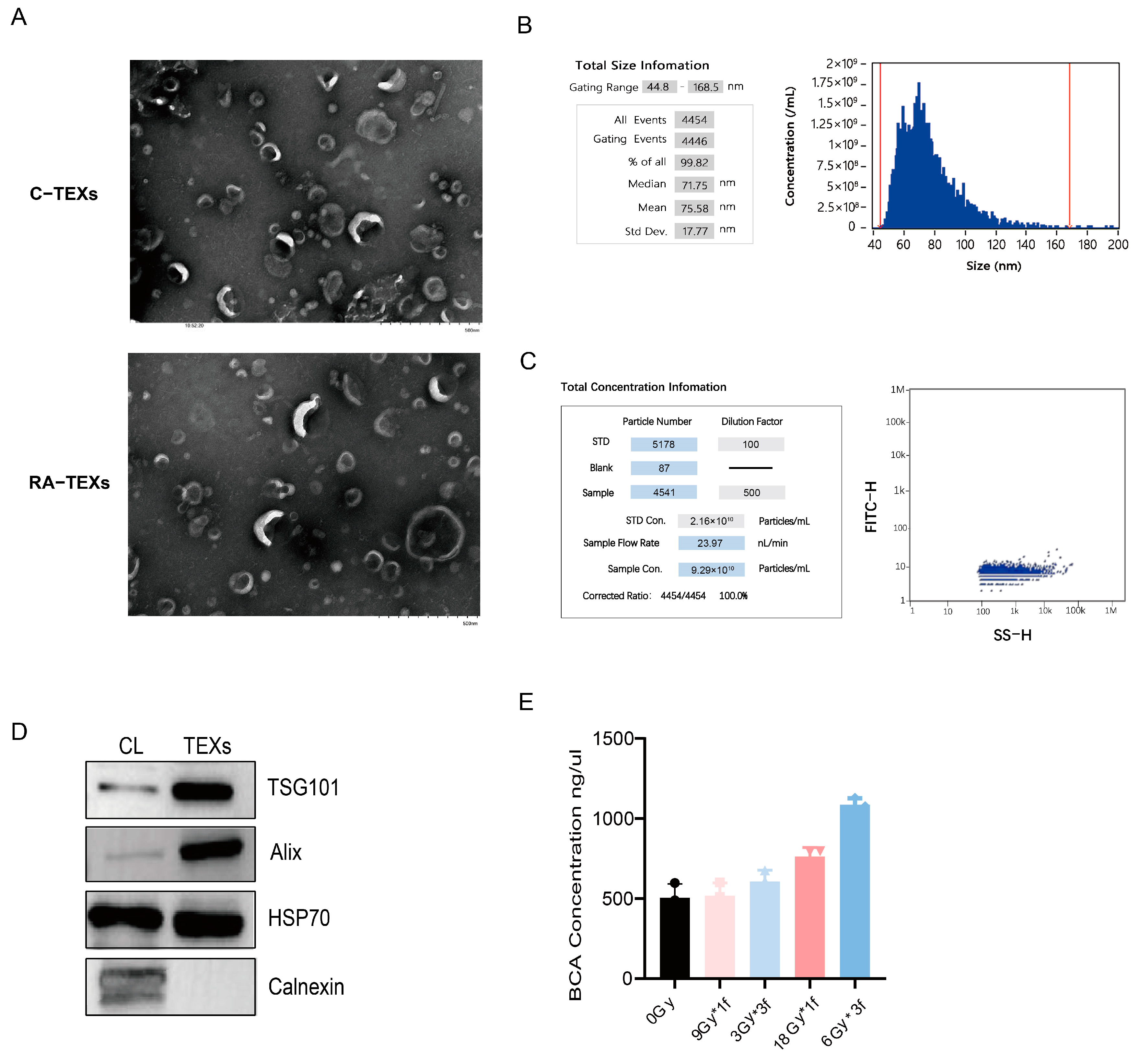
2.5. EV Isolation and Characterization
2.6. Analysis of DCs Activation Induced by EVs
2.7. T Cell Proliferation and Tumor-Specific CTL Response In Vitro
2.8. Animal Model and Group Treatment
2.9. Animals Sampling
2.10. Flow Cytometry Detection
2.11. Immunohistochemical Staining and Analysis
2.12. ELISA Analysis
2.13. Western Blot Analysis
2.14. Data Analysis
3. Results
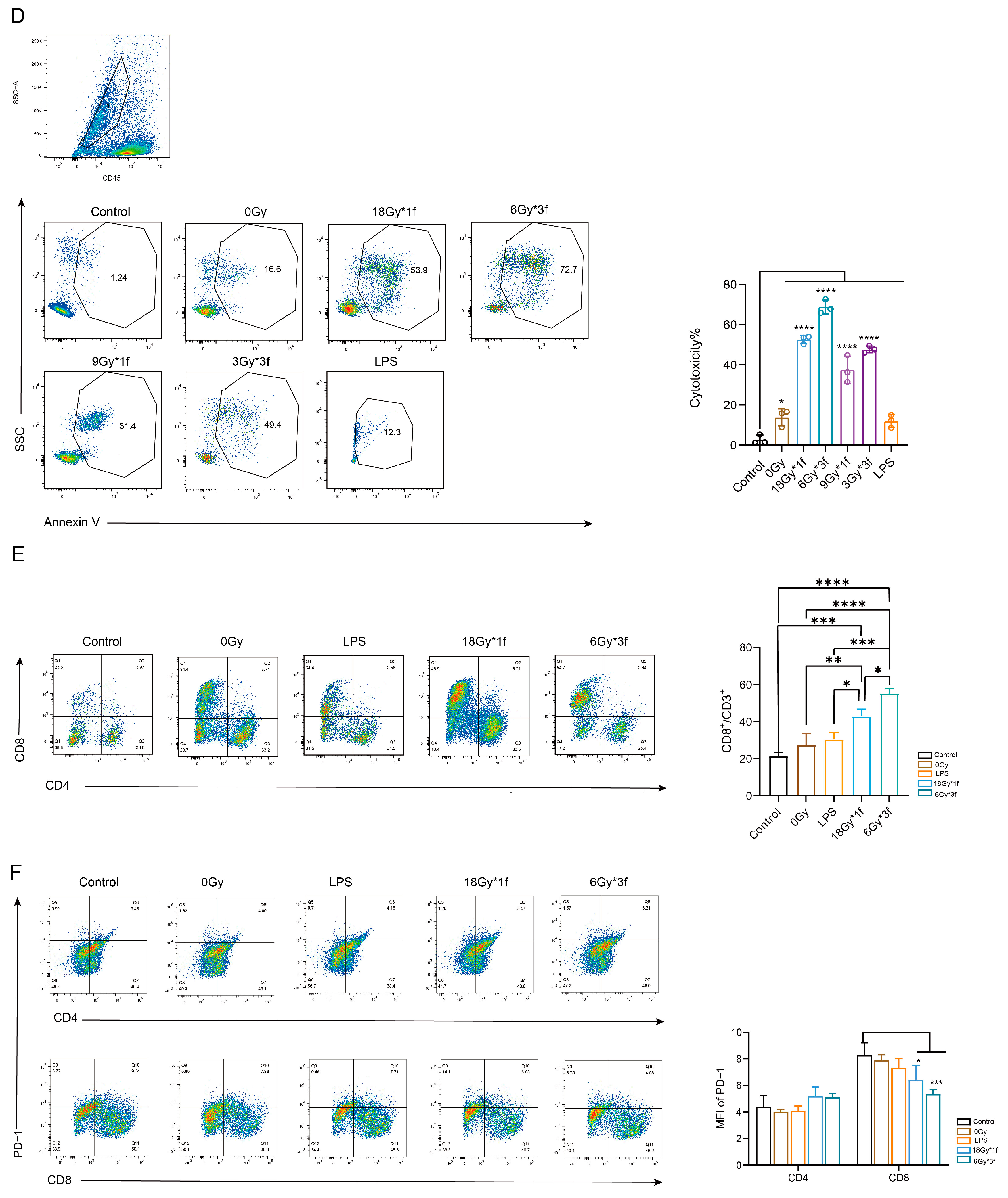
3.1. Radiation Dose-Dependently Promoted Apoptosis, Autophagy, and Immunogenic Cell Death (ICD) of Tumor Cells and the Radiation Fraction Matters
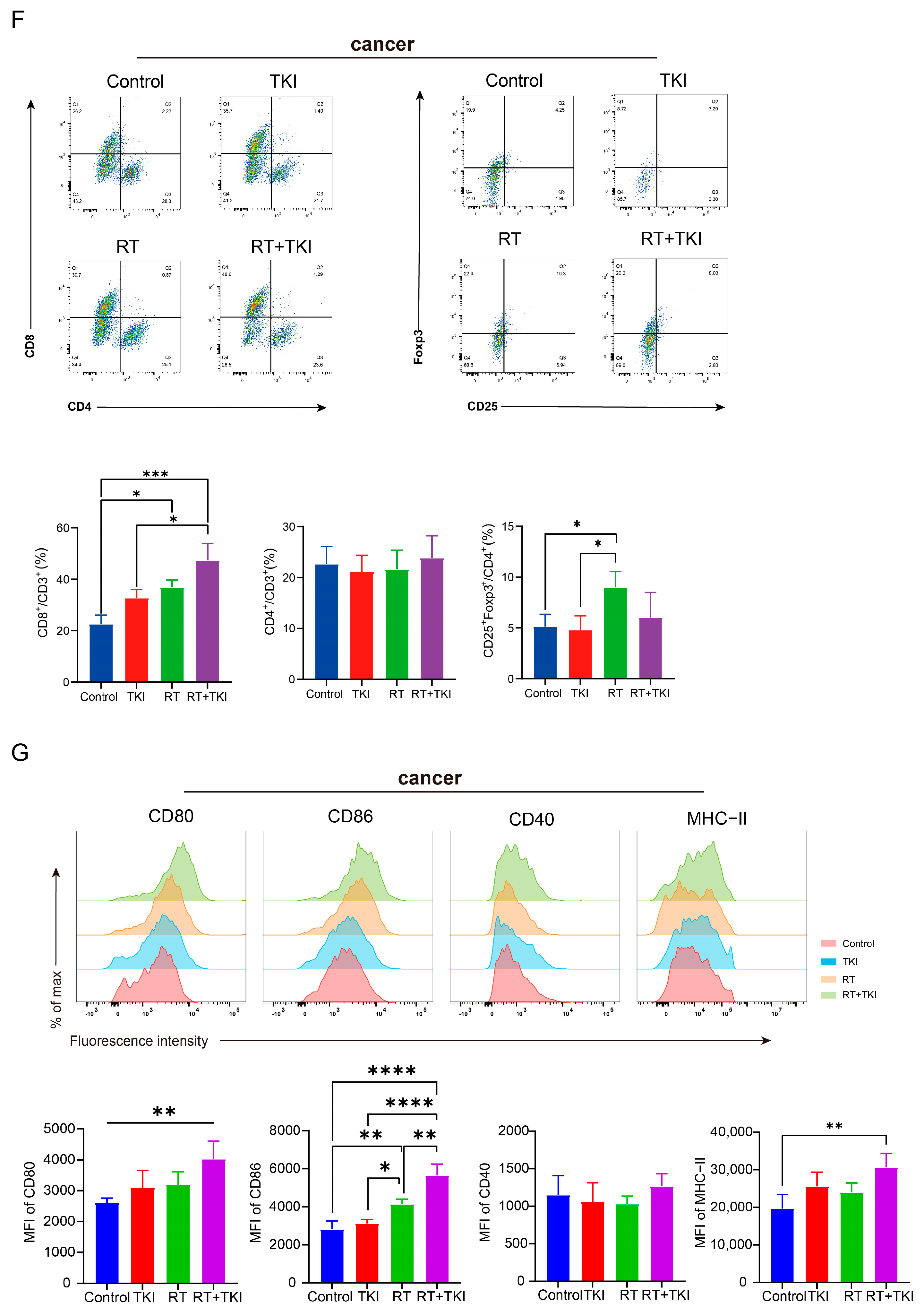
3.2. Radiation Therapy Optimizes the Efficacy of TKIs and Increases CD8+ T Cell Infiltration in the TME but Is Associated with a Potential Risk of Radiation Lung Damage
3.3. Identification of EVs Secreted by Tumor Cells
3.4. Radiation-Induced EVs Promote the Expression of Surface Co-Stimulatory Molecules and Cytokine Secretion During BMDC Maturation
3.5. Radiation-Induced EVs Enhance the Ability of DCs to Promote T Lymphocyte Proliferation and Killing In Vitro
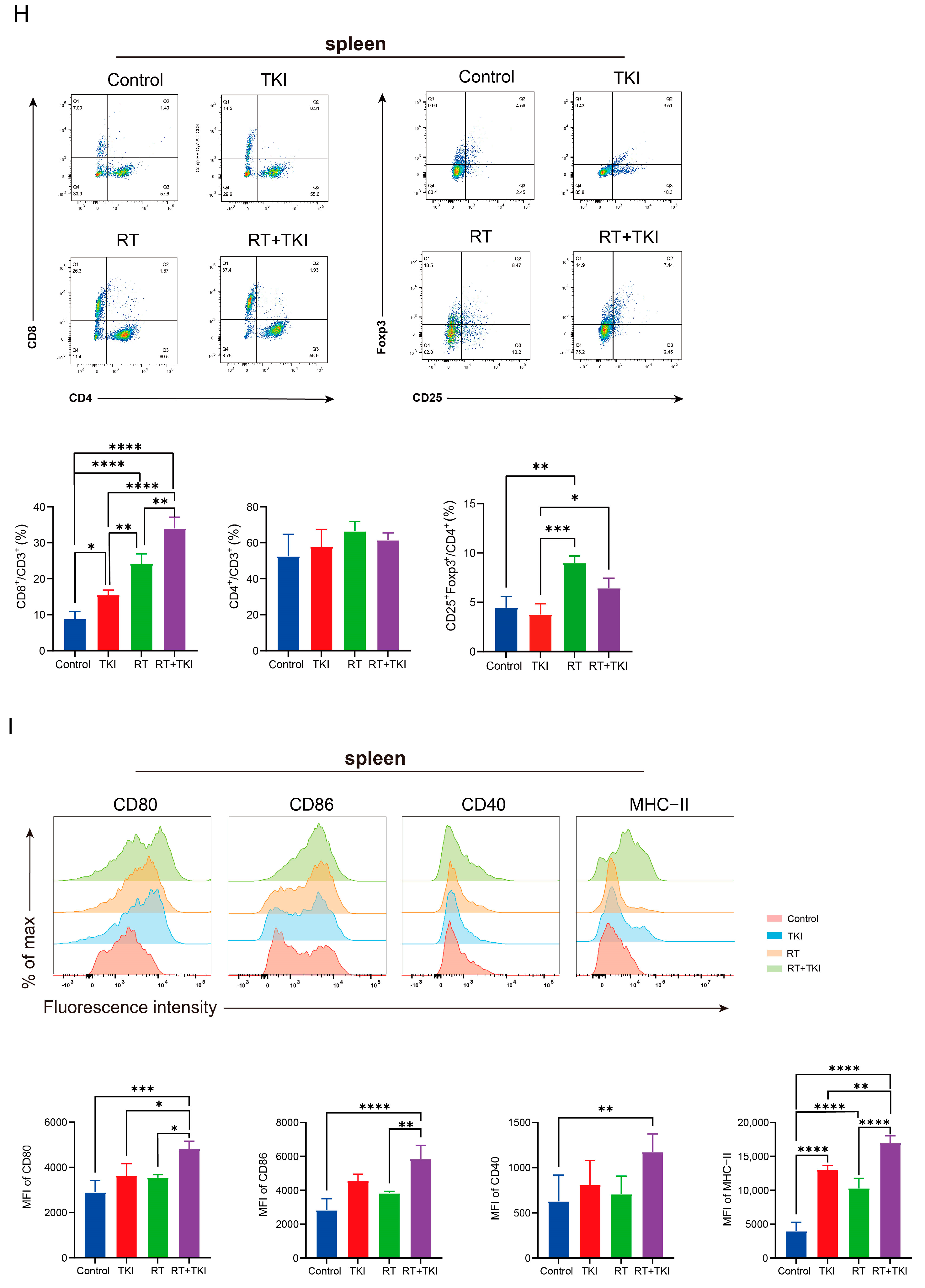
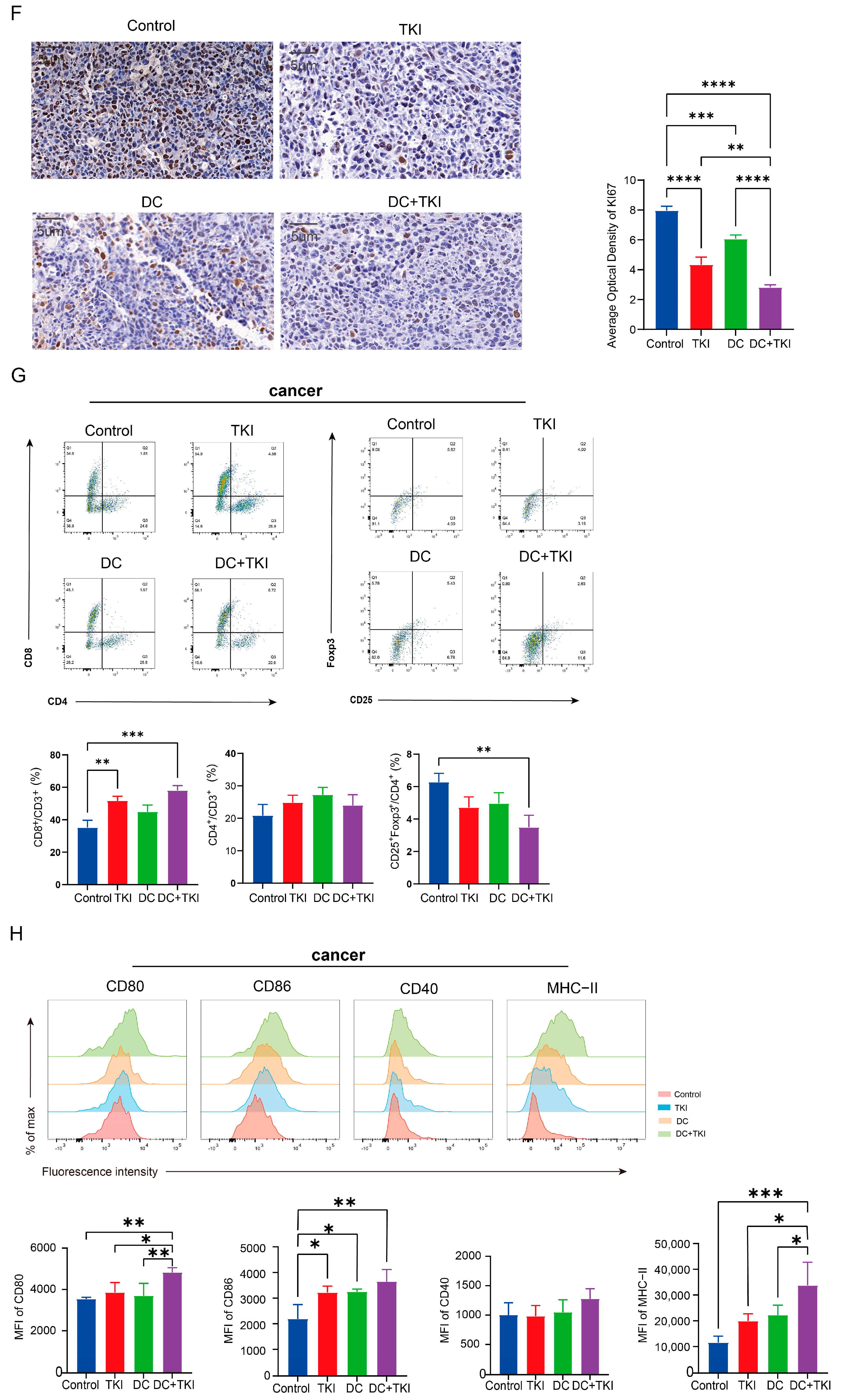
3.6. EVs Secreted by Radial Cells Enhance DC-Mediated Anti-Tumor Responses in C57BL/6 Mice WRP Avoiding Adverse Effects Such as Lungs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alduais, Y.; Zhang, H.; Fan, F.; Chen, J.; Chen, B. Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine 2023, 102, e32899. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M. Repurposing established cyclic adenosine monophosphate reducing agents for the prevention and therapy of epidermal growth factor receptor inhibitor resistance in non-small cell lung cancer. Transl. Lung Cancer Res. 2018, 7, S117–S122. [Google Scholar] [CrossRef] [PubMed]
- Al Olayan, A.; Al Hussaini, H.; Jazieh, A.R. The roles of epidermal growth factor receptor (EGFR) inhibitors in the management of lung cancer. J. Infect. Public. Health 2012, 5 (Suppl. 1), S50–S60. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Nagasaka, M.; Zhu, V.W.; Lim, S.M.; Greco, M.; Wu, F.; Ou, S.I. Beyond Osimertinib: The Development of Third-Generation EGFR Tyrosine Kinase Inhibitors For Advanced EGFR+ NSCLC. J. Thorac. Oncol. 2021, 16, 740–763. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Wilkins, A.C.; Patin, E.C.; Harrington, K.J.; Melcher, A.A. The immunological consequences of radiation-induced DNA damage. J. Pathol. 2019, 247, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Onyshchenko, K.; Luo, R.; Rao, X.; Zhang, X.; Gaedicke, S.; Grosu, A.L.; Firat, E.; Niedermann, G. Hypofractionated radiotherapy combined with lenalidomide improves systemic antitumor activity in mouse solid tumor models. Theranostics 2024, 14, 2573–2588. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Naidoo, J.; Antonia, S.; Wu, Y.L.; Cho, B.C.; Thiyagarajah, P.; Mann, H.; Newton, M.; Faivre-Finn, C. Brief Report: Durvalumab After Chemoradiotherapy in Unresectable Stage III EGFR-Mutant NSCLC: A Post Hoc Subgroup Analysis From PACIFIC. J. Thorac. Oncol. 2023, 18, 657–663. [Google Scholar] [CrossRef]
- Xing, L.; Wu, G.; Wang, L.; Li, J.; Wang, J.; Yuan, Z.; Chen, M.; Xu, Y.; Fu, X.; Zhu, Z.; et al. Erlotinib Versus Etoposide/Cisplatin With Radiation Therapy in Unresectable Stage III Epidermal Growth Factor Receptor Mutation-Positive Non-Small Cell Lung Cancer: A Multicenter, Randomized, Open-Label, Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, X.; Wu, Y.; Wang, J.; Li, F.; Guo, G. Tumor Cell-associated Exosomes Robustly Elicit Anti-tumor Immune Responses through Modulating Dendritic Cell Vaccines in Lung Tumor. Int. J. Biol. Sci. 2020, 16, 633–643. [Google Scholar] [CrossRef]
- Yang, Z.; Zhong, W.; Yang, L.; Wen, P.; Luo, Y.; Wu, C. The emerging role of exosomes in radiotherapy. Cell Commun. Signal 2022, 20, 171. [Google Scholar] [CrossRef]
- Lei, Q.Q.; Sui, J.D.; Jin, F.; Luo, H.L.; Shan, J.J.; Tang, L.; Wang, Y.; Wu, Y.Z. Impact of high-dose rate radiotherapy on B and natural killer (NK) cell polarization in peripheral blood mononuclear cells (PBMCs) via inducing non-small cell lung cancer (NSCLC)-derived exosomes. Transl. Cancer Res. 2021, 10, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Sha, H.; Qin, X.; Chen, Y.; Li, X.; Shi, M.; Feng, J. EGFR E746-A750 deletion in lung cancer represses antitumor immunity through the exosome-mediated inhibition of dendritic cells. Oncogene 2020, 39, 2643–2657. [Google Scholar] [CrossRef]
- Gurupadaswamy, H.D.; Thirusangu, P.; Vijay Avin, B.R.; Vigneshwaran, V.; Prashanth Kumar, M.V.; Abhishek, T.S.; Lakshmi Ranganatha, V.; Khanum, S.A.; Prabhakar, B.T. DAO-9 (2,5-di(4-aryloylaryloxymethyl)-1,3,4-oxadiazole) exhibits p53 induced apoptogenesis through caspase-3 mediated endonuclease activity in murine carcinoma. Biomed. Pharmacother. 2014, 68, 791–797. [Google Scholar] [CrossRef]
- Li, B.; Lei, Z.; Lichty, B.D.; Li, D.; Zhang, G.M.; Feng, Z.H.; Wan, Y.; Huang, B. Autophagy facilitates major histocompatibility complex class I expression induced by IFN-γ in B16 melanoma cells. Cancer Immunol. Immunother. 2010, 59, 313–321. [Google Scholar] [CrossRef]
- Chan, O.S.H.; Lee, V.H.F.; Mok, T.S.K.; Mo, F.; Chang, A.T.Y.; Yeung, R.M.W. The Role of Radiotherapy in Epidermal Growth Factor Receptor Mutation-positive Patients with Oligoprogression: A Matched-cohort Analysis. Clin. Oncol. (R. Coll. Radiol.) 2017, 29, 568–575. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Zhuang, H.; Yuan, Z.; Chang, J.Y.; Wang, J.; Pang, Q.; Zhao, L.; Wang, P. Radiation pneumonitis in patients with non--small-cell lung cancer treated with erlotinib concurrent with thoracic radiotherapy. J. Thorac. Oncol. 2014, 9, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ranoa, D.R.E.; Huang, X.; Hou, Y.; Yang, K.; Poli, E.C.; Beckett, M.A.; Fu, Y.X.; Weichselbaum, R.R. RIG-I-Like Receptor LGP2 Is Required for Tumor Control by Radiotherapy. Cancer Res. 2020, 80, 5633–5641. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, H.; Taira, T.; Sonoda, H.; Sasaki, K.; Murono, K.; Emoto, S.; Yokoyama, Y.; Nagai, Y.; Abe, S.; Ishihara, S. Enhancement of radiation therapy by indoleamine 2,3 dioxygenase 1 inhibition through multimodal mechanisms. BMC Cancer 2023, 23, 62. [Google Scholar] [CrossRef]
- Ploeg, E.M.; Samplonius, D.F.; Xiong, X.; Ke, X.; Hendriks, M.; Britsch, I.; van Wijngaarden, A.P.; Zhang, H.; Helfrich, W. Bispecific antibody CD73xEGFR more selectively inhibits the CD73/adenosine immune checkpoint on cancer cells and concurrently counteracts pro-oncogenic activities of CD73 and EGFR. J. Immunother. Cancer 2023, 11, e006837. [Google Scholar] [CrossRef] [PubMed]
- Caronni, N.; Piperno, G.M.; Simoncello, F.; Romano, O.; Vodret, S.; Yanagihashi, Y.; Dress, R.; Dutertre, C.A.; Bugatti, M.; Bourdeley, P.; et al. TIM4 expression by dendritic cells mediates uptake of tumor-associated antigens and anti-tumor responses. Nat. Commun. 2021, 12, 2237. [Google Scholar] [CrossRef]
- Wang, F.; Ullah, A.; Fan, X.; Xu, Z.; Zong, R.; Wang, X.; Chen, G. Delivery of nanoparticle antigens to antigen-presenting cells: From extracellular specific targeting to intracellular responsive presentation. J. Control Release 2021, 333, 107–128. [Google Scholar] [CrossRef]
- Mateu-Jimenez, M.; Curull, V.; Pijuan, L.; Sánchez-Font, A.; Rivera-Ramos, H.; Rodríguez-Fuster, A.; Aguiló, R.; Gea, J.; Barreiro, E. Systemic and Tumor Th1 and Th2 Inflammatory Profile and Macrophages in Lung Cancer: Influence of Underlying Chronic Respiratory Disease. J. Thorac. Oncol. 2017, 12, 235–248. [Google Scholar] [CrossRef]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Advances in Hypofractionated Irradiation-Induced Immunosuppression of Tumor Microenvironment. Front. Immunol. 2020, 11, 612072. [Google Scholar] [CrossRef]
- Aredo, J.V.; Mambetsariev, I.; Hellyer, J.A.; Amini, A.; Neal, J.W.; Padda, S.K.; McCoach, C.E.; Riess, J.W.; Cabebe, E.C.; Naidoo, J.; et al. Durvalumab for Stage III EGFR-Mutated NSCLC After Definitive Chemoradiotherapy. J. Thorac. Oncol. 2021, 16, 1030–1041. [Google Scholar] [CrossRef]
- Yen, Y.C.; Hsu, H.L.; Chang, J.H.; Lin, W.C.; Chang, Y.C.; Chang, C.L.; Chow, J.M.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Efficacy of thoracic radiotherapy in patients with stage IIIB-IV epidermal growth factor receptor-mutant lung adenocarcinomas who received and responded to tyrosine kinase inhibitor treatment. Radiother. Oncol. 2018, 129, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.H.; Melosky, B.; Shih, J.Y.; Wang, J.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study. Ann. Oncol. 2024, 35, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liang, J.; Zhang, T.; Zhou, Z.; Chen, D.; Feng, Q.; Xiao, Z.; Hui, Z.; Lu, J.; Wang, X.; et al. Clinical outcomes and radiation pneumonitis after concurrent EGFR-tyrosine kinase inhibitors and radiotherapy for unresectable stage III non-small cell lung cancer. Thorac. Cancer 2021, 12, 814–823. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, H.; Wang, S.; Yang, H.; Kong, F.S. Treatment-Related Pneumonitis of EGFR Tyrosine Kinase Inhibitors Plus Thoracic Radiation Therapy in Patients With Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 415–426. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Meng, X.; Wang, J.; Zhu, L.; Liu, C.; Li, S.; Zheng, L.; Yang, Z.; Xing, L.; et al. Osimertinib (AZD9291) increases radio-sensitivity in EGFR T790M non-small cell lung cancer. Oncol. Rep. 2019, 41, 77–86. [Google Scholar] [CrossRef]
- Tachi, H.; Shiozawa, T.; Sakai, C.; Kasuga, M.; Nakazawa, K.; Morishima, Y.; Satoh, H.; Hizawa, N.; Sakashita, S.; Sekine, I. Osimertinib-Induced Interstitial Lung Disease Presenting as Eosinophilic Pneumonia. J. Thorac. Oncol. 2017, 12, e118–e120. [Google Scholar] [CrossRef]
- Kepp, O.; Tesniere, A.; Schlemmer, F.; Michaud, M.; Senovilla, L.; Zitvogel, L.; Kroemer, G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis 2009, 14, 364–375. [Google Scholar] [CrossRef]
- Demaria, S.; Formenti, S.C. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front. Oncol. 2012, 2, 153. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Schartz, N.E.; Movassagh, M.; Flament, C.; Pautier, P.; Morice, P.; Pomel, C.; Lhomme, C.; Escudier, B.; Le Chevalier, T.; et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002, 360, 295–305. [Google Scholar] [CrossRef]
- Diamond, J.M.; Vanpouille-Box, C.; Spada, S.; Rudqvist, N.P.; Chapman, J.R.; Ueberheide, B.M.; Pilones, K.A.; Sarfraz, Y.; Formenti, S.C.; Demaria, S. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol. Res. 2018, 6, 910–920. [Google Scholar] [CrossRef]
- Brummel, K.; Eerkens, A.L.; de Bruyn, M.; Nijman, H.W. Tumour-infiltrating lymphocytes: From prognosis to treatment selection. Br. J. Cancer 2023, 128, 451–458. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Long, Y.; Ge, X.; Zhang, P.; Li, T.; Wu, L.; Fan, H.; Du, Z.; Liu, Q.; Hu, Y. Radiation-Induced Tumor-Derived Extracellular Vesicles Combined with Tyrosine Kinase Inhibitors: An Effective and Safe Therapeutic Approach for Lung Adenocarcinoma with EGFR19Del. Vaccines 2024, 12, 1412. https://doi.org/10.3390/vaccines12121412
Li Y, Long Y, Ge X, Zhang P, Li T, Wu L, Fan H, Du Z, Liu Q, Hu Y. Radiation-Induced Tumor-Derived Extracellular Vesicles Combined with Tyrosine Kinase Inhibitors: An Effective and Safe Therapeutic Approach for Lung Adenocarcinoma with EGFR19Del. Vaccines. 2024; 12(12):1412. https://doi.org/10.3390/vaccines12121412
Chicago/Turabian StyleLi, Yao, Yaping Long, Xiangwei Ge, Pengfei Zhang, Tao Li, Liangliang Wu, Hao Fan, Zhijuan Du, Qiaowei Liu, and Yi Hu. 2024. "Radiation-Induced Tumor-Derived Extracellular Vesicles Combined with Tyrosine Kinase Inhibitors: An Effective and Safe Therapeutic Approach for Lung Adenocarcinoma with EGFR19Del" Vaccines 12, no. 12: 1412. https://doi.org/10.3390/vaccines12121412
APA StyleLi, Y., Long, Y., Ge, X., Zhang, P., Li, T., Wu, L., Fan, H., Du, Z., Liu, Q., & Hu, Y. (2024). Radiation-Induced Tumor-Derived Extracellular Vesicles Combined with Tyrosine Kinase Inhibitors: An Effective and Safe Therapeutic Approach for Lung Adenocarcinoma with EGFR19Del. Vaccines, 12(12), 1412. https://doi.org/10.3390/vaccines12121412





