Automated, Quantitative Capillary Western Blots to Analyze Host Cell Proteins in COVID-19 Vaccine Produced in Vero Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Commercial Reagents
2.2. V590 COVID-19 Vaccine HCP Samples from Vero Cell
2.3. Detection and Quantitation of HCP Using SW in Live Virus COVID-19 Vaccine Samples
2.4. Spike Protein SW Method
2.5. HCP ELISA
2.6. SDS PAGE
2.7. Total Protein Assay
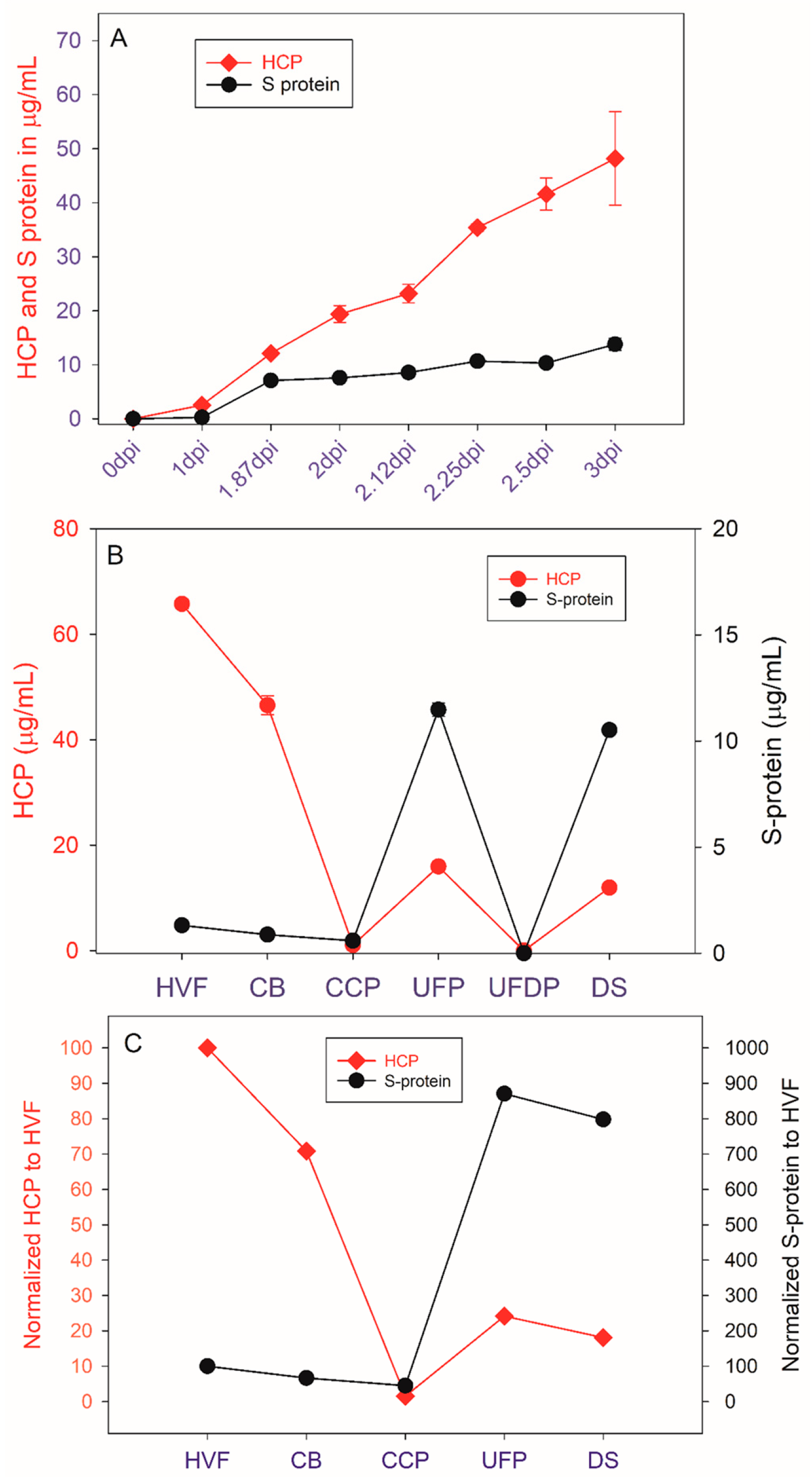
3. Results and Discussion
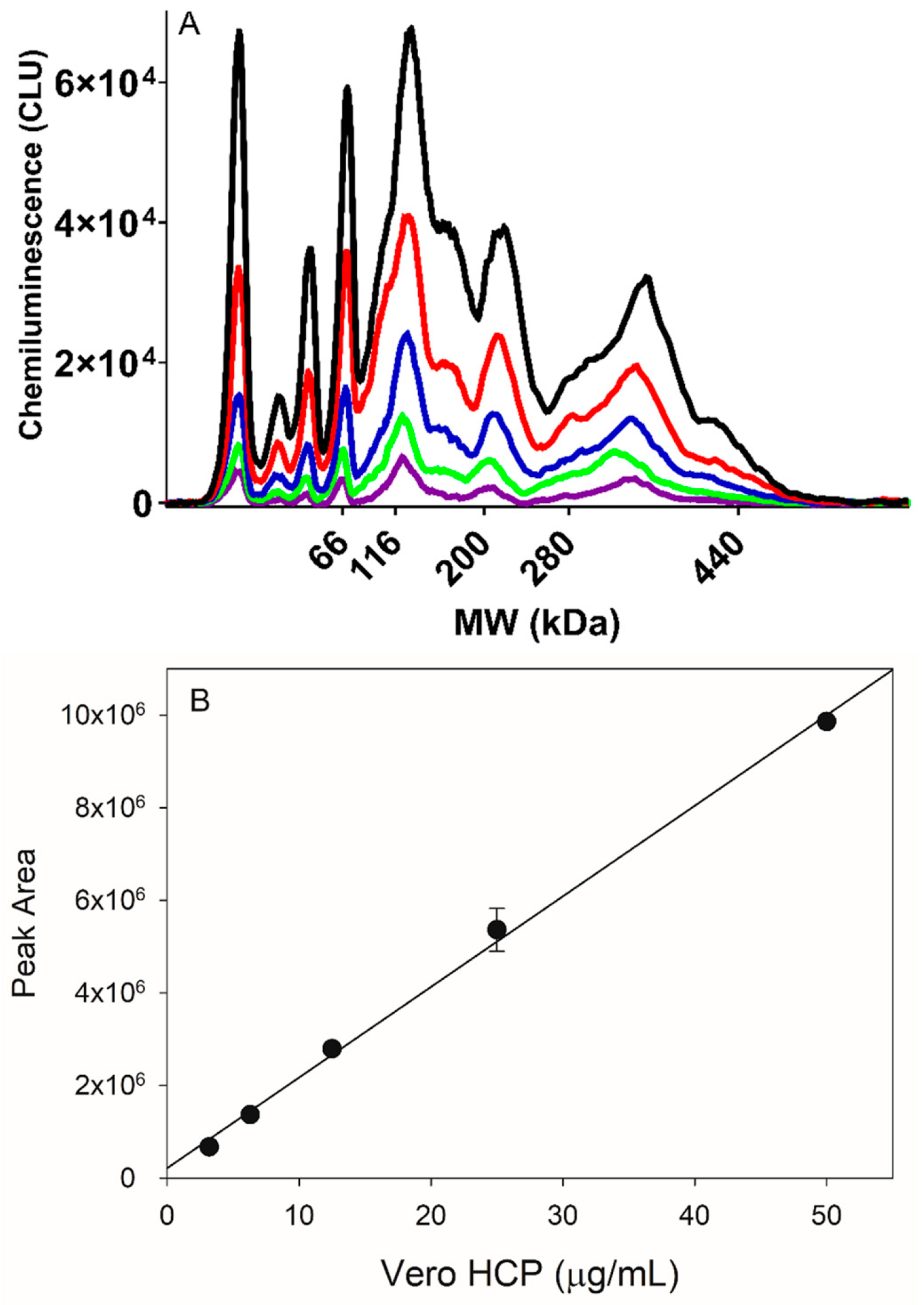
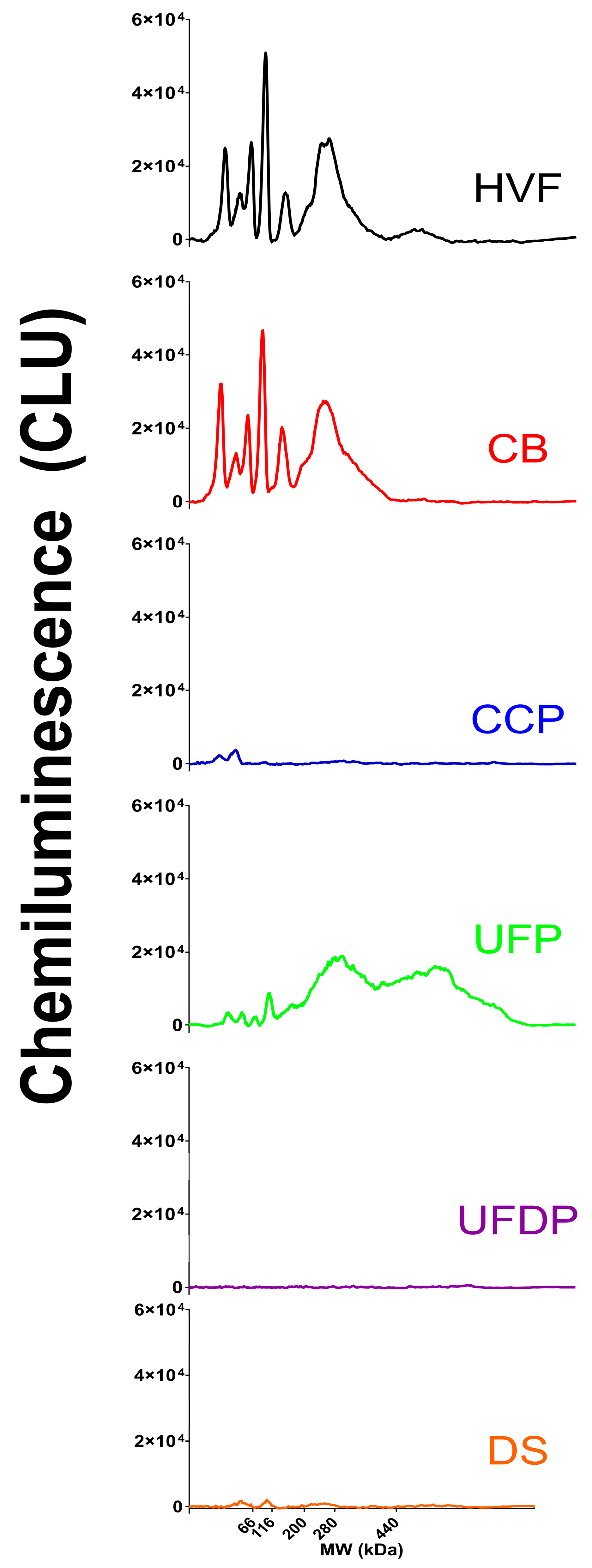
3.1. HCP Capillary Western Method Assay Development
3.2. Comparison of HCP SW and ELISA Testing
3.3. SDS PAGE HCP Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| UFP Sample | % rVSV Purity | Total Protein in µg/mL | rVSV in µg/mL | HCP (SDS PAGE) in µg/mL | HCP (SW) in µg/mL | HCP (ELISA) in µg/mL |
|---|---|---|---|---|---|---|
| Lot 1 | 40.3 | 22.0 | 8.90 | 13.1 | 12.9 | NT |
| Lot 2 | 25.6 | 90.0 | 23.0 | 67.0 | 67.8 | NT |
| Lot 3 | 47.5 | 72.0 | 34.2 | 37.8 | 32.6 | 1.07 |
| Lot 4 | 60.2 | 172 | 104 | 68.5 | 53.5 | 4.46 |
| Lot 5 | 74.8 | 70.0 | 52.4 | 17.6 | 9.60 | 0.720 |
| Lot 6 | 17.5 | 40.0 | 7.00 | 33.0 | 22.0 | 5.73 |
| Lot 7 | 53.1 | 49.0 | 26.0 | 23.0 | 27.3 | 6.48 |
| Lot 8 | 77.5 | 131 | 102 | 29.5 | 16.6 | 1.06 |
| Lot 9 | 77.2 | 24.0 | 18.5 | 5.50 | 3.70 | 1.64 |
| Lot10 | 74.8 | 27.0 | 20.2 | 6.80 | 4.50 | 1.98 |
| Lot 11 | 66.2 | 38.0 | 25.2 | 12.8 | 12.1 | 2.35 |
| Lot 12 | 77.6 | 96.0 | 74.5 | 21.5 | 10.2 | 0.500 |
| Lot 13 | 74.0 | 31.0 | 22.9 | 8.10 | 9.10 | 2.67 |
| Lot 14 | 78.0 | 77.0 | 60.1 | 16.9 | 7.60 | 0.820 |
| Lot 15 | 77.6 | 32.0 | 24.8 | 7.20 | 4.70 | 0.930 |
| Lot 16 | 78.0 | 79.0 | 61.6 | 17.4 | 15.2 | 3.85 |
| Lot 17 | 80.0 | 79.0 | 63.2 | 15.8 | 15.9 | 3.96 |
| Lot 18 | NT | 48.5 | NT | NT | 1.50 | 0.290 |
| Lot 19 | 78.4 | 50.0 | 39.2 | 10.8 | 6.70 | 0.350 |
| Lot 20 | 81.9 | 69.3 | 56.8 | 12.5 | 6.90 | 1.35 |
| Lot 21 | 80.8 | 75.0 | 60.6 | 14.4 | 15.3 | 3.77 |
| Lot 22 | 62.5 | 114 | 71.3 | 42.8 | 38.4 | 10.2 |
| Lot 23 | 90.2 | 69.0 | 62.2 | 6.80 | 4.60 | 0.770 |
| Lot 24 | 92.0 | 69.0 | 63.5 | 5.50 | 4.50 | 1.19 |
| Lot 25 | 82.9 | 120. | 99.5 | 20.5 | 18.9 | 4.69 |
| Lot 26 | 90.6 | 52.0 | 47.1 | 4.90 | 2.50 | 0.620 |
| Lot 27 | 90.6 | 65.0 | 58.9 | 6.10 | 4.40 | 0.550 |
| Lot 28 | 88.3 | 66.0 | 58.3 | 7.70 | 4.60 | 0.520 |
| Lot 29 | 75.6 | 40.0 | 30.20 | 9.80 | 5.80 | NT |
References
- Wang, F.; Li, X.; Swanson, M.; Guetschow, E.; Winston, M.; Smith, J.P.; Hoyt, E.; Liu, Z.; Richardson, D.; Bu, X.; et al. Holistic analytical characterization and risk assessment of residual host cell protein impurities in an active pharmaceutical ingredient synthesized by biocatalysts. Biotechnol. Bioeng. 2022, 119, 2088–2104. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, K.; Hamamura-Yasuno, E.; Nishimiya, D.; Soma, M.; Imaoka, M.; Kai, K.; Mori, K. Host cell proteins induce inflammation and immunogenicity as adjuvants in an integrated analysis of in vivo and in vitro assay systems. J. Pharmacol. Toxicol. Methods 2020, 103, 106694. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Palackal, N.; Wang, F.; Gaza-Bulseco, G.; Hurkmans, K.; Zhao, Y.; Chitikila, C.; Clavier, S.; Liu, S.; Menesale, E.; et al. “High-risk” host cell proteins (HCPs): A multi-company collaborative view. Biotechnol. Bioeng. 2021, 118, 2870–2885. [Google Scholar] [CrossRef]
- United States Pharmacopoeia. Residual Host Cell Protein Measurement in Biopharmaceuticals; United States Pharmacopoeia: North Bethesda, MD, USA, 2015. [Google Scholar] [CrossRef]
- Food and Drug Administration. CFR 610.13 Purity; Food and Drug Administration: Silver Spring, MD, USA, 2024.
- Host-Cell Protein Assays; European Pharmacopoeia: Amsterdam, The Netherlands, 2017.
- ICH. Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. In Proceedings of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; ICH: Geneva, Switzerland, 1999. [Google Scholar]
- Committee for Medicinal Products for Human Use. CPMP Position Statement on DNA and Host Cell Proteins (HCP) Impurities, Routine Testing versus Validation Studies; The European Agency for the Evaluation of Medicinal Products: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Krutzke, L.; Rösler, R.; Allmendinger, E.; Engler, T.; Wiese, S.; Kochanek, S. Process- and product-related impurities in the ChAdOx1 nCov-19 vaccine. eLife 2022, 11, e78513. [Google Scholar] [CrossRef] [PubMed]
- Zhu-Shimoni, J.; Yu, C.; Nishihara, J.; Wong, R.M.; Gunawan, F.; Lin, M.; Krawitz, D.; Liu, P.; Sandoval, W.; Vanderlaan, M. Host cell protein testing by ELISAs and the use of orthogonal methods. Biotechnol. Bioeng. 2014, 111, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Toinon, A.; Fontaine, C.; Thion, L.; Gajewska, B.; Carpick, B.; Nougarede, N.; Uhlrich, S. Host cell protein testing strategy for hepatitis B antigen in Hexavalent vaccine—Towards a general testing strategy for recombinant vaccines. Biologicals 2018, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Michalik, S.; Siegerist, F.; Palankar, R.; Franzke, K.; Schindler, M.; Reder, A.; Seifert, U.; Cammann, C.; Wesche, J.; Steil, L.; et al. Comparative analysis of ChAdOx1 nCoV-19 and Ad26.COV2.S SARS-CoV-2 vector vaccines. Haematologica 2022, 107, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, V.; Toll, H.; Mayer, R.E.; Visser, J.; Wolschin, F. A mass spectrometry-based approach to host cell protein identification and its application in a comparability exercise. Anal. Biochem. 2014, 463, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Obrstar, D.; Kroener, F.; Japelj, B.; Bojic, L.; Anderka, O. Host Cell Protein Profiling in Biopharmaceutical Harvests. Anal. Chem. 2018, 90, 11240–11247. [Google Scholar] [CrossRef] [PubMed]
- Berkelman, T.; Harbers, A.; Bandhakavi, S. 2-D Western blotting for evaluation of antibodies developed for detection of host cell protein. Methods Mol. Biol. 2015, 1295, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Szapiel, N.; Zhang, J.; Hickey, J.; Ghose, S. Profiling of host cell proteins by two-dimensional difference gel electrophoresis (2D-DIGE): Implications for downstream process development. Biotechnol. Bioeng. 2010, 105, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.; Squaglia, N.; Boge, A.; Fung, P.A. The Simple Western™: A gel-free, blot-free, hands-free Western blotting reinvention. Nat. Methods 2011, 8, v–vi. [Google Scholar] [CrossRef]
- Gillespie, P.F.; Wang, Y.; Hofmann, C.; Kuczynski, L.E.; Winters, M.A.; Teyral, J.L.; Tubbs, C.M.; Shiflett, K.; Patel, N.; Rustandi, R.R. Understanding the Spike Protein in COVID-19 Vaccine in Recombinant Vesicular Stomatitis Virus (rVSV) Using Automated Capillary Western Blots. ACS Omega 2023, 8, 3319–3328. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.; Wang, Y.; Alkurdi, E.; Zhang, Y.; Yin, K.; Rustandi, R.R.; Loughney, J.W. Quantitation of host cell proteins in biopharmaceuticals from chinese hamster ovarian and vero cell lines using capillary electrophoresis western blots. J. Pharm. Biomed. Anal. 2023, 233, 115420. [Google Scholar] [CrossRef] [PubMed]
- Espeseth, A.S.; Yuan, M.; Citron, M.; Reiserova, L.; Morrow, G.; Wilson, A.; Horton, M.; Rukhman, M.; Kinek, K.; Hou, F.; et al. Preclinical immunogenicity and efficacy of a candidate COVID-19 vaccine based on a vesicular stomatitis virus-SARS-CoV-2 chimera. EBioMedicine 2022, 82, 104203. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, L.E.; Shallow, J.R.; Watson, M.P.; Homsy, M.L.; Svab, T.; Gruber, A.; Rustandi, R.R.; Hu, J.; Winters, M.A. Adaptation of an rVSV Ebola vaccine purification process for rapid development of a viral vaccine candidate for SARS-CoV-2. Biotechnol. J. 2023, 19, e2300041. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, F.; Nishihara, J.; Liu, P.; Sandoval, W.; Vanderlaan, M.; Zhang, H.; Krawitz, D. Comparison of platform host cell protein ELISA to process-specific host cell protein ELISA. Biotechnol. Bioeng. 2017, 115, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Feldmann, H. Recombinant Vesicular Stomatitis Virus–Based Vaccines Against Ebola and Marburg Virus Infections. J. Infect. Dis. 2011, 204 (Suppl. S3), S1075–S1081. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Barenholz, Y.; Wagner, R.R. Vesicular stomatitis virus membrane proteins and their interactions with lipid bilayers. Biochim. Et Biophys. Acta BBA-Rev. Biomembr. 1987, 906, 175–193. [Google Scholar] [CrossRef] [PubMed]

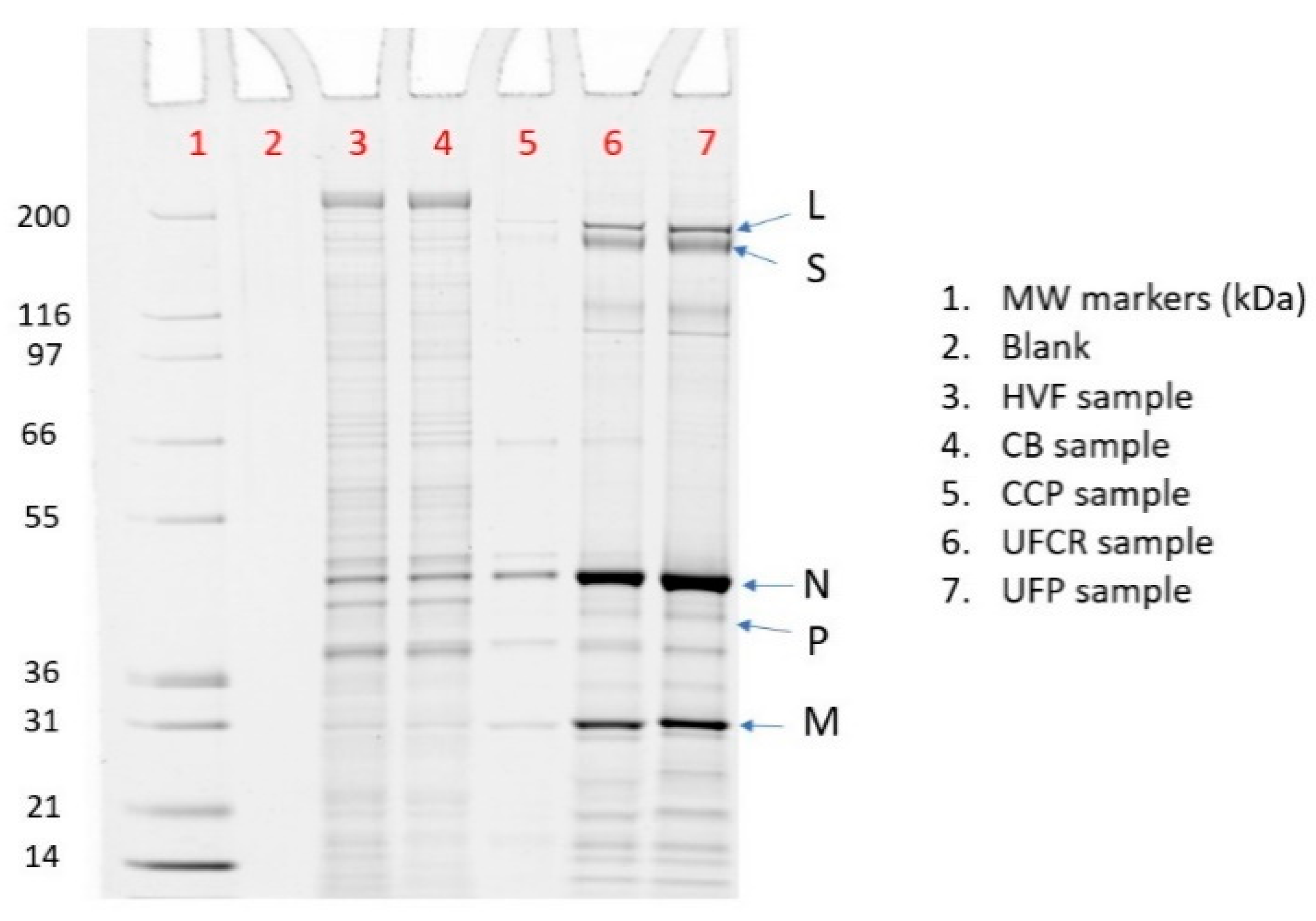


| Lane | Sample | % rVSV | % L | % S | % N | % P | % M |
|---|---|---|---|---|---|---|---|
| 3 | HVF | 16% | 1% | 2% | 12% | 0% | 2% |
| 4 | CB | 18% | 1% | 2% | 14% | 0% | 2% |
| 5 | CCP | 67% | 5% | 0% | 50% | 0% | 11% |
| 6 | UFCR | 78% | 5% | 8% | 46% | 2% | 16% |
| 7 | UFP | 80% | 7% | 8% | 46% | 2% | 17% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillespie, P.F.; Wang, Y.; Yin, K.; Groegler, E.; Cunningham, N.; Stiving, A.Q.; Raffaele, J.; Marusa, N.; Tubbs, C.M.; Loughney, J.W.; et al. Automated, Quantitative Capillary Western Blots to Analyze Host Cell Proteins in COVID-19 Vaccine Produced in Vero Cell Line. Vaccines 2024, 12, 1373. https://doi.org/10.3390/vaccines12121373
Gillespie PF, Wang Y, Yin K, Groegler E, Cunningham N, Stiving AQ, Raffaele J, Marusa N, Tubbs CM, Loughney JW, et al. Automated, Quantitative Capillary Western Blots to Analyze Host Cell Proteins in COVID-19 Vaccine Produced in Vero Cell Line. Vaccines. 2024; 12(12):1373. https://doi.org/10.3390/vaccines12121373
Chicago/Turabian StyleGillespie, Paul F., Yanjie Wang, Kuo Yin, Emily Groegler, Nicholas Cunningham, Alyssa Q. Stiving, Jessica Raffaele, Natalia Marusa, Christopher M. Tubbs, John W. Loughney, and et al. 2024. "Automated, Quantitative Capillary Western Blots to Analyze Host Cell Proteins in COVID-19 Vaccine Produced in Vero Cell Line" Vaccines 12, no. 12: 1373. https://doi.org/10.3390/vaccines12121373
APA StyleGillespie, P. F., Wang, Y., Yin, K., Groegler, E., Cunningham, N., Stiving, A. Q., Raffaele, J., Marusa, N., Tubbs, C. M., Loughney, J. W., Winters, M. A., & Rustandi, R. R. (2024). Automated, Quantitative Capillary Western Blots to Analyze Host Cell Proteins in COVID-19 Vaccine Produced in Vero Cell Line. Vaccines, 12(12), 1373. https://doi.org/10.3390/vaccines12121373






