Antibody Avidity Maturation Following Booster Vaccination with an Intranasal Adenovirus Salnavac Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Volunteers
2.3. Vaccines
2.4. SARS-CoV-2 Pseudovirus Production
2.5. SARS-CoV-2 S-Pseudotyped Lentivirus Neutralization Assay (pVNA)
2.6. Source of Recombinant Proteins
2.7. Biolayer Interferometry Measurement of KD, kon, and koff for Serum Antibodies
2.8. Avidity ELISA
2.9. Surrogate Virus Neutralization Assay on Chips (sVNT)
2.10. Statistical Analysis
3. Results
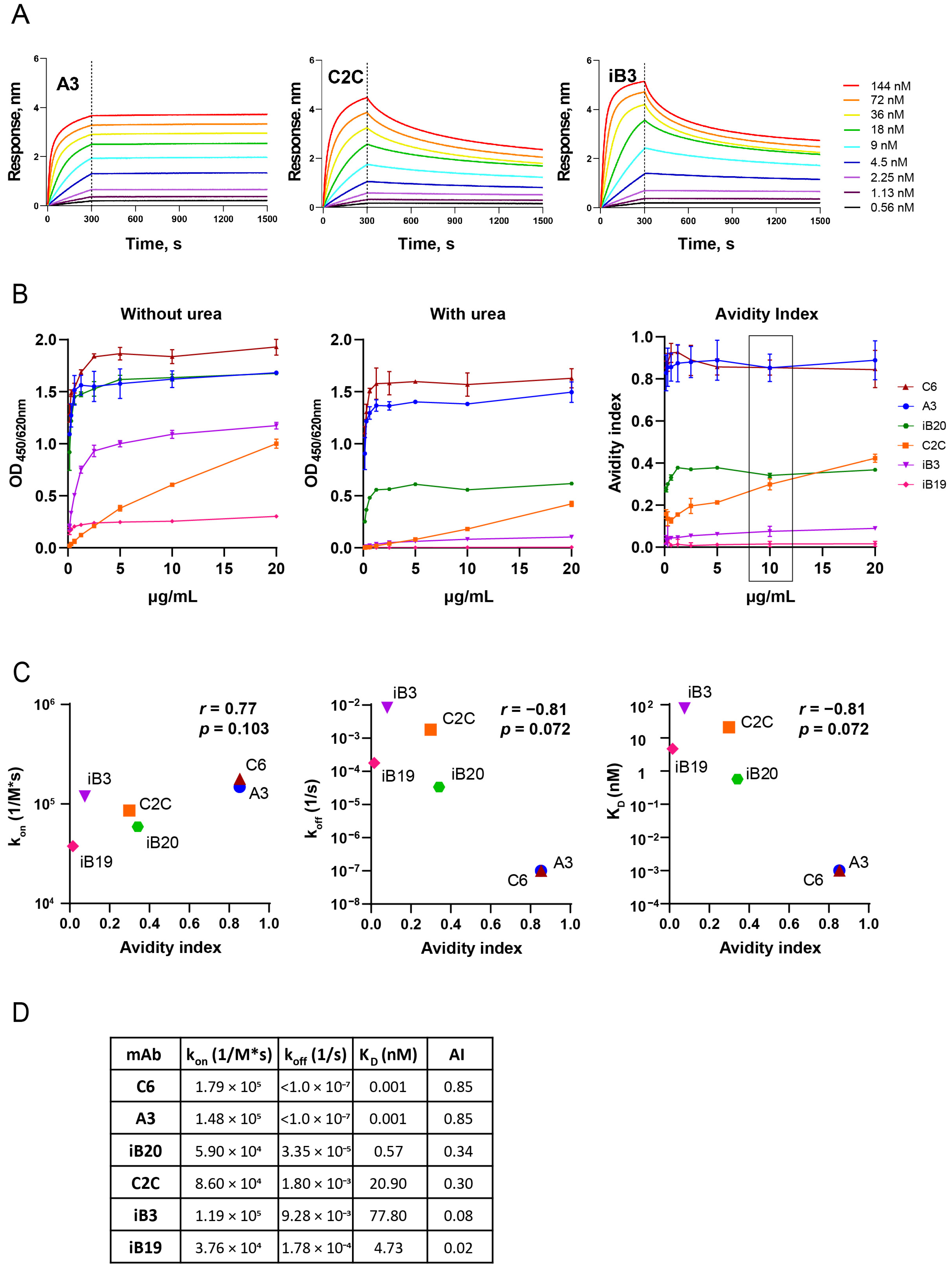
3.1. The Avidity of RBD-Specific mAbs
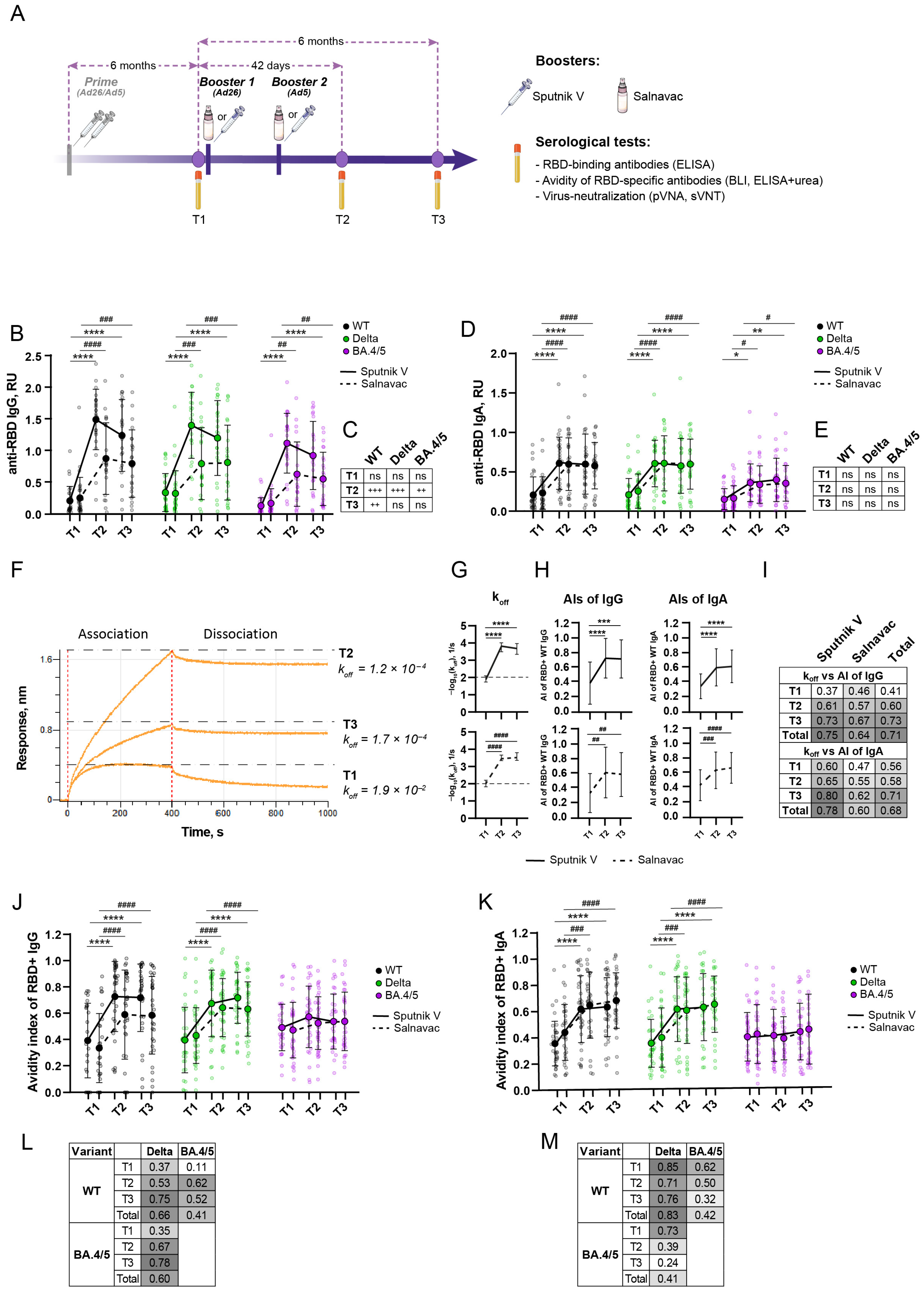
3.2. Study Design and Blood Sampling
3.3. A Strong Anti-RBD Humoral Immune Response Lasts at Least Six Months After a Booster with Sputnik V/Salnavac
3.4. Avidity Indexes of Polyclonal Sera Measured by BLI and ELISA with Urea Show High Concordance
3.5. Booster Vaccination Induced an Increase in Avidity Index for WT and Delta RBD but Not BA.4/5 RBD
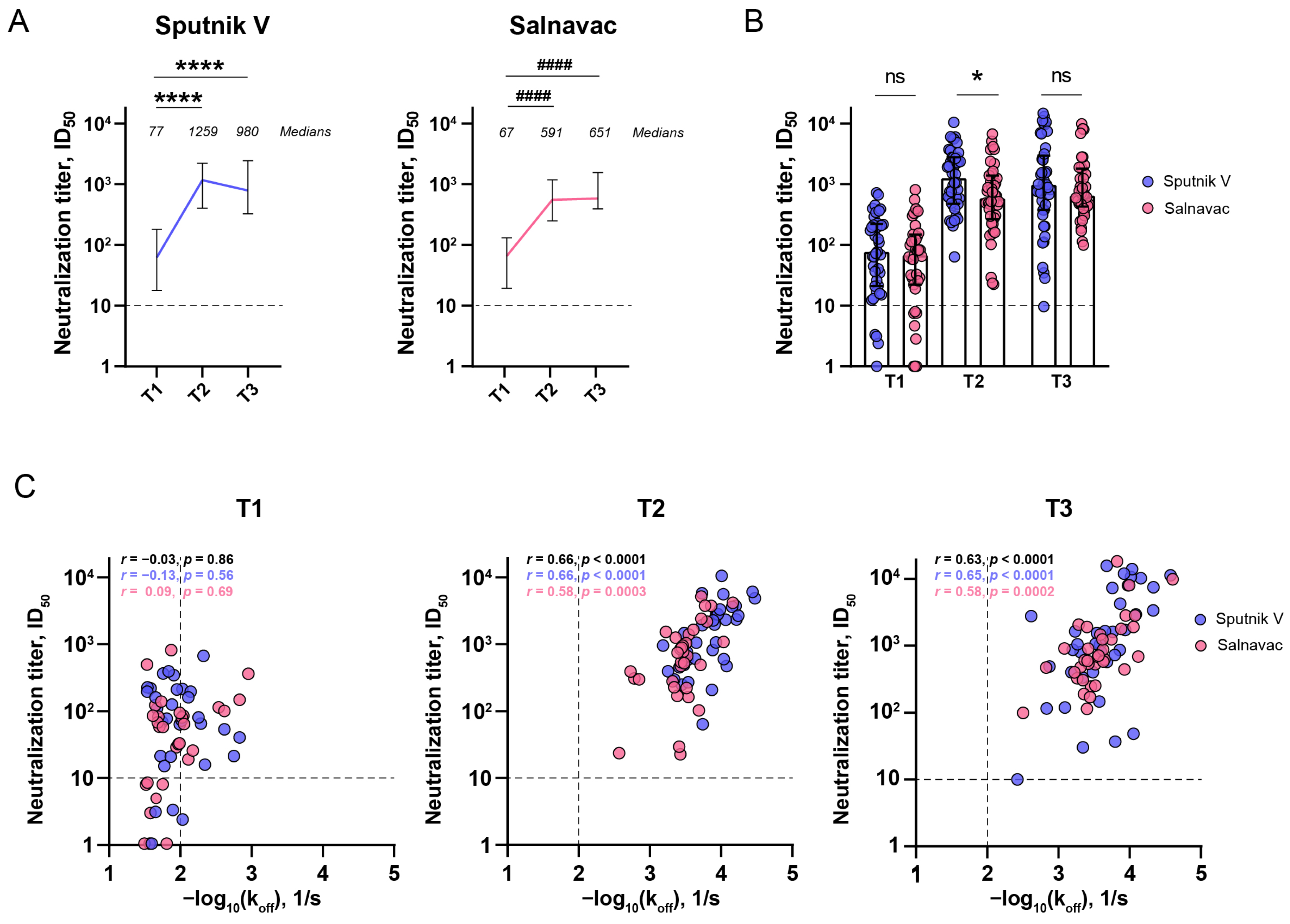
3.6. The Relationship Between the Serum Neutralizing Activity and Avidity After Sputnik and Salnavac Booster Vaccination
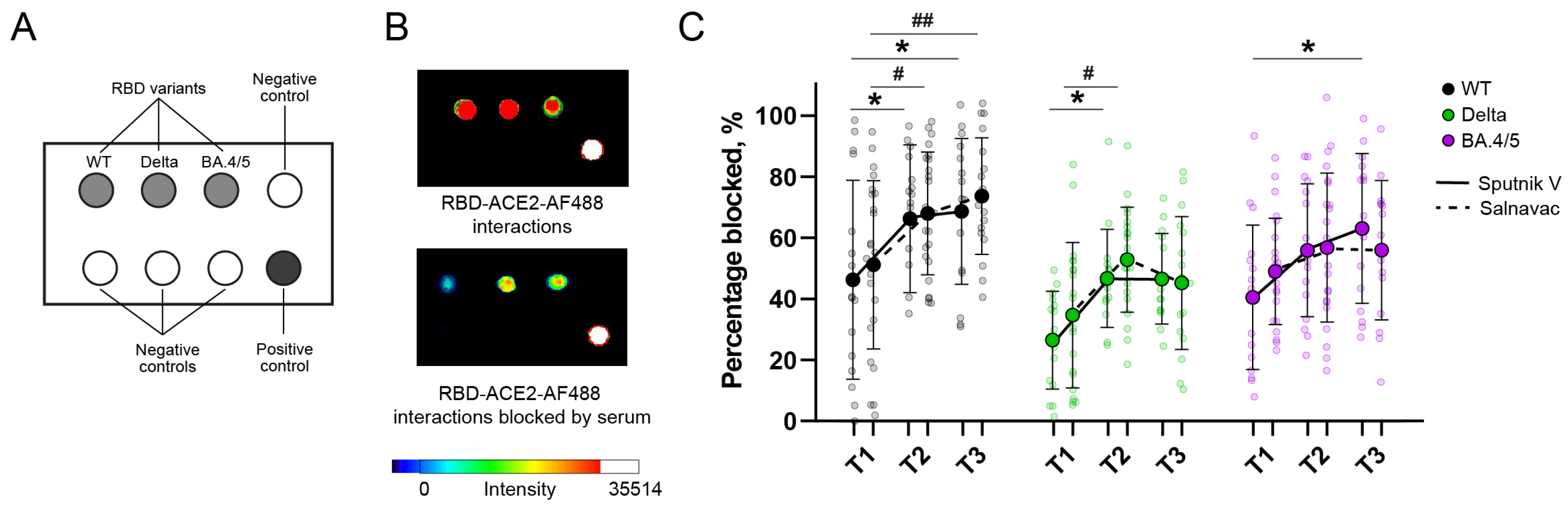
3.7. Surrogate Chip-Based Virus Neutralization Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plotkin, S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising Antibody Titres as Predictors of Protection against SARS-CoV-2 Variants and the Impact of Boosting: A Meta-Analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Mcdonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative Systematic Review and Meta-Analysis of Reactogenicity, Immunogenicity and Efficacy of Vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 74. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal Centers. Annu. Rev. Ofimmunol. 2022, 40, 413–442. [Google Scholar] [CrossRef]
- Rajewsky, K. Clonal Selection and Learning in the Antibody System. Nature 1996, 381, 751–758. [Google Scholar] [CrossRef]
- Oostindie, S.C.; Lazar, G.A.; Schuurman, J.; Parren, P.W.H.I. Avidity in Antibody Effector Functions and Biotherapeutic Drug Design. Nat. Rev. 2022, 21, 715–735. [Google Scholar] [CrossRef]
- Doria-rose, N.A.; Joyce, M.G. Strategies to Guide the Antibody Affinity Maturation Process. Curr. Opin. Virol. 2015, 11, 137–147. [Google Scholar] [CrossRef]
- Polack, F.P.; Teng, M.N.; Collins, P.L.; Prince, G.A.; Exner, M.; Regele, H.; Lirman, D.D.; Rabold, R.; Hoffman, S.J.; Karp, C.L.; et al. A Role for Immune Complexes in Enhanced Respiratory Syncytial Virus Disease. J. Exp. Med. 2002, 196, 859–865. [Google Scholar] [CrossRef]
- Alexander, M.R.; Ringe, R.; Sanders, R.W.; Voss, J.E.; Moore, J.P.; Klasse, J. What Do Chaotrope-Based Avidity Assays for Antibodies to HIV-1 Envelope Glycoproteins Measure? J. Virol. 2015, 89, 5981–5995. [Google Scholar] [CrossRef]
- Pullen, G.R.; Fitzgerald, M.G.; Hosking, C.S. Antibody Avidity Determination by ELISA Using Thiocyanate Elution. J. Immunol. Methods 1986, 86, 83–87. [Google Scholar] [CrossRef]
- Khurana, S.; Verma, N.; Yewdell, J.W.; Hilbert, A.K.; Castellino, F.; Lattanzi, M.; Del Giudice, G.; Rappuoli, R.; Golding, H. MF59 Adjuvant Enhances Diversity and Affinity of Antibody-Mediated Immune Response to Pandemic Influenza Vaccines. Sci. Transl. Med. 2011, 3, 85ra48. [Google Scholar] [CrossRef]
- Narita, M.; Matsuzono, Y.; Takekoshi, Y.; Yamada, S.; Itakura, O.; Kubota, M.; Kikuta, H.; Togashi, T. Analysis of Mumps Vaccine Failure by Means of Avidity Testing for Mumps Virus-Specific Immunoglobulin G. Clin. Diagn. Lab. Immunol. 1998, 5, 799–803. [Google Scholar] [CrossRef]
- Tsuji, I.; Dominguez, D.; Egan, M.A.; Dean, H.J. Development of a Novel Assay to Assess the Avidity of Dengue Virus-Specific Antibodies Elicited in Response to a Tetravalent Dengue Vaccine. J. Infect. Dis. 2022, 225, 1533–1544. [Google Scholar] [CrossRef]
- Ravichandran, S.; Hahn, M.; Belaunzarán-Zamudio, P.F.; Ramos-Castañeda, J.; Nájera-Cancino, G.; Caballero-Sosa, S.; Navarro-fuentes, K.R.; Ruiz-Palacios, G.; Golding, H.; Beigel, J.H.; et al. Differential Human Antibody Repertoires Following Zika Infection and the Implications for Serodiagnostics and Disease Outcome. Nat. Commun. 2019, 10, 1943. [Google Scholar] [CrossRef]
- Monsalvo, A.C.; Batalle, J.P.; Lopez, M.F.; Krause, J.C.; Klemenc, J.; Hernandez, J.Z.; Maskin, B.; Bugna, J.; Rubinstein, C.; Aguilar, L.; et al. Severe Pandemic 2009 H1N1 Influenza Disease Due to Pathogenic Immune Complexes. Nat. Med. 2011, 17, 195–200. [Google Scholar] [CrossRef]
- Tang, J.; Ravichandran, S.; Lee, Y.; Grubbs, G.; Coyle, E.M.; Klenow, L.; Genser, H.; Golding, H.; Khurana, S. Antibody Affinity Maturation and Plasma IgA Associate with Clinical Outcome in Hospitalized COVID-19 Patients. Nat. Commun. 2021, 12, 1221. [Google Scholar] [CrossRef]
- Hendriks, J.; Schasfoort, R.; Koerselman, M.; Dannenberg, M.; Cornet, A.D.; Beishuizen, A.; van der Palen, J.; Krabbe, J.; Mulder, A.H.L.; Karperien, M. High Titers of Low Affinity Antibodies in COVID-19 Patients Are Associated with Disease Severity. Front. Immunol. 2022, 13, 867716. [Google Scholar] [CrossRef]
- Bauer, G. The Potential Significance of High Avidity Immunoglobulin G (IgG) for Protective Immunity towards SARS-CoV-2. Int. J. Infect. Dis. 2021, 106, 61–64. [Google Scholar] [CrossRef]
- Pušnik, J.; König, J.; Mai, K.; Richter, E.; Zorn, J.; Proksch, H.; Schulte, B.; Alter, G.; Streeck, H. Persistent Maintenance of Atypical Memory B Cells Following SARS-CoV-2 Infection and Vaccination Recall Response. SSRN Electron. J. 2022, 96, e00760-22. [Google Scholar] [CrossRef]
- Infection, S.-; Tauzin, A.; Gendron-lepage, G.; Nayrac, M.; Anand, S.P.; Bourassa, C.; Medjahed, H.; Goyette, G.; Dub, M.; Kaufmann, D.E. Evolution of Anti-RBD IgG Avidity Following SARS-CoV-2 Infection. Viruses 2022, 14, 532. [Google Scholar] [CrossRef]
- Bullock, J.L.; Hickey, T.E.; Kemp, T.J.; Metz, J.; Loftus, S.; Haynesworth, K.; Castro, N.; Luke, B.T.; Lowy, D.R.; Pinto, L.A. Longitudinal Assessment of BNT162b2- and mRNA-1273-Induced Anti-SARS-CoV-2 Spike IgG Levels and Avidity Following Three Doses of Vaccination. Vaccines 2024, 12, 516. [Google Scholar] [CrossRef]
- Scheiblauer, H.; Micha, C.; Wolf, T.; Khodamoradi, Y.; Bellinghausen, C.; Sonntagbauer, M.; Esser-nobis, K.; Filomena, A.; Mahler, V.; Jürgen, T.; et al. Antibody Response to SARS-CoV-2 for More than One Year − Kinetics and Persistence of Detection Are Predominantly Determined by Avidity Progression and Test Design. J. Clin. Virol. 2022, 146, 105052. [Google Scholar] [CrossRef]
- Tauzin, A.; Gong, S.Y.; Chatterjee, D.; Ding, S.; Painter, M.M.; Goel, R.R.; Beaudoin-Bussières, G.; Marchitto, L.; Boutin, M.; Laumaea, A.; et al. A Boost with SARS-CoV-2 BNT162b2 mRNA Vaccine Elicits Strong Humoral Responses Independently of the Interval between the First Two Doses. Cell Rep. 2022, 41, 111554. [Google Scholar] [CrossRef]
- Kashte, S.; Gulbake, A.; El, S.F.; Iii, A.; Gupta, A. COVID-19 Vaccines: Rapid Development, Implications, Challenges and Future Prospects Indian Council of Medical Research. Hum. Cell 2021, 34, 711–733. [Google Scholar] [CrossRef]
- Krammer, B.F.; Ellebedy, A.H. Variant-Adapted COVID-19 Booster Vaccines. Science 2023, 382, 157–159. [Google Scholar] [CrossRef]
- Nicolas, A.; Sannier, G.; Dubé, M.; Nayrac, M.; Tauzin, A.; Painter, M.M.; Goel, R.R.; Laporte, M.; Gendron-Lepage, G.; Medjahed, H.; et al. An Extended SARS-CoV-2 MRNA Vaccine Prime-Boost Interval Enhances B Cell Immunity with Limited Impact on T Cells. iScience 2023, 26, 105904. [Google Scholar] [CrossRef]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of Standard and Extended Dosing Intervals of BNT162b2 mRNA Vaccine. Cell 2021, 184, 5699–5714. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal Vaccines—Fortifying the Frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Pilapitiya, D.; Wheatley, A.K.; Tan, H. Review Mucosal Vaccines for SARS-CoV-2: Triumph of Hope over Experience. eBioMedicine 2023, 92, 104585. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Purushotham, J.N.; Schulz, J.E.; Holbrook, M.G.; Bushmaker, T.; Carmody, A.; Port, J.R.; Yinda, C.K.; Okumura, A.; Saturday, G.; et al. Intranasal ChAdOx1 NCoV-19/AZD1222 Vaccination Reduces Viral Shedding after SARS-CoV-2 D614G Challenge in Preclinical Models. Sci. Transl. Med. 2021, 13, eabh0755. [Google Scholar] [CrossRef]
- Hassan, A.O.; Shrihari, S.; Gorman, M.J.; Curiel, D.T.; Alter, G.; Diamond, M.S.; Hassan, A.O.; Shrihari, S.; Gorman, M.J.; Ying, B.; et al. Article An Intranasal Vaccine Durably Protects against SARS-CoV-2 Variants in Mice Ll An Intranasal Vaccine Durably Protects against SARS-CoV-2 Variants in Mice. Cell Rep. 2021, 36, 109452. [Google Scholar] [CrossRef]
- Zuev, E.V.; Markova, O.A.; Kulemzin, S.V.; Poteryaev, D.A.; Litvinova, N.A.; Korotkevich, I.A.; Grigoryeva, T.V.; Khamitov, R.A. Virus Neutralizing Antibodies in Pseudovirus Particle Neutralization Reaction as a Bioanalytical Part of a Salnavac® Vaccine Clinical Trial. Russ. J. Infect. Immun. 2023, 13, 853–863. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, Tolerability, and Immunogenicity of an Aerosolised Adenovirus Type-5 Vector-Based COVID-19 Vaccine (Ad5-NCoV) in Adults: Preliminary Report of an Open-Label and Randomised Phase 1 Clinical Trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- Aase, A.; Næss, L.M.; Sandin, R.H.; Herstad, T.K.; Oftung, F.; Holst, J.; Haugen, I.L.; Høiby, E.A.; Michaelsen, T.E. Comparison of Functional Immune Responses in Humans after Intranasal and Intramuscular Immunisations with Outer Membrane Vesicle Vaccines against Group B Meningococcal Disease. Vaccine 2003, 21, 2042–2051. [Google Scholar] [CrossRef]
- Rothen, D.A.; Krenger, P.S.; Nonic, A.; Balke, I.; Vogt, C.S.; Chang, X.; Manenti, A.; Vedovi, F.; Resevica, G.; Walton, S.M.; et al. Intranasal Administration of a Virus like Particles- Based Vaccine Induces Neutralizing Antibodies against SARS-CoV 2 and Variants of Concern. Allergy 2022, 77, 2446–2458. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Mantooth, S.M.; Vrabel, M.R.; Zaharoff, D.A. Intranasal Delivery of Thermostable Subunit Vaccine for Cross-Reactive Mucosal and Systemic Antibody Responses Against SARS-CoV-2. Front. Immunol. 2022, 13, 858904. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Q.; Chang, H. Vaccines for COVID-19: A Systematic Review of Immunogenicity, Current Development, and Future Prospects. Front. Immunol. 2022, 13, 843928. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccine 2023, 11, 432. [Google Scholar] [CrossRef]
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Atluri, P. Review of COVID-19 Viral Vector-Based Vaccines and COVID-19 Variants. Le. Infez. Med. 2021, 29, 328. [Google Scholar] [CrossRef]
- Bellusci, L.; Grubbs, G.; Zahra, F.T.; Forgacs, D.; Golding, H.; Ross, T.M.; Khurana, S. Antibody Affinity and Cross-Variant Neutralization of SARS-CoV-2 Omicron BA.1, BA.2 and BA.3 Following Third mRNA Vaccination. Nat. Commun. 2022, 13, 4617. [Google Scholar] [CrossRef]
- Singh, G.; Abbad, A.; Tcheou, J.; Mendu, D.R.; Firpo-Betancourt, A.; Gleason, C.; Srivastava, K.; Cordon-Cardo, C.; Simon, V.; Krammer, F.; et al. Binding and Avidity Signatures of Polyclonal Sera From Individuals With Different Exposure Histories to Severe Acute Respiratory Syndrome Coronavirus 2 Infection, Vaccination, and Omicron Breakthrough Infections. J. Infect. Dis. 2023, 228, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.; Brugger, S.D.; Von, A.; Emmenegger, M.; Fiedler, S.; Brugger, S.D.; Devenish, S.R.A.; Morgunov, A.S.; Meisl, G.; Lynn, A.K.; et al. Both COVID-19 Infection and Vaccination Induce High-Affinity Cross-Clade Responses to SARS-CoV-2 Variants. iScience 2022, 25, 104766. [Google Scholar] [CrossRef]
- Gorchakov, A.A.; Kulemzin, S.V.; Guselnikov, S.V.; Baranov, K.O.; Belovezhets, T.N.; Mechetina, L.V.; Volkova, O.Y.; Najakshin, A.M.; Chikaev, N.A.; Chikaev, A.N.; et al. Isolation of a Panel of Ultra-Potent Human Antibodies Neutralizing SARS-CoV-2 and Viral Variants of Concern. Cell Discov. 2021, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Hahn, M.; Coyle, E.M.; King, L.R.; Lin, T.; Treanor, J.; Sant, A.; Golding, H. Repeat Vaccination Reduces Antibody Affinity Maturation across Different Influenza Vaccine Platforms in Humans. Nat. Commun. 2019, 10, 3338. [Google Scholar] [CrossRef]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 Vaccines: From Bench to Bed. eBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, H.S.; Karbaschi, M.; Racine-Brzostek, S.E.; Li, P.; Zuk, R.; Yang, Y.J.; Klasse, P.J.; Shi, Y.; Zhao, Z. Measurements of SARS-CoV-2 Antibody Dissociation Rate Constant by Chaotrope-Free Biolayer Interferometry in Serum of COVID-19 Convalescent Patients. Biosens. Bioelectron. 2022, 209, 114237. [Google Scholar] [CrossRef]
- Dimitrov, J.D.; Lacroix-Desmazes, S.; Kaveri, S.V. Important Parameters for Evaluation of Antibody Avidity by Immunosorbent Assay. Anal. Biochem. 2011, 418, 149–151. [Google Scholar] [CrossRef]
- Eltanbouly, M.A.; Ramos, V.; Maclean, A.J.; Chen, S.T.; Loewe, M.; Steinbach, S.; Tanfous, T.B.; Johnson, B.; Cipolla, M.; Gazumyan, A.; et al. Role of Affinity in Plasma Cell Development in the Germinal Center Light Zone. J. Exp. Med. 2024, 221, e20231838. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Hentenaar, I.T.; Morrison-Porter, A.; Solano, D.; Haddad, N.S.; Castrillon, C.; Runnstrom, M.C.; Lamothe, P.A.; Andrews, J.; Roberts, D.; et al. SARS-CoV-2-Specific Plasma Cells Are Not Durably Established in the Bone Marrow Long-Lived Compartment after mRNA Vaccination. Nat. Med. 2024, 1–10. [Google Scholar] [CrossRef]
- Klasse, P.J. How to Assess the Binding Strength of Antibodies Elicited by Vaccination against HIV and Other Viruses. Expert. Rev. Vaccines 2016, 15, 295–311. [Google Scholar] [CrossRef]
- Li, K.; Dodds, M.; Spreng, R.L.; Abraha, M.; Huntwork, R.H.C.; Dahora, L.C.; Nyanhete, T.; Dutta, S.; Wille-Reece, U.; Jongert, E.; et al. A Tool for Evaluating Heterogeneity in Avidity of Polyclonal Antibodies. Front. Immunol. 2023, 14, 1049673. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.M.; Unger, E.R.; Panicker, G. Description of a Novel Multiplex Avidity Assay for Evaluating HPV Antibodies. J. Immunol. Methods 2017, 447, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Hoffman, S.J.; Crujeiras, G.; Griffin, D.E. A Role for Nonprotective Complement-Fixing Antibodies with Low Avidity for Measles Virus in Atypical Measles. Nat. Med. 2003, 9, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Portilho, A.I.; Santos, J.S.; Trzewikoswki de Lima, G.; Lima, G.G.; De Gaspari, E. Study of Avidity-ELISA: Comparison of Chaotropic Agents, Incubation Temperature and Affinity Maturation after Meningococcal Immunization. J. Immunol. Methods 2023, 512, 113387. [Google Scholar] [CrossRef]
- Correa, V.A.; Rodrigues, T.S.; Portilho, A.I.; Trzewikoswki de Lima, G.; De Gaspari, E. Modified ELISA for Antibody Avidity Evaluation: The Need for Standardization. Biomed. J. 2021, 44, 433–438. [Google Scholar] [CrossRef]
- Hollander, Z.; Katchalski-Katzir, E. Use of Monoclonal Antibodies to Detect Conformational Alterations in Lactate Dehydrogenase Isoenzyme 5 on Heat Denaturation and on Adsorption to Polystyrene Plates. Mol. Immunol. 1986, 23, 927–933. [Google Scholar] [CrossRef]
- Butler, J.E.; Navarro, P.; Sun, J. Adsorption-induced antigenic changes and their significance in ELISA and immunological disorders. In Immunological and Molecular Diagnosis of Infectious Disease; CRC Press: Boca Raton, FL, USA, 1997; pp. 53–68. [Google Scholar]
- Butler, J.E.; Ni, L.; Nessler, R.; Joshi, K.S.; Suter, M.; Rosenberg, B.; Chang, J.; Brown, W.R.; Cantarero, L.A. The physical and functional behavior of capture antibodies adsorbed on polystyrene. J. Immunol. Methods. 1992, 150, 77–90. [Google Scholar] [CrossRef]
- Kwong, J.C.; Chung, H.; Jung, J.K.H.; Buchan, S.A.; Campigotto, A.; Campitelli, M.A.; Crowcroft, N.S.; Gubbay, J.B.; Karnauchow, T.; Katz, K.; et al. The Impact of Repeated Vaccination Using 10-Year Vaccination History on Protection against Influenza in Older Adults: A Test-Negative Design Study across the 2010/11 to 2015/16 Influenza Seasons in Ontario, Canada. Eurosurveillance 2020, 25, 1900245. [Google Scholar] [CrossRef]
- Baumgarth, N. How Specific Is Too Specific? B-Cell Responses to Viral Infections Reveal the Importance of Breadth over Depth. Immunol. Rev. 2013, 255, 82–94. [Google Scholar] [CrossRef]
- Horndler, L.; Delgado, P.; Romeropinedo, S.; Llamas, M.A.; Almendrova, P. Decreased Breadth of the Antibody Response to the Spike Protein of SARS-CoV-2 after Repeated Vaccination. Front. Immunol. 2023, 14, 1157263. [Google Scholar] [CrossRef]
- Astakhova, E.A.; Morozov, A.A.; Byazrova, M.G.; Sukhova, M.M.; Mikhailov, A.A.; Minnegalieva, A.R.; Gorchakov, A.A.; Filatov, A.V. Antigenic Cartography Indicates That the Omicron BA.1 and BA.4/BA.5 Variants Remain Antigenically Distant to Ancestral SARS-CoV-2 after Sputnik V Vaccination Followed by Homologous (Sputnik V) or Heterologous (Comirnaty) Revaccination. Int. J. Mol. Sci. 2023, 24, 10493. [Google Scholar] [CrossRef] [PubMed]
- Edara, V.V.; Manning, K.E.; Ellis, M.; Lai, L.; Moore, K.M.; Foster, S.L.; Floyd, K.; Davis-Gardner, M.E.; Mantus, G.; Nyhoff, L.E.; et al. mRNA-1273 and BNT162b2 mRNA Vaccines Have Reduced Neutralizing Activity against the SARS-CoV-2 Omicron Variant. Cell Rep. Med. 2022, 3, 100529. [Google Scholar] [CrossRef] [PubMed]
- Muecksch, F.; Weisblum, Y.; Barnes, C.O.; Bjorkman, P.J.; Hatziioannou, T.; Bieniasz, P.D.; Muecksch, F.; Weisblum, Y.; Barnes, C.O.; Schmidt, F.; et al. Article Affinity Maturation of SARS-CoV-2 Neutralizing Antibodies Confers Potency, Breadth, and Resilience to Viral Escape Mutations Ll Affinity Maturation of SARS-CoV-2 Neutralizing Antibodies Confers Potency, Breadth, and Resilience to Viral Escape. Immunity 2021, 54, 1853–1868.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Q.; Vishwanath, S.; Carnell, G.W.; Chun, A.; Chan, Y.; Heeney, J.L. Immune Imprinting and Next-Generation Coronavirus Vaccines. Nat. Microbiol. 2023, 8, 1971–1985. [Google Scholar] [CrossRef] [PubMed]
- Nakagama, Y.; Candray, K.; Kaku, N.; Komase, Y.; Rodriguez-Funes, M.V.; Dominguez, R.; Tsuchida, T.; Kunishima, H.; Nagai, E.; Adachi, E.; et al. Antibody Avidity Maturation Following Recovery From Infection or the Booster Vaccination Grants Breadth of SARS-CoV-2 Neutralizing Capacity. J. Infect. Dis. 2023, 227, 780–787. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 Surrogate Virus Neutralization Test Based on Antibody-Mediated Blockage of ACE2–Spike Protein–Protein Interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Mariën, J.; Michiels, J.; Heyndrickx, L.; Nkuba-Ndaye, A.; Ceulemans, A.; Bartholomeeusen, K.; Madinga, J.; Mbala-Kingebeni, P.; Vanlerberghe, V.; Ahuka-Mundeke, S.; et al. Evaluation of a Surrogate Virus Neutralization Test for High-Throughput Serosurveillance of SARS-CoV-2. J. Virol. Methods 2021, 297, 114228. [Google Scholar] [CrossRef]
- Santos da Silva, E.; Servais, J.Y.; Kohnen, M.; Arendt, V.; Staub, T.; Krüger, R.; Fagherazzi, G.; Wilmes, P.; Hübschen, J.M.; Ollert, M.; et al. Validation of a SARS-CoV-2 Surrogate Neutralization Test Detecting Neutralizing Antibodies against the Major Variants of Concern. Int. J. Mol. Sci. 2023, 24, 14965. [Google Scholar] [CrossRef]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Srimuan, D.; Thatsanatorn, T.; et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and mRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines 2022, 10, 86. [Google Scholar] [CrossRef]
- Kim, S.J.; Yao, Z.; Marsh, M.C.; Eckert, D.M.; Kay, M.S.; Lyakisheva, A.; Pasic, M.; Bansal, A.; Birnboim, C.; Jha, P.; et al. Homogeneous Surrogate Virus Neutralization Assay to Rapidly Assess Neutralization Activity of Anti-SARS-CoV-2 Antibodies. Nat. Commun. 2022, 13, 3716. [Google Scholar] [CrossRef]
- Lynch, K.L.; Zhou, S.; Kaul, R.; Walker, R.; Wu, A.H. Evaluation of Neutralizing Antibodies against SARS-CoV-2 Variants after Infection and Vaccination Using a Multiplexed Surrogate Virus Neutralization Test. Clin. Chem. 2022, 68, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Heggestad, J.T.; Britton, R.J.; Kinnamon, D.S.; Wall, S.A.; Joh, D.Y.; Hucknall, A.M.; Olson, L.B.; Anderson, J.G.; Mazur, A.; Wolfe, C.R.; et al. Rapid Test to Assess the Escape of SARS-CoV-2 Variants of Concern. Sci. Adv. 2021, 7, eabl7682. [Google Scholar] [CrossRef] [PubMed]
- McDade, T.W.; Demonbreun, A.R.; Sancilio, A.; Mustanski, B.; D’Aquila, R.T.; McNally, E.M. Durability of Antibody Response to Vaccination and Surrogate Neutralization of Emerging Variants Based on SARS-CoV-2 Exposure History. Sci. Rep. 2021, 11, 17325. [Google Scholar] [CrossRef] [PubMed]
- Springer, D.N.; Höltl, E.; Prüger, K.; Puchhammer-Stöckl, E.; Aberle, J.H.; Stiasny, K.; Weseslindtner, L. Measuring Variant-Specific Neutralizing Antibody Profiles after Bivalent SARS-CoV-2 Vaccinations Using a Multivariant Surrogate Virus Neutralization Microarray. Vaccines 2024, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, X.; Wang, H.; Du, G.; Sun, X. Recent Advances in Respiratory Immunization: A Focus on COVID-19 Vaccines. J. Control Release 2023, 355, 655–674. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Wang, Q.; Yang, Q.; Yin, L.; Ning, L.; Chen, Z.; Tang, J.; Deng, W.; He, P.; et al. Intranasal Adenovirus-Vectored Omicron Vaccine Induced Nasal Immunoglobulin A Has Superior Neutralizing Potency than Serum Antibodies. Signal Transduct. Target. Ther. 2024, 9, 190. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astakhova, E.A.; Baranov, K.O.; Shilova, N.V.; Polyakova, S.M.; Zuev, E.V.; Poteryaev, D.A.; Taranin, A.V.; Filatov, A.V. Antibody Avidity Maturation Following Booster Vaccination with an Intranasal Adenovirus Salnavac Vaccine. Vaccines 2024, 12, 1362. https://doi.org/10.3390/vaccines12121362
Astakhova EA, Baranov KO, Shilova NV, Polyakova SM, Zuev EV, Poteryaev DA, Taranin AV, Filatov AV. Antibody Avidity Maturation Following Booster Vaccination with an Intranasal Adenovirus Salnavac Vaccine. Vaccines. 2024; 12(12):1362. https://doi.org/10.3390/vaccines12121362
Chicago/Turabian StyleAstakhova, Ekaterina A., Konstantin O. Baranov, Nadezhda V. Shilova, Svetlana M. Polyakova, Evgeniy V. Zuev, Dmitry A. Poteryaev, Alexander V. Taranin, and Alexander V. Filatov. 2024. "Antibody Avidity Maturation Following Booster Vaccination with an Intranasal Adenovirus Salnavac Vaccine" Vaccines 12, no. 12: 1362. https://doi.org/10.3390/vaccines12121362
APA StyleAstakhova, E. A., Baranov, K. O., Shilova, N. V., Polyakova, S. M., Zuev, E. V., Poteryaev, D. A., Taranin, A. V., & Filatov, A. V. (2024). Antibody Avidity Maturation Following Booster Vaccination with an Intranasal Adenovirus Salnavac Vaccine. Vaccines, 12(12), 1362. https://doi.org/10.3390/vaccines12121362




